 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Palabras clave: atherosclerosis, statins, epigenetics, immunomodulation, cytokines
Atherosclerosis is an immune-inflammatory disease, in which pathophysiological mechanisms include inflammation patterns and epigenetic changes that alter gene expression of several inflammatory and non-inflammatory mediators. Epigenetics is offering explanations on how diet, environmental factors and lifestyle can influence the onset and progression of the disease, and how these alterations can be transmitted to the following generations without any changes in DNA sequences. Statins, through their pleiotropic effects, provide a useful tool in controlling the progression of plaques and their subsequent impact.
Atherosclerosis is a progressive disease, type immune inflammatory, characterized by the accumulation of lipids in the media layer of large and medium arteries walls. It is now considered a common disease in which, plaques and fatty deposits appear in the inner layer of the arteries [1]. The formation of these plaques begins with the deposition of small crystals in the intima and its underlying muscle layer, which grows with the proliferation of fibrous tissue and smooth muscle tissue around it, heading into the arteries lumen with the consequent reduction in blood flow.
The reduction of connective tissue fibroblasts and deposition of calcium in the injury causes sclerosis of the arteries [1]. Finally, an uneven surface leads to clotting and thrombosis, resulting in a sudden blockage of blood flow [2].
Among the risk factors are described: hyperlipidemia, diabetes mellitus, hypertension and smoking. Therefore, atherosclerosis represents the cause of cardiovascular morbidity most important worldwide. Understanding its pathophysiological mechanisms has allowed the development of therapeutic strategies producing a substantial reduction of clinical manifestations and acute complications of this entity [2].
Competitive inhibitors of 3-hydroxy-3 methylglutaryl CoA reductase, better known as statins have demonstrated over the years an ability to reduce the onset and progression of atherosclerosis. This makes them an alternative for prevention and progression of atherosclerosis [3]. Among the studies that demonstrate their use, the Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) showed a reduction of 44% in the rate of major vascular events: myocardial infarction, cerebral vascular accident, revascularization, hospitalization for unstable angina and vascular death. Thus, their use is justified to reduce cardiovascular risk factors and to prevent major events [4].
The mechanisms through which statins exert this effect at the vascular level, is beyond their power for inhibition of endogenous cholesterol production. Several pleiotropic effects involving immunological, inflammatory and endothelial function components, favoring risk reduction in treated cardiovascular patients have been found [3].
This review aims to update pathophysiological concepts of atherosclerosis, and the pleiotropic effects of statins on cardiovascular diseases of atherosclerotic origin.
For this review, scientific articles were retrieved through MEDLINE, Medscape and UpToDate search engines. We made particular emphasis in the current pathophysiological concept, in order to develop references regarding epigenetic and the effect of statins, beyond the current concept we have of it.
Hematopoietic cells and atherosclerosis
It has been known for several years that patients with hypercholesterolemia present elevated inflammatory cell counts (neutrophils and monocytes) in peripheral blood, which are associated with high risk of acute coronary events [1],[5]. It has also been observed that hypercholesterolemia, specifically elevated levels of oxidized LDL cholesterol (oxcLDL), induces monocyte migration to the atherosclerotic plaque. This favors the growth that leads to instability thereof and the resulting complications. This situation has been confirmed by subsequent studies, where it has been observed that hypercholesterolemia caused by diet promotes monocyte migration, identified by clusters of differentiation CD16─CD14+, to injury atheromatous [6],[7]. Other animal models of hypercholesterolemia have shown that the number of monocytes in peripheral blood is directly related to the size of the atheromatous [8] damage. Similarly thus, hypercholesterolemia induces the release of the granulocyte colonies growth factor with subsequent elevation of neutrophils, which have a direct relation with the onset, progression and severity of atherosclerosis [7],[9].
It has been shown in animal models that hypercholesterolemia induces proliferation of precursor inflammatory cells, which are released into the general circulation because of stimulation of the LDL [9] receptor. These precursor cells migrate to the spleen, which serves as a reservoir where they receive stimulation by interleukin 3 (IL-3) and factor granulocyte colony stimulating macrophage (GMCFS). These proliferate and differentiate into monocytes, who then are recruited in atheromatous lesions. These findings suggest that cholesterol is able to mobilize inflammatory progenitor cells outside the bone marrow, a fact that promotes myelopoiesis and extramedullary rapid progression of vascular lesions [10] (Figure 1).
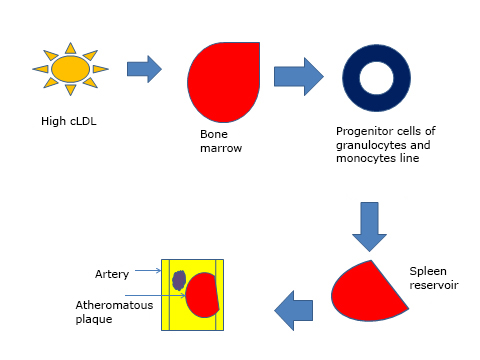
Figure 1. Effects of high cholesterol levels on bone marrow and spleen arteries
Inflammation and atherosclerosis
Atherosclerosis is considered a chronic inflammatory disease of the arterial wall. This assertion is based on the findings of abundance of phagocytic cells within atheromatous plaques [11]. Furthermore, elevated levels of inflammatory markers in the blood of patients with cardiovascular disease and atherosclerosis have been found [12]. Traditionally monocytes can be divided into two subpopulations, known as classic or inflammatory Act- 6C (high), and non-classical or resident Law-6C (low). There are substantial differences in the type and amount of chemokines receptors in the membrane of each population. However, studies mapping populations of monocytes/macrophages in murine models show that resident monocytes necessarily come from inflammatory lymphocytes [12],[13].
The immune-mediated inflammation is a major component of atherosclerotic process. The complement system, Toll-like (TLR) receptors and their interactions, may be of interest with respect to the pathogenesis and therapeutic targets in atherosclerosis. Animal studies indicate that inhibition of C3 to C5 reduces atherosclerosis. Meanwhile, clinical studies have shown that complement and Toll-Like are overexpressed in atherosclerotic disease, but interventional clinical trials have failed in this regard [14],[15].
Similarly, there is an increase in serum levels of chemokines, which are responsible for the chemotaxis of phagocytic cells within atheromatous plaques [12]. It is interesting to see how there is functional redundancy in the actions of various chemokines given by the ability of some to interact with multiple receptors simultaneously, and by the ability of receptors to be activated with different ligands. In general, there are three combinations of chemokines (ligand: receptor) relating to the Act-6C migration (high), which include molecules known as CCL2: CCR2, CX3CL1: CX3CR1, and CCL5: CCR5. Deficiency of these three chemokines, in animal models has led to the complete attenuation in murine models of atherosclerosis. Migration of resident populations of monocytes occurs less and it is often related to the axis CX3CL1: CX3CR1 [16],[17]. However, chemokines functions are not limited to the chemotactic function, but also they have metabolic effects related to the inhibition of reverse transport of cholesterol, which is mediated by CCL2chemokine. This induces suppression of key proteins involved in the efflux of cell cholesterol [8]. Here is one of the pleiotropic effects of statins, which attenuate the expression of chemokines and their receptors [18].
Epigenetics and atherosclerosis
Epigenetic (Figure 2) provides an explanation of how the diet, environmental factors and lifestyle may induce aberrations in gene expression, altering methylation acetylation patterns of DNA and histones at a nuclear level. Processes related to the expression and repression of genes, are based on the state of acetylation and methylation of DNA and histones, respectively. A hypermethylated gene cannot be transcribed. Acetylated histones allow the passage of heterochromatin (dense) to euchromatin (loose or open) allowing gene transcription. Methylated genes are surrounded by not acetylated histones. These processes can last the life of the individual and even transmitted to subsequent generations [19]. The importance of methylation as a contributing factor in the pathogenesis of atherosclerosis was demonstrated in a study that linked general DNA hypermethylation in inflammatory cells of peripheral blood, with predisposition and natural history of atherosclerosis. However, this study did not assess epigenetic changes associated with vascular endothelial cells and vascular smooth muscle cells [16]. Hypermethylation states of the genome are observed in patients having high levels of C reactive protein, which relates to the chronic inflammatory status accompanying cardiovascular diseases [20].
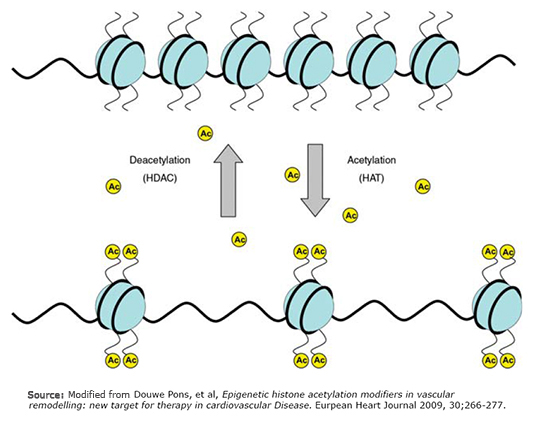
Figure 2. Epigenetic processes.
Besides DNA methylation within the pathophysiological spectrum, another mechanism immersed in the origin and predisposition to atherosclerosis is described, non-coding RNAs as epigenetic regulators [21]. Transcription of the genome in various forms of RNA, including antisense RNA, is currently well established. Noncoding RNAs are considered epigenetic marks because they participate in the control of gene expression without involving changes in the nucleotide sequence or in DNA copy number [22]. Non coding RNAs do not produce changes on the DNA molecule, they are responsible for monitoring the post-transcriptional gene expression by binding to target messenger RNAs. In this way, they promote, degrade or inhibit their translation, avoiding gene expression in all cases.
Noncoding RNAs best studied and characterized are microRNAs in charge of finely setting and directing translation of up to 60% of the genes encoding human proteins. At present, there are more than 1000 described human microRNAs. In recent years the important role they play in cardiovascular diseases has been noted, not only as molecules involved in the pathogenesis of different cardiovascular phenotypes, but also as potential biomarkers of risk and progression of heart disease and acute myocardial infarction, cardiovascular disease or heart failure [23]. In this context, an important outcome that explains microRNAs crucial function in the development of the cardiovascular system is describing that the elimination of mice Dicer (RNase that processes the pre-miRNAs) involves defects in angiogenesis, vessel formation and cardiac development [24].
The study of microRNAs has allowed relating them to many of the processes or basic mechanisms involved in the development of arteriosclerosis. Thus, it has been shown that microRNAs function as key drivers of gene expression, and therefore, of functionality of the different cell types involved in arteriosclerosis (endothelial cells, vascular smooth muscle cells, monocytes, and macrophages). In addition, they have been implicated in the control of processes such as inflammation, heart and lipoprotein metabolism, cholesterol biosynthesis and uptake, cardiac remodeling, endothelial dysfunction, angiogenesis or differentiation, migration and cell proliferation.
Along with regulating intracellular targets, it is described that microRNAs are present in physiologically significant blood concentrations. Although stimuli that trigger the secretion of RNA into microcirculation are unclear, circulating micro RNAs can be stably transported and delivered to destination cells thanks to their incorporation into apoptotic bodies [22], exosomes, HDL particles and the formation of Argonaut complexes [22]. More studies are needed to clarify whether endogenous circulating microRNAs may be directed to target cells to facilitate communication between different organs and thus regulate the gene expression systemically. Yet, the role of circulating micro RNA as specific biomarkers of disease is much more studied [22].
The challenge in these studies is to identify such micro accurate and reproducible specific RNAs, or a set of them to be used as biomarkers of risk or progression of disease, and to identify those patients susceptible of developing the disease.
Finally, it is reported that some microRNAs may increase the proliferation and differentiation of human cardiomyocytes from embryonic stem cells or human progenitor cells [25]. Moreover, using these specific microRNAs can increase reprogramming adult fibroblasts, directly into cardiomyocyte lineage cells [26]. It has also been reported that overexpression cluster -17-92 microRNA in mice increases proliferation of cardiac progenitor cells and facilitates heart regeneration after acute myocardial infarction [25]. Therefore, one might think that these micro RNAs can become therapeutic targets for repairing heart damage and for heart regeneration.
Statins and atherosclerosis
Statins main mechanism of action is the inhibition of 3-OH-3-methylglutaryl CoA reductase enzyme, which is key in the process of endogenous cholesterol synthesis. Pleiotropic effects of statins occur at the immunologic level where they regulate the inflammatory response at different levels [27].
Statins are related to the regulation of the expression of class II molecules of the major histocompatibility complex, which is mediated by interferon gamma (INF γ) [2]. This effect of this pharmacological group is mediated by the inhibition of the expression of PIV transactivator of major class II histocompatibility complex, a main promoter of the expression of major class II histocompatibility complex, needing nanomolar and micromolar concentrations of statins. From all statins, atorvastatin has the highest effect in inhibiting the mentioned promoter [23]. However, the inhibition effect on the major histocompatibility complex is specific for class II, since no effects have been seen in class I major histocompatibility complex [23].
Statins also affect costimulatory signals of T cells activation, similarly, to what they do with the major histocompatibility complex. Statins regulate the expression of costimulatory molecules such as CD40, CD80, and CD86 in lymphocytes, macrophages, microglia and endothelial cells. In turn, statins also suppress cytokine-induced maturation of dendritic cells, which results in a decreased receptor expression of chemokine CC7, CD40, CD83, CD86 and HLA-DR, and thus activation of T cells, [4],[28].
Regarding the leukocyte adhesion molecules, statins can block a leukocyte surface protein called LFA1, which joins the molecule of intercellular adhesion 1 (ICAM1), which besides facilitating leukocyte diapedesis also provides costimulatory signals for T lymphocytes [4].
Atherosclerosis is the leading cause of cardiovascular morbidity and mortality in western society. Advances in the understanding of its pathophysiology have allowed to not only improve treatment and prevent complications, but to develop new treatments aimed at therapeutic targets related to control inflammation and epigenetic changes observed in this pathology. New therapeutic tools ahead for the control and stabilization of atheromatous plaques. However, statins remain the main weapon for this purpose since their mechanism of action on plasma cholesterol level, and pleiotropic effects offer a very broad utility, with an optimum safety and cost profile.
From the editor
This article was originally submitted in Spanish and was translated into English by the authors. The Journal has not copyedited this version.
Conflicts of interest
The authors completed the conflict of interests declaration form from the ICMJE, translated into Spanish by Medwave, and declared not having any conflict of interests with the matter dealt herein. Forms can be requested to the responsible author or the editorial direction of the Journal.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Atherosclerosis is an immune-inflammatory disease, in which pathophysiological mechanisms include inflammation patterns and epigenetic changes that alter gene expression of several inflammatory and non-inflammatory mediators. Epigenetics is offering explanations on how diet, environmental factors and lifestyle can influence the onset and progression of the disease, and how these alterations can be transmitted to the following generations without any changes in DNA sequences. Statins, through their pleiotropic effects, provide a useful tool in controlling the progression of plaques and their subsequent impact.
 Autores:
Marcelo Storino Farina[1,2], Jairo Rojano Rada[1], Antony Molina Garrido[1], Xiomara Martínez[1], Alfredo Pulgar[1], Roxanna Paniagua[1], Jorge Garrido[1]
Autores:
Marcelo Storino Farina[1,2], Jairo Rojano Rada[1], Antony Molina Garrido[1], Xiomara Martínez[1], Alfredo Pulgar[1], Roxanna Paniagua[1], Jorge Garrido[1]

Citación: Storino Farina M, Rojano Rada J, Molina Garrido A, Martínez X, Pulgar A, Paniagua R, et al. Statins and atherosclerosis: the role of epigenetics. Medwave 2015 Nov;15(10):e6324 doi: 10.5867/medwave.2015.10.6324
Fecha de envío: 27/7/2015
Fecha de aceptación: 8/11/2015
Fecha de publicación: 26/11/2015
Origen: no solicitado
Tipo de revisión: con revisión por cinco pares revisores externos, a doble ciego

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Lang JK, Cimato TR. Cholesterol and hematopoietic stem cells: inflammatory mediators of atherosclerosis. Stem Cells Transl Med. 2014 May;3(5):549-52. | CrossRef | PubMed |
Lang JK, Cimato TR. Cholesterol and hematopoietic stem cells: inflammatory mediators of atherosclerosis. Stem Cells Transl Med. 2014 May;3(5):549-52. | CrossRef | PubMed | Guardiola M, Vallvé JC, Zaina S, Ribalta J. [Epigenetics in atherosclerosis]. Clin Investig Arterioscler. 2015 Jun 15. pii: S0214-9168(15)00054-6. | CrossRef | PubMed |
Guardiola M, Vallvé JC, Zaina S, Ribalta J. [Epigenetics in atherosclerosis]. Clin Investig Arterioscler. 2015 Jun 15. pii: S0214-9168(15)00054-6. | CrossRef | PubMed | Chow SC. Immunomodulation by statins: mechanisms and potential impact on autoimmune diseases. Arch Immunol Ther Exp (Warsz). 2009 Jul-Aug;57(4):243-51. | CrossRef | PubMed |
Chow SC. Immunomodulation by statins: mechanisms and potential impact on autoimmune diseases. Arch Immunol Ther Exp (Warsz). 2009 Jul-Aug;57(4):243-51. | CrossRef | PubMed | Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008 Nov 20;359(21):2195-207. | CrossRef | PubMed |
Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008 Nov 20;359(21):2195-207. | CrossRef | PubMed | Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013 Jan 11;339(6116):161-6. | CrossRef | PubMed |
Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013 Jan 11;339(6116):161-6. | CrossRef | PubMed | Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007 Jan;117(1):195-205. | PubMed |
Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007 Jan;117(1):195-205. | PubMed | Berg KE, Ljungcrantz I, Andersson L, Bryngelsson C, Hedblad B, Fredrikson GN, et al. Elevated CD14++CD16- monocytes predict cardiovascular events. Circ Cardiovasc Genet. 2012 Feb 1;5(1):122-31. | CrossRef | PubMed |
Berg KE, Ljungcrantz I, Andersson L, Bryngelsson C, Hedblad B, Fredrikson GN, et al. Elevated CD14++CD16- monocytes predict cardiovascular events. Circ Cardiovasc Genet. 2012 Feb 1;5(1):122-31. | CrossRef | PubMed | Combadière C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C (lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008 Apr 1;117(13):1649-57. | CrossRef | PubMed |
Combadière C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C (lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008 Apr 1;117(13):1649-57. | CrossRef | PubMed | Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010 Nov 2;122(18):1837-45. | CrossRef | PubMed |
Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010 Nov 2;122(18):1837-45. | CrossRef | PubMed | Gomes AL, Carvalho T, Serpa J, Torre C, Dias S. Hypercholesterolemia promotes bone marrow cell mobilization by perturbing the SDF-1:CXCR4 axis. Blood. 2010 May 13;115(19):3886-94. | CrossRef | PubMed |
Gomes AL, Carvalho T, Serpa J, Torre C, Dias S. Hypercholesterolemia promotes bone marrow cell mobilization by perturbing the SDF-1:CXCR4 axis. Blood. 2010 May 13;115(19):3886-94. | CrossRef | PubMed | Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005 Apr 21;352(16):1685-95. | PubMed |
Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005 Apr 21;352(16):1685-95. | PubMed | Zernecke A, Weber C. Chemokines in atherosclerosis: proceedings resumed. Arterioscler Thromb Vasc Biol. 2014 Apr;34(4):742-50. | CrossRef | PubMed |
Zernecke A, Weber C. Chemokines in atherosclerosis: proceedings resumed. Arterioscler Thromb Vasc Biol. 2014 Apr;34(4):742-50. | CrossRef | PubMed | Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013 Jan 24;38(1):79-91. | CrossRef | PubMed |
Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013 Jan 24;38(1):79-91. | CrossRef | PubMed | Rafieian-Kopaei M, Setorki M, Doudi M, Baradaran A, Nasri H. Atherosclerosis: process, indicators, risk factors and new hopes. Int J Prev Med. 2014 Aug;5(8):927-46. | PubMed |
Rafieian-Kopaei M, Setorki M, Doudi M, Baradaran A, Nasri H. Atherosclerosis: process, indicators, risk factors and new hopes. Int J Prev Med. 2014 Aug;5(8):927-46. | PubMed | Hovland A, Jonasson L, Garred P, Yndestad A, Aukrust P, Lappegård KT, et al. The complement system and toll-like receptors as integrated players in the pathophysiology of atherosclerosis. Atherosclerosis. 2015 Aug;241(2):480-94. | CrossRef | PubMed |
Hovland A, Jonasson L, Garred P, Yndestad A, Aukrust P, Lappegård KT, et al. The complement system and toll-like receptors as integrated players in the pathophysiology of atherosclerosis. Atherosclerosis. 2015 Aug;241(2):480-94. | CrossRef | PubMed | Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013 Oct;13(10):709-21. | CrossRef | PubMed |
Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013 Oct;13(10):709-21. | CrossRef | PubMed | Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007 Sep;7(9):678-89. | PubMed |
Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007 Sep;7(9):678-89. | PubMed | Nie P, Li D, Hu L, Jin S, Yu Y, Cai Z, et al. Atorvastatin improves plaque stability in ApoE-knockout mice by regulating chemokines and chemokine receptors. PLoS One. 2014 May 9;9(5):e97009. | CrossRef | PubMed |
Nie P, Li D, Hu L, Jin S, Yu Y, Cai Z, et al. Atorvastatin improves plaque stability in ApoE-knockout mice by regulating chemokines and chemokine receptors. PLoS One. 2014 May 9;9(5):e97009. | CrossRef | PubMed | Wierda RJ, Geutskens SB, Jukema JW, Quax PH, van den Elsen PJ. Epigenetics in atherosclerosis and inflammation. J Cell Mol Med. 2010 Jun;14(6A):1225-40. | CrossRef | PubMed |
Wierda RJ, Geutskens SB, Jukema JW, Quax PH, van den Elsen PJ. Epigenetics in atherosclerosis and inflammation. J Cell Mol Med. 2010 Jun;14(6A):1225-40. | CrossRef | PubMed | Stenvinkel P, Karimi M, Johansson S, Axelsson J, Suliman M, Lindholm B, et al. Impact of inflammation on epigenetic DNA methylation - a novel risk
factor for cardiovascular disease? J Intern Med. 2007 May;261(5):488-99. | PubMed |
Stenvinkel P, Karimi M, Johansson S, Axelsson J, Suliman M, Lindholm B, et al. Impact of inflammation on epigenetic DNA methylation - a novel risk
factor for cardiovascular disease? J Intern Med. 2007 May;261(5):488-99. | PubMed | Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009 Jan;19(1):92-105. | CrossRef | PubMed |
Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009 Jan;19(1):92-105. | CrossRef | PubMed | van Rooij E, Olson EN. MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets. J Clin Invest. 2007 Sep;117(9):2369-76.
| PubMed |
van Rooij E, Olson EN. MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets. J Clin Invest. 2007 Sep;117(9):2369-76.
| PubMed | Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalia mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92-105.
Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalia mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92-105.  Chen J, Huang ZP, Seok HY, Ding J, Kataoka M, Zhang Z, et al. mir-17-92 cluster is required for and sufficient to induce cardiomyocyte proliferation in postnatal and adult hearts. Circ Res. 2013 Jun 7;112(12):1557-66.
| CrossRef | PubMed |
Chen J, Huang ZP, Seok HY, Ding J, Kataoka M, Zhang Z, et al. mir-17-92 cluster is required for and sufficient to induce cardiomyocyte proliferation in postnatal and adult hearts. Circ Res. 2013 Jun 7;112(12):1557-66.
| CrossRef | PubMed | Jayawardena TM, Egemnazarov B, Finch EA, Zhang L, Payne JA, Pandya K, et al. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res. 2012 May 25;110(11):1465-73.
| CrossRef | PubMed |
Jayawardena TM, Egemnazarov B, Finch EA, Zhang L, Payne JA, Pandya K, et al. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res. 2012 May 25;110(11):1465-73.
| CrossRef | PubMed | Sek C. Chow. Immunomodulation by Statins: mechanisms and potential impact on autoimmune disease, Arch. Immunol. Ther. Exp., 2009, 57, 243–251.
Sek C. Chow. Immunomodulation by Statins: mechanisms and potential impact on autoimmune disease, Arch. Immunol. Ther. Exp., 2009, 57, 243–251.  Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000 Dec;6(12):1399-402.
| PubMed |
Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000 Dec;6(12):1399-402.
| PubMed | Weitz-Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, Bruns C, et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001 Jun;7(6):687-92. | PubMed |
Weitz-Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, Bruns C, et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001 Jun;7(6):687-92. | PubMed |