 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Palabras clave: Retinopathy of prematurity (ROP), Anti-vascular endothelial growth factor (anti-VEGF), laser coagulation, Epistemonikos, GRADE.
INTRODUCTION
Retinopathy of prematurity (ROP) is a condition that affects preterm infants, being the second leading cause of childhood blindness worldwide. The most commonly used treatments are cryotherapy or laser photocoagulation, but over the last few years, anti-vascular endothelial growth factor (anti-VEGF) drugs have gradually gained more adherents, mainly in the treatment of patients with type 1 retinopathy of prematurity (ET- ROP) Therefore, it is important to summarize the existing evidence to evaluate the efficacy and safety of anti-VEGF drugs in type 1 retinopathy of prematurity.
METHODS
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified six systematic reviews including 15 studies overall, of which five were randomized trials.
We conclude that the use of anti-VEGF compared to laser photocoagulation, probably reduces the risk of refractive errors. On the other hand, the use of anti-VEGF may result in little or no difference in the mortality at hospital discharge, lens or corneal opacity requiring surgery, and complete or partial retinal detachment, but the certainty of the evidence is low. Finally, it is not possible to clearly establish whether anti-VEGF compared with laser photocoagulation, increases the recurrence of retinopathy of prematurity, because the certainty of the existing evidence has been assessed as very low.
Retinopathy of prematurity (ROP) is a vasoproliferative disorder of the retina that affects preterm newborns (PTNB) resulting in the second cause of childhood blindness in the world. Ablation of the avascular retina, with cryotherapy or laser photocoagulation has been considered useful and effective treatments for retinopathy of prematurity [1] [2].
The role and association of vascular endothelial growth factor (VEGF) in the pathogenesis of retinopathy of prematurity has been studied extensively. It has been found that the retinal neovascularization that causes the traction ultimately leading to retinal detachment is mainly due to the development and effect of vascular endothelial growth factor (VEGF) [3], [4], [5]. Given the above, the use of intravitreal anti-VEGF agents has gradually gained popularity, and has even been recommended by some ophthalmologists as first-line therapy to treat aggressive posterior retinopathy of prematurity and zone I disease. The objective of this summary is to evaluate the efficacy and safety of anti-VEGF drugs as monotherapy in preterm infants with type 1 retinopathy of prematurity compared to laser photocoagulation therapy.
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
|
What is the evidence. |
The use of anti-VEGF compared to laser photocoagulation therapy probably reduces the risk of refractive errors .The use of anti-VEGF compared to laser photocoagulation therapy may make little or no difference in terms of treatment safety, whether in hospital discharge mortality, opacity of the lens or cornea that requires surgery and in partial or complete retinal detachment (certainty of low evidence).We are uncertain whether anti-VEGF compared to laser photocoagulation therapy reduces the recurrence of retinopathy of prematurity, as the certainty of the evidence has been assessed as very low. |
|
What type of patients did the studies include? * |
All trials included preterm infants with type 1 retinopathy of prematurity [13], [15], [19], [25], [29]. |
|
What type of interventions did the studies include? * |
All trials compared anti-VEGF therapy versus laser photocoagulation therapy (standard treatment) [13], [15], [19], [25], [29].The anti-VEGF used were bevacizumab in four trials [13], [15], [25], [27] and ranibizumab in one trial [19]. |
|
What type of outcomes did they measure |
The trials reported multiple outcomes, which were grouped by systematic reviews as follows:
The average follow-up of the trials was six months with a range between eight weeks and two years [13], [15], [19], [25], [29]. |
*Information on primary studies is extracted from the identified systematic reviews, not directly from the studies unless otherwise specified.
The information on the effects of anti-VEGF drugs is based on five randomized trials that include premature patients with type 1 retinopathy of prematurity [13], [15], [19], [25], [27]. Two trials measured the outcome recurrence of retinopathy of prematurity (193 patients) [13], [19]. Two trials measured the outcome mortality at hospital discharge (229 patients) [13], [27]. Four trials measured the outcome total or partial retinal detachment (393 patients) [13], [15], [19], [27]. Three trials measured the outcome opacity of the lens (cataract) that requires removal (544 patients) [13], [19], [27]. One trial evaluated the outcome corneal opacity requiring corneal transplantation (286 patients) [13]. One trial measured the outcome refractive error (myopia) (211 patients) [13].
The summary of the results is as follows:
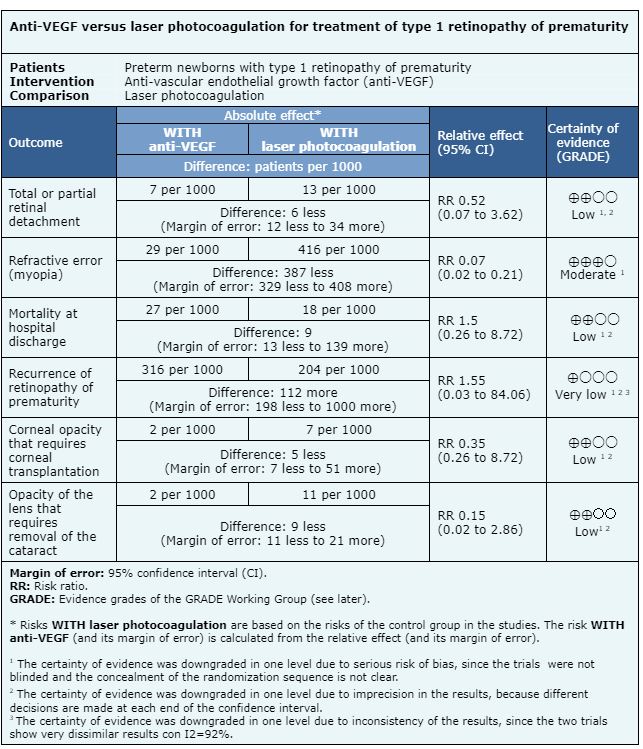
Follow the link to access the interactive version of this table (Interactive Summary of Findings - iSoF)
| To whom this evidence applies and to whom it does not apply. |
|
|
About the outcomes included in this summary |
|
|
Harm/benefit balance and certainty in evidence |
|
|
Resource considerations |
|
|
What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
|
Could this information change in the future? |
|
We collected all the relevant evidence for this question and presented it in an evidence matrix using automated and collaborative methods.
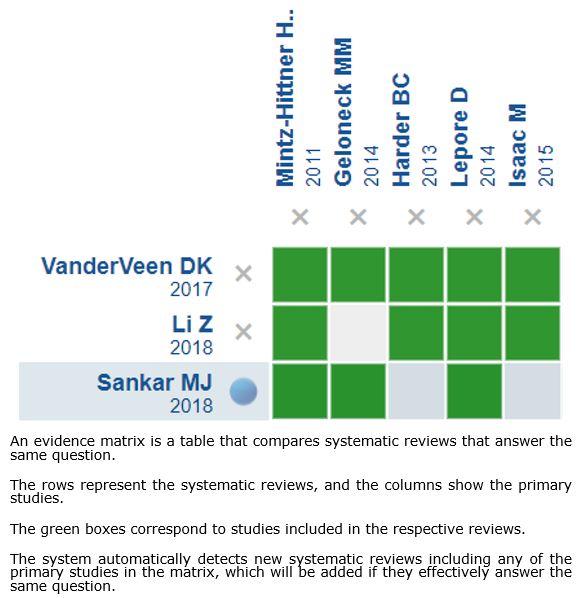
Follow the link to access the interactive version: Anti-VEGF compared to laser photocoagulation for type 1 retinopathy of prematurity
Notes
If new systematic reviews on this topic are published after the publication of this abstract, a "new evidence" notification will be displayed at the top of the matrix. While the project provides regular updates of these abstracts, users are invited to comment on the Medwave website or contact the authors by e-mail if they believe evidence warrants an earlier update.
After creating an Epistemonikos account, by saving the matrices, you will receive automatic notifications whenever there is new evidence that potentially answers this question.
This article is part of the Epistemonikos evidence synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and an internal peer review process. Each of these articles corresponds to a summary, called FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence of a specific question, in a friendly manner for physicians. The main resources are based on the Epistemonikos evidence matrix and the analysis of the result is based on the GRADE methodology. Further details of this FRISBEE elaboration method are described here.
The Epistemonikos Foundation is an organization that seeks to bring information closer to health decision-makers through the use of technologies. Its main source is the Epistemonikos database (www.epistemonikos.org).
Competing interest
The authors declare no competing interest.

INTRODUCTION
Retinopathy of prematurity (ROP) is a condition that affects preterm infants, being the second leading cause of childhood blindness worldwide. The most commonly used treatments are cryotherapy or laser photocoagulation, but over the last few years, anti-vascular endothelial growth factor (anti-VEGF) drugs have gradually gained more adherents, mainly in the treatment of patients with type 1 retinopathy of prematurity (ET- ROP) Therefore, it is important to summarize the existing evidence to evaluate the efficacy and safety of anti-VEGF drugs in type 1 retinopathy of prematurity.
METHODS
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified six systematic reviews including 15 studies overall, of which five were randomized trials.
We conclude that the use of anti-VEGF compared to laser photocoagulation, probably reduces the risk of refractive errors. On the other hand, the use of anti-VEGF may result in little or no difference in the mortality at hospital discharge, lens or corneal opacity requiring surgery, and complete or partial retinal detachment, but the certainty of the evidence is low. Finally, it is not possible to clearly establish whether anti-VEGF compared with laser photocoagulation, increases the recurrence of retinopathy of prematurity, because the certainty of the existing evidence has been assessed as very low.
 Autores:
Raúl González C.[1,2], Marcela Díaz C.[2,3], Rodolfo Garretón C.[1,2]
Autores:
Raúl González C.[1,2], Marcela Díaz C.[2,3], Rodolfo Garretón C.[1,2]

Citación: González C. , Díaz C. , Garretón C. . Anti-vascular endothelial growth factor (VEGF) drugs compared to laser photocoagulation for treatment of type 1 retinopathy of prematurity. Medwave 2022;22(01):e8506 doi: DOI 10.5867/medwave.2022.01.8507
Fecha de envío: 8/10/2019
Fecha de aceptación: 26/12/2019
Fecha de publicación: 17/1/2022
Origen: Este artículo es producto del Epistemonikos Evidence Synthesis Project de la Fundación Epistemonikos, en colaboración con Medwave para su publicación.
Tipo de revisión: Con revisión por pares sin ciego por parte del equipo metodológico del Centro Evidencia UC Synthesis Project

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity. Preliminary results. Arch Ophthalmol. 1988;106:471-479.
Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity. Preliminary results. Arch Ophthalmol. 1988;106:471-479.  Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the Early Treatment for Retinopathy of Prematurity randomized trial. Arch Ophthalmol. 2003;121: 1684-1694.
Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the Early Treatment for Retinopathy of Prematurity randomized trial. Arch Ophthalmol. 2003;121: 1684-1694.  Smith LE. IGF-1 and retinopathy of prematurity in the preterm infant. Biol Neonate. 2005;88:237-244.
Smith LE. IGF-1 and retinopathy of prematurity in the preterm infant. Biol Neonate. 2005;88:237-244.  Pierce EA, Avery RL, Foley ED, et al. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA. 1995;92:905-909.
Pierce EA, Avery RL, Foley ED, et al. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA. 1995;92:905-909.  Hartnett ME. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology. 2015;122:200-210.
Hartnett ME. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology. 2015;122:200-210.  Sankar, M. J., Sankar, J., & Chandra, P. Anti-vascular endothelial growth factor (VEGF) drugs for treatment of retinopathy of prematurity. Cochrane Database of Systematic Reviews. 2018 Jan 8;1:CD009734.
Sankar, M. J., Sankar, J., & Chandra, P. Anti-vascular endothelial growth factor (VEGF) drugs for treatment of retinopathy of prematurity. Cochrane Database of Systematic Reviews. 2018 Jan 8;1:CD009734.  VanderVeen, D. K., Melia, M., Yang, M. B., Hutchinson, A. K., Wilson, L. B., & Lambert, S. Anti-Vascular Endothelial Growth Factor Therapy for Primary Treatment of Type 1 Retinopathy of Prematurity: A Report by the American Academy of Ophthalmology.Ophthalmology, 2017; 24(5), 619–633.
VanderVeen, D. K., Melia, M., Yang, M. B., Hutchinson, A. K., Wilson, L. B., & Lambert, S. Anti-Vascular Endothelial Growth Factor Therapy for Primary Treatment of Type 1 Retinopathy of Prematurity: A Report by the American Academy of Ophthalmology.Ophthalmology, 2017; 24(5), 619–633.  Abri Aghdam, K., Khadamy, J., Falavarjani, K. G., & Tsui, I. Refractive outcomes following the treatment of retinopathy of prematurity in the anti-VEGF era: a literature review. Journal of American Association for Pediatric Ophthalmology and Strabismus, 2016;20(6), 539–540.
Abri Aghdam, K., Khadamy, J., Falavarjani, K. G., & Tsui, I. Refractive outcomes following the treatment of retinopathy of prematurity in the anti-VEGF era: a literature review. Journal of American Association for Pediatric Ophthalmology and Strabismus, 2016;20(6), 539–540.  Pertl, L., Steinwender, G., Mayer, C., Hausberger, S., Pöschl, E.-M., Wackernagel, W., Haas, A. A Systematic Review and Meta-Analysis on the Safety of Vascular Endothelial Growth Factor (VEGF) Inhibitors for the Treatment of Retinopathy of Prematurity. PLOS ONE, 2015,10(6)
Pertl, L., Steinwender, G., Mayer, C., Hausberger, S., Pöschl, E.-M., Wackernagel, W., Haas, A. A Systematic Review and Meta-Analysis on the Safety of Vascular Endothelial Growth Factor (VEGF) Inhibitors for the Treatment of Retinopathy of Prematurity. PLOS ONE, 2015,10(6)  Mihai Mititelu, MD, MPH; Khurram M. Chaudhary, MD; Ronni M. Lieberman, MD.An Evidence-Based Meta-analysis of Vascular Endothelial Growth Factor Inhibition in Pediatric Retinal Diseases: Part 1. Retinopathy of Prematurity.Journal of Pediatric Ophthalmology and Strabismus. 2012;49(6):332-340
Mihai Mititelu, MD, MPH; Khurram M. Chaudhary, MD; Ronni M. Lieberman, MD.An Evidence-Based Meta-analysis of Vascular Endothelial Growth Factor Inhibition in Pediatric Retinal Diseases: Part 1. Retinopathy of Prematurity.Journal of Pediatric Ophthalmology and Strabismus. 2012;49(6):332-340  Li Z, Zhang Y, Liao Y, Zeng R, Zeng P, Lan Y. Comparison of efficacy between anti-vascular endothelial growth factor (VEGF) and laser treatment in Type-1 and threshold retinopathy of prematurity (ROP). BMC Ophthalmology. 2018;18(1).
Li Z, Zhang Y, Liao Y, Zeng R, Zeng P, Lan Y. Comparison of efficacy between anti-vascular endothelial growth factor (VEGF) and laser treatment in Type-1 and threshold retinopathy of prematurity (ROP). BMC Ophthalmology. 2018;18(1).  Geloneck, M. M., Chuang, A. Z., Clark, W. L., Hunt, M. G., Norman, A. A., Packwood, E. A.,Mintz-Hittner, H. A. (2014). Refractive Outcomes Following Bevacizumab Monotherapy Compared With Conventional Laser Treatment. JAMA Ophthalmology, 132(11), 1327
Geloneck, M. M., Chuang, A. Z., Clark, W. L., Hunt, M. G., Norman, A. A., Packwood, E. A.,Mintz-Hittner, H. A. (2014). Refractive Outcomes Following Bevacizumab Monotherapy Compared With Conventional Laser Treatment. JAMA Ophthalmology, 132(11), 1327  Mintz-Hittner, H. A., Kennedy, K. A., & Chuang, A. Z. (2011). Efficacy of Intravitreal Bevacizumab for Stage 3+ Retinopathy of Prematurity. New England Journal of Medicine, 364(7), 603–615.
Mintz-Hittner, H. A., Kennedy, K. A., & Chuang, A. Z. (2011). Efficacy of Intravitreal Bevacizumab for Stage 3+ Retinopathy of Prematurity. New England Journal of Medicine, 364(7), 603–615.  Harder, B. C., Schlichtenbrede, F. C., von Baltz, S., Jendritza, W., Jendritza, B., & Jonas, J. B. (2013). Intravitreal Bevacizumab for Retinopathy of Prematurity: Refractive Error Results. American Journal of Ophthalmology, 155(6), 1119–1124.
Harder, B. C., Schlichtenbrede, F. C., von Baltz, S., Jendritza, W., Jendritza, B., & Jonas, J. B. (2013). Intravitreal Bevacizumab for Retinopathy of Prematurity: Refractive Error Results. American Journal of Ophthalmology, 155(6), 1119–1124.  Lepore, D., Quinn, G. E., Molle, F., Baldascino, A., Orazi, L., Sammartino, M. Romagnoli, C. (2014). Intravitreal Bevacizumab versus Laser Treatment in Type 1 Retinopathy of Prematurity. Ophthalmology, 121(11), 2212–2219.
Lepore, D., Quinn, G. E., Molle, F., Baldascino, A., Orazi, L., Sammartino, M. Romagnoli, C. (2014). Intravitreal Bevacizumab versus Laser Treatment in Type 1 Retinopathy of Prematurity. Ophthalmology, 121(11), 2212–2219.  Isaac, M., Mireskandari, K., & Tehrani, N. (2015). Treatment of type 1 retinopathy of prematurity with bevacizumab versus laser. Journal of American Association for Pediatric Ophthalmology and Strabismus, 19(2), 140–144.
Isaac, M., Mireskandari, K., & Tehrani, N. (2015). Treatment of type 1 retinopathy of prematurity with bevacizumab versus laser. Journal of American Association for Pediatric Ophthalmology and Strabismus, 19(2), 140–144.  Harder, B. C., von Baltz, S., Schlichtenbrede, F. C., & Jonas, J. B. (2012). Early Refractive Outcome After Intravitreous Bevacizumab for Retinopathy of Prematurity. Archives of Ophthalmology, 130(6).
Harder, B. C., von Baltz, S., Schlichtenbrede, F. C., & Jonas, J. B. (2012). Early Refractive Outcome After Intravitreous Bevacizumab for Retinopathy of Prematurity. Archives of Ophthalmology, 130(6).  Moran, S., O’Keefe, M., Hartnett, C., Lanigan, B., Murphy, J., & Donoghue, V. (2014). Bevacizumab versus diode laser in stage 3 posterior retinopathy of prematurity. Acta Ophthalmologica, 92(6), e496–e497.
Moran, S., O’Keefe, M., Hartnett, C., Lanigan, B., Murphy, J., & Donoghue, V. (2014). Bevacizumab versus diode laser in stage 3 posterior retinopathy of prematurity. Acta Ophthalmologica, 92(6), e496–e497.  Zhang, G., Yang, M., Zeng, J., Vakros, G., Su, K., Chen, M. Zhuang, R. (2017). Comparison of intravitreal injection of Ranibizumab versus laser therapy for Zone II treatment- requiring retinopathy of prematury . Retina, 37(4), 710–717.
Zhang, G., Yang, M., Zeng, J., Vakros, G., Su, K., Chen, M. Zhuang, R. (2017). Comparison of intravitreal injection of Ranibizumab versus laser therapy for Zone II treatment- requiring retinopathy of prematury . Retina, 37(4), 710–717.  Hwang, C. K., Hubbard, G. B., Hutchinson, A. K., & Lambert, S. R. (2015). Outcomes after Intravitreal Bevacizumab versus Laser Photocoagulation for Retinopathy of Prematurity. Ophthalmology, 122(5), 1008–1015.
Hwang, C. K., Hubbard, G. B., Hutchinson, A. K., & Lambert, S. R. (2015). Outcomes after Intravitreal Bevacizumab versus Laser Photocoagulation for Retinopathy of Prematurity. Ophthalmology, 122(5), 1008–1015.  Lee, J. Y., Chae, J. B., Yang, S. J., Yoon, Y. H., & Kim, J.-G. (2010). Effects of intravitreal bevacizumab and laser in retinopathy of prematurity therapy on the development of peripheral retinal vessels. Graefe’s Archive for Clinical and Experimental Ophthalmology, 248(9), 1257–1262
Lee, J. Y., Chae, J. B., Yang, S. J., Yoon, Y. H., & Kim, J.-G. (2010). Effects of intravitreal bevacizumab and laser in retinopathy of prematurity therapy on the development of peripheral retinal vessels. Graefe’s Archive for Clinical and Experimental Ophthalmology, 248(9), 1257–1262  Gunay, M., Celik, G., Tuten, A., Karatekin, G., Bardak, H., & Ovali, F. (2016). Characteristics of Severe Retinopathy of Prematurity in Infants with Birth Weight above 1500 Grams at a Referral Center in Turkey. PLOS ONE, 11(8), e0161692.
Gunay, M., Celik, G., Tuten, A., Karatekin, G., Bardak, H., & Ovali, F. (2016). Characteristics of Severe Retinopathy of Prematurity in Infants with Birth Weight above 1500 Grams at a Referral Center in Turkey. PLOS ONE, 11(8), e0161692.  Helen Mintz-Hittner, MD, The University of Texas Health Science Center, Houston. Bevacizumab Eliminates the Angiogenic Threat for Retinopathy of Prematurity (BEAT-ROP).ClinicalTrials.gov Identifier: NCT00622726 June 6, 2017
Helen Mintz-Hittner, MD, The University of Texas Health Science Center, Houston. Bevacizumab Eliminates the Angiogenic Threat for Retinopathy of Prematurity (BEAT-ROP).ClinicalTrials.gov Identifier: NCT00622726 June 6, 2017
 Mueller, B., Salchow, D. J., Waffenschmidt, E., Joussen, A. M., Schmalisch, G., Czernik, C.,Winterhalter, S. (2016). Treatment of type I ROP with intravitreal bevacizumab or laser photocoagulation according to retinal zone. British Journal of Ophthalmology, bjophthalmol–2016
Mueller, B., Salchow, D. J., Waffenschmidt, E., Joussen, A. M., Schmalisch, G., Czernik, C.,Winterhalter, S. (2016). Treatment of type I ROP with intravitreal bevacizumab or laser photocoagulation according to retinal zone. British Journal of Ophthalmology, bjophthalmol–2016  O'Keeffe N, Murphy J, O'Keefe M, Lanigan B.Bevacizumab compared with diode laser in stage 3 posterior retinopathy of prematurity: A 5 year follow up.Ir Med J. 2016 Feb 19;109(2):355.
O'Keeffe N, Murphy J, O'Keefe M, Lanigan B.Bevacizumab compared with diode laser in stage 3 posterior retinopathy of prematurity: A 5 year follow up.Ir Med J. 2016 Feb 19;109(2):355.  Gunay, M., Sukgen, E. A., Celik, G., & Kocluk, Y. (2016). Comparison of Bevacizumab, Ranibizumab, and Laser Photocoagulation in the Treatment of Retinopathy of Prematurity in Turkey. Current Eye Research, 42(3), 462–469.
Gunay, M., Sukgen, E. A., Celik, G., & Kocluk, Y. (2016). Comparison of Bevacizumab, Ranibizumab, and Laser Photocoagulation in the Treatment of Retinopathy of Prematurity in Turkey. Current Eye Research, 42(3), 462–469.  Karkhaneh, R., Khodabande, A., Riazi-Eafahani, M., Roohipoor, R., Ghassemi, F., Imani, M.,Torabi, H. (2016). Efficacy of intravitreal bevacizumab for zone-II retinopathy of prematurity. Acta Ophthalmologica, 94(6), e417–e420.
Karkhaneh, R., Khodabande, A., Riazi-Eafahani, M., Roohipoor, R., Ghassemi, F., Imani, M.,Torabi, H. (2016). Efficacy of intravitreal bevacizumab for zone-II retinopathy of prematurity. Acta Ophthalmologica, 94(6), e417–e420.  Kuo, H.-K., Sun, I.-T., Chung, M.-Y., & Chen, Y.-H. (2015). Refractive Error in Patients with Retinopathy of Prematurity after Laser Photocoagulation or Bevacizumab Monotherapy. Ophthalmologica, 234(4), 211–217.
Kuo, H.-K., Sun, I.-T., Chung, M.-Y., & Chen, Y.-H. (2015). Refractive Error in Patients with Retinopathy of Prematurity after Laser Photocoagulation or Bevacizumab Monotherapy. Ophthalmologica, 234(4), 211–217.  American Academy of Ophthalmology, Weisenthal RW, Staff AA of O, Ophthalmology EB of. 2018-2019 Basic and Clinical Science Course (BCSC) [Internet]. American Academy of Ophthalmology; 2018. (Basic and clinical science course). | Link |
American Academy of Ophthalmology, Weisenthal RW, Staff AA of O, Ophthalmology EB of. 2018-2019 Basic and Clinical Science Course (BCSC) [Internet]. American Academy of Ophthalmology; 2018. (Basic and clinical science course). | Link | MINISTERIO DE SALUD. Guía Clínica RETINOPATÍA DEL PREMATURO. Santiago: Minsal, 2010.
MINISTERIO DE SALUD. Guía Clínica RETINOPATÍA DEL PREMATURO. Santiago: Minsal, 2010.  Longueira C, Perapoch J, Mart N. Retinopatía de la prematuridad. 2008
Longueira C, Perapoch J, Mart N. Retinopatía de la prematuridad. 2008