 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Palabras clave: Central serous chorioretinopathy, mineralocorticoid receptor antagonists, eplerenone, spironolactone, Epistemonikos, GRADE.
INTRODUCTION
Central serous chorioretinopathy consists of the leakage of fluid from the choroid and its accumulation into the subretinal space. Its chronic form is associated with permanent vision loss. Mineralocorticoid receptor antagonists are an alternative treatment for this condition, although there is no clear evidence about their effectiveness.
METHODS
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified three systematic reviews including 22 studies overall and four of them are randomized trials. We concluded that in chronic central serous chorioretinopathy, mineralocorticoid receptor antagonists probably make little or no difference to best-corrected visual acuity. We are uncertain whether this intervention reduces subretinal fluid height because the certainty of the evidence is very low. Furthermore, this intervention may make little or no difference in terms of adverse effects, but the certainty of the evidence is low.
Central serous chorioretinopathy consists of the leakage of fluid from the choroid and the retinal pigment epithelium into the subretinal space, which generate a serous retinal detachment. There is not a universally accepted classification for this disease, but in general terms, it is chronic when its course is longer than three months. In this case, there is a high risk of permanent vision loss.
There is not a standard treatment for chronic central serous chorioretinopathy and there are several alternatives of management: photodynamic therapy, focal laser photocoagulation, micropulse diode laser, intravitreal anti‐vascular endothelial growth factor agents (anti-VEGF) and mineralocorticoid receptor antagonists (eplerenone and spironolactone). The association between central serous chorioretinopathy and the overactivation of mineralocorticoid receptors localized in the choroid supports the employment of these drugs.
Mineralocorticoid receptor antagonists are an affordable treatment especially when there is not a leakage point in fluorescein angiography, or when it has a macular localization. Nevertheless, their effectiveness is unclear.
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
|
What is the evidence. |
We identified three systematic reviews [1], [2], [3] with 22 primary studies [4], [5],[6],[7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], four of which are randomized control trials [4], [5], [6], [7]. The table and summary are based on the randomized trials, as the observational studies did not increase the level of certainty of the evidence nor added any additional relevant information. |
|
What types of patients were included* |
All trials included patients with chronic central serous chorioretinopathy, defined by the systematic reviews as central serous chorioretinopathy with a course longer than three months. The best-corrected visual acuity for the control group (placebo or observation) on average was 0.24 logMAR scale units. For the intervention group, this parameter was 0.36 logMAR [4], [5], [6], [7]. The baseline subretinal fluid height was on average 198.5 micrometres (μm) for the control group and 210.5 μm for the intervention group [4], [5], [6], [7]. |
|
What types of interventions were included* |
All trials evaluated mineralocorticoid receptor antagonists. Two of them [4], [5] evaluated eplerenone, one trial [6] evaluated spironolactone and another trial [7] evaluated the combination of spironolactone and eplerenone. Two trials [4], [5] evaluated the administration of 25 mg daily of oral eplerenone for one week followed by 50 mg daily for nine weeks [4] or 12 weeks [5]. Another trial [6], evaluated the administration of 50 mg daily of spironolactone for four weeks. In the last trial [7], the patients received 50 mg daily of eplerenone, followed by 50 mg daily of spironolactone for four weeks. Three trials [4], [5], [6] compared the intervention with placebo and one trial [7] compared it with placebo and spironolactone.. |
|
What types of outcomes |
The trials evaluated several outcomes grouped by the systematic reviews as follows:
The average follow-up was 8,5 weeks with an interval between four to 24 weeks.
|
* Information about primary studies is not extracted directly from primary studies but from identified systematic reviews, unless otherwise stated.
The information about the effect of mineralocorticoid receptor antagonists (spironolactone and eplerenone) in chronic central serous chorioretinopathy is based in four randomized controlled trials (75 eyes) [4], [5], [6], [7].
Four trials measured the outcomes best-corrected visual acuity (logMAR scale) and subretinal fluid height (μm) (75 eyes) [4], [5], [6], [7].
None of the systematic reviews enabled data extraction regarding adverse effects to perform a meta-analysis. In consequence, the information about this outcome is presented as a narrative synthesis.
The summary of findings is as follows:
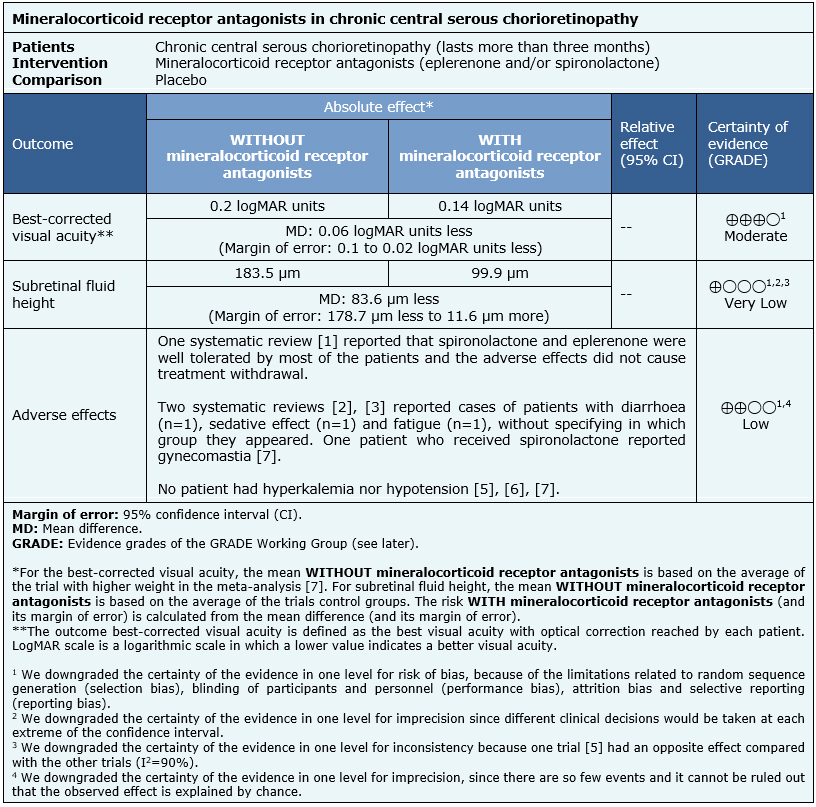
| Follow the link to access the interactive version of this table (Interactive Summary of Findings – iSoF) |

|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
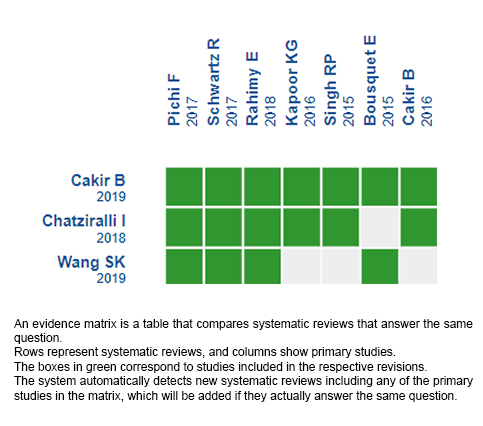
Follow the link to access the interactive version: Mineralocorticoid receptor antagonists in chronic central serous chorioretinopathy.
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCTION
Central serous chorioretinopathy consists of the leakage of fluid from the choroid and its accumulation into the subretinal space. Its chronic form is associated with permanent vision loss. Mineralocorticoid receptor antagonists are an alternative treatment for this condition, although there is no clear evidence about their effectiveness.
METHODS
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified three systematic reviews including 22 studies overall and four of them are randomized trials. We concluded that in chronic central serous chorioretinopathy, mineralocorticoid receptor antagonists probably make little or no difference to best-corrected visual acuity. We are uncertain whether this intervention reduces subretinal fluid height because the certainty of the evidence is very low. Furthermore, this intervention may make little or no difference in terms of adverse effects, but the certainty of the evidence is low.
 Autores:
Ana Sanhueza[1,2], Raúl González[2,3]
Autores:
Ana Sanhueza[1,2], Raúl González[2,3]

Citación: Sanhueza A, González R. Mineralocorticoid receptor antagonists in chronic central serous chorioretinopathy. Medwave 2020;20(08):e8035 doi: 10.5867/medwave.2020.08.8035
Fecha de envío: 5/12/2019
Fecha de aceptación: 12/12/2020
Fecha de publicación: 29/9/2020
Origen: Este artículo es producto del Epistemonikos Evidence Synthesis Project de la Fundación Epistemonikos, en colaboración con Medwave para su publicación.
Tipo de revisión: Con revisión por pares sin ciego por parte del equipo metodológico del Centro Evidencia UC en colaboración con Epistemonikos Evidence Synthesis Project.

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Wang SK, Sun P, Tandias RM, Seto BK, Arroyo JG. Mineralocorticoid Receptor Antagonists in Central Serous Chorioretinopathy: A Meta-Analysis of Randomized Controlled Trials. Ophthalmol Retina. 2019 Feb;3(2):154-160. | CrossRef | PubMed |
Wang SK, Sun P, Tandias RM, Seto BK, Arroyo JG. Mineralocorticoid Receptor Antagonists in Central Serous Chorioretinopathy: A Meta-Analysis of Randomized Controlled Trials. Ophthalmol Retina. 2019 Feb;3(2):154-160. | CrossRef | PubMed | Cakir B, Agostini H, Lange C. [Treatment of central serous chorioretinopathy with mineralocorticoid receptor antagonists]. Ophthalmologe. 2019 Feb;116(2):189-200. | CrossRef | PubMed |
Cakir B, Agostini H, Lange C. [Treatment of central serous chorioretinopathy with mineralocorticoid receptor antagonists]. Ophthalmologe. 2019 Feb;116(2):189-200. | CrossRef | PubMed | Chatziralli I, Vlachodimitropoulou A, Daoula C, Vrettou C, Galani E, Theodossiadis G, Theodossiadis P. Eplerenone in the treatment of central serous chorioretinopathy: a review of the literature. Int J Retina Vitreous. 2018 Sep 19;4:33. | CrossRef | PubMed | PMC |
Chatziralli I, Vlachodimitropoulou A, Daoula C, Vrettou C, Galani E, Theodossiadis G, Theodossiadis P. Eplerenone in the treatment of central serous chorioretinopathy: a review of the literature. Int J Retina Vitreous. 2018 Sep 19;4:33. | CrossRef | PubMed | PMC | Rahimy E, Pitcher JD 3rd, Hsu J, Adam MK, Shahlaee A, Samara WA, Vander JF, Kaiser RS, Chiang A, Spirn MJ, Fineman MS. A randomized double-blind placebo-control pilot study of eplerenone for the treatment of central serous chorioretinopathy (ECSELSIOR). Retina. 2018 May;38(5):962-969. | CrossRef | PubMed |
Rahimy E, Pitcher JD 3rd, Hsu J, Adam MK, Shahlaee A, Samara WA, Vander JF, Kaiser RS, Chiang A, Spirn MJ, Fineman MS. A randomized double-blind placebo-control pilot study of eplerenone for the treatment of central serous chorioretinopathy (ECSELSIOR). Retina. 2018 May;38(5):962-969. | CrossRef | PubMed | Schwartz R, Habot-Wilner Z, Martinez MR, Nutman A, Goldenberg D, Cohen S, Shulman S, Guzner-Gur H, Loewenstein A, Goldstein M. Eplerenone for chronic central serous chorioretinopathy-a randomized controlled prospective study. Acta Ophthalmol. 2017 Nov;95(7):e610-e618. | CrossRef | PubMed |
Schwartz R, Habot-Wilner Z, Martinez MR, Nutman A, Goldenberg D, Cohen S, Shulman S, Guzner-Gur H, Loewenstein A, Goldstein M. Eplerenone for chronic central serous chorioretinopathy-a randomized controlled prospective study. Acta Ophthalmol. 2017 Nov;95(7):e610-e618. | CrossRef | PubMed | Bousquet E, Beydoun T, Rothschild PR, Bergin C, Zhao M, Batista R, Brandely ML, Couraud B, Farman N, Gaudric A, Chast F, Behar-Cohen F. Spironolactone for nonresolving central serous chorioretinopathy: a randomized controlled crossover study. Retina. 2015 Dec;35(12):2505-15. | CrossRef | PubMed | PMC |
Bousquet E, Beydoun T, Rothschild PR, Bergin C, Zhao M, Batista R, Brandely ML, Couraud B, Farman N, Gaudric A, Chast F, Behar-Cohen F. Spironolactone for nonresolving central serous chorioretinopathy: a randomized controlled crossover study. Retina. 2015 Dec;35(12):2505-15. | CrossRef | PubMed | PMC | Pichi F, Carrai P, Ciardella A, Behar-Cohen F, Nucci P; Central Serous Chorioretinopathy Study Group. Comparison of two mineralcorticosteroids receptor antagonists for the treatment of central serous chorioretinopathy. Int Ophthalmol. 2017 Oct;37(5):1115-1125. | CrossRef | PubMed |
Pichi F, Carrai P, Ciardella A, Behar-Cohen F, Nucci P; Central Serous Chorioretinopathy Study Group. Comparison of two mineralcorticosteroids receptor antagonists for the treatment of central serous chorioretinopathy. Int Ophthalmol. 2017 Oct;37(5):1115-1125. | CrossRef | PubMed | Kapoor KG, Wagner AL. Mineralocorticoid Antagonists in the Treatment of Central Serous Chorioretinopathy: A Comparative Analysis. Ophthalmic Res. 2016;56(1):17-22. | CrossRef | PubMed |
Kapoor KG, Wagner AL. Mineralocorticoid Antagonists in the Treatment of Central Serous Chorioretinopathy: A Comparative Analysis. Ophthalmic Res. 2016;56(1):17-22. | CrossRef | PubMed | Singh RP, Sears JE, Bedi R, Schachat AP, Ehlers JP, Kaiser PK. Oral eplerenone for the management of chronic central serous chorioretinopathy. Int J Ophthalmol. 2015 Apr 18;8(2):310-4. | CrossRef | PubMed | PMC |
Singh RP, Sears JE, Bedi R, Schachat AP, Ehlers JP, Kaiser PK. Oral eplerenone for the management of chronic central serous chorioretinopathy. Int J Ophthalmol. 2015 Apr 18;8(2):310-4. | CrossRef | PubMed | PMC | Cakir B, Fischer F, Ehlken C, Bühler A, Stahl A, Schlunck G, Böhringer D, Agostini H, Lange C. Clinical experience with eplerenone to treat chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2016 Nov;254(11):2151-2157. Epub 2016 May 5. | PubMed |
Cakir B, Fischer F, Ehlken C, Bühler A, Stahl A, Schlunck G, Böhringer D, Agostini H, Lange C. Clinical experience with eplerenone to treat chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2016 Nov;254(11):2151-2157. Epub 2016 May 5. | PubMed | Chin EK, Almeida DR, Roybal CN, Niles PI, Gehrs KM, Sohn EH, Boldt HC, Russell SR, Folk JC. Oral mineralocorticoid antagonists for recalcitrant central serous chorioretinopathy. Clin Ophthalmol. 2015 Aug 11;9:1449-56. | CrossRef | PubMed | PMC |
Chin EK, Almeida DR, Roybal CN, Niles PI, Gehrs KM, Sohn EH, Boldt HC, Russell SR, Folk JC. Oral mineralocorticoid antagonists for recalcitrant central serous chorioretinopathy. Clin Ophthalmol. 2015 Aug 11;9:1449-56. | CrossRef | PubMed | PMC | Leisser C, Hirnschall N, Hackl C, Plasenzotti P, Findl O. Eplerenone in patients with chronic recurring central serous chorioretinopathy. Eur J Ophthalmol. 2016 Aug 4;26(5):479-84. | CrossRef | PubMed |
Leisser C, Hirnschall N, Hackl C, Plasenzotti P, Findl O. Eplerenone in patients with chronic recurring central serous chorioretinopathy. Eur J Ophthalmol. 2016 Aug 4;26(5):479-84. | CrossRef | PubMed | Salz DA, Pitcher JD 3rd, Hsu J, Regillo CD, Fineman MS, Elliott KS, Vander JF, Fischer DH, Spirn MJ. Oral eplerenone for treatment of chronic central serous chorioretinopathy: a case series. Ophthalmic Surg Lasers Imaging Retina. 2015 Apr;46(4):439-44. | CrossRef | PubMed |
Salz DA, Pitcher JD 3rd, Hsu J, Regillo CD, Fineman MS, Elliott KS, Vander JF, Fischer DH, Spirn MJ. Oral eplerenone for treatment of chronic central serous chorioretinopathy: a case series. Ophthalmic Surg Lasers Imaging Retina. 2015 Apr;46(4):439-44. | CrossRef | PubMed | Ghadiali Q, Jung JJ, Yu S, Patel SN, Yannuzzi LA. Central serous chorioretinopathy treated with mineralocorticoid antagonists: a one-year pilot study. Retina. 2016 Mar;36(3):611-8. | CrossRef | PubMed |
Ghadiali Q, Jung JJ, Yu S, Patel SN, Yannuzzi LA. Central serous chorioretinopathy treated with mineralocorticoid antagonists: a one-year pilot study. Retina. 2016 Mar;36(3):611-8. | CrossRef | PubMed | Gergely R, Kovács I, Schneider M, Resch M, Papp A, Récsán Z, Nagy ZZ, Ecsedy M. Mineralocorticoid receptor antagonist treatment in bilateral chronic central serous chorioretinopathy: a comparative study of exudative and nonexudative fellow eyes. Retina. 2017 Jun;37(6):1084-1091. | CrossRef | PubMed |
Gergely R, Kovács I, Schneider M, Resch M, Papp A, Récsán Z, Nagy ZZ, Ecsedy M. Mineralocorticoid receptor antagonist treatment in bilateral chronic central serous chorioretinopathy: a comparative study of exudative and nonexudative fellow eyes. Retina. 2017 Jun;37(6):1084-1091. | CrossRef | PubMed | Bousquet E, Beydoun T, Zhao M, Hassan L, Offret O, Behar-Cohen F. Mineralocorticoid receptor antagonism in the treatment of chronic central serous chorioretinopathy: a pilot study. Retina. 2013 Nov-Dec;33(10):2096-102. | CrossRef | PubMed |
Bousquet E, Beydoun T, Zhao M, Hassan L, Offret O, Behar-Cohen F. Mineralocorticoid receptor antagonism in the treatment of chronic central serous chorioretinopathy: a pilot study. Retina. 2013 Nov-Dec;33(10):2096-102. | CrossRef | PubMed | Breukink MB, den Hollander AI, Keunen JE, Boon CJ, Hoyng CB. The use ofeplerenone in therapy-resistant chronic central serous chorioretinopathy. Acta Ophthalmol. 2014 Sep;92(6):e488-90. | CrossRef | PubMed |
Breukink MB, den Hollander AI, Keunen JE, Boon CJ, Hoyng CB. The use ofeplerenone in therapy-resistant chronic central serous chorioretinopathy. Acta Ophthalmol. 2014 Sep;92(6):e488-90. | CrossRef | PubMed | Maier M, Stumpfe S, Feucht N, Strobl P, Rath V, Lohmann CP. Mineralocorticoid receptor antagonists as treatment option for acute and chronic central serous chorioretinopathy. Ophthalmologe. 2014 Feb;111(2):173-80. | CrossRef | PubMed |
Maier M, Stumpfe S, Feucht N, Strobl P, Rath V, Lohmann CP. Mineralocorticoid receptor antagonists as treatment option for acute and chronic central serous chorioretinopathy. Ophthalmologe. 2014 Feb;111(2):173-80. | CrossRef | PubMed | Herold TR, Rist K, Priglinger SG, Ulbig MW, Wolf A. Long-term results and recurrence rates after spironolactone treatment in non-resolving central serous chorio-retinopathy (CSCR). Graefes Arch Clin Exp Ophthalmol. 2017 Feb;255(2):221-229. | CrossRef | PubMed |
Herold TR, Rist K, Priglinger SG, Ulbig MW, Wolf A. Long-term results and recurrence rates after spironolactone treatment in non-resolving central serous chorio-retinopathy (CSCR). Graefes Arch Clin Exp Ophthalmol. 2017 Feb;255(2):221-229. | CrossRef | PubMed | Sampo M, Soler V, Gascon P, Ho Wang Yin G, Hoffart L, Denis D, Matonti F. Eplerenone treatment in chronic central serous chorioretinopathy]. J Fr Ophtalmol. 2016 Jun;39(6):535-42. | CrossRef | PubMed |
Sampo M, Soler V, Gascon P, Ho Wang Yin G, Hoffart L, Denis D, Matonti F. Eplerenone treatment in chronic central serous chorioretinopathy]. J Fr Ophtalmol. 2016 Jun;39(6):535-42. | CrossRef | PubMed | Lee JH, Lee SC, Kim H, Lee CS. Comparison of short-term efficacy between oral spironolactone treatment and photodynamic therapy for the treatment of nonresolving central serous chorioretinopathy. Retina. 2019 Jan;39(1):127-133. | CrossRef | PubMed |
Lee JH, Lee SC, Kim H, Lee CS. Comparison of short-term efficacy between oral spironolactone treatment and photodynamic therapy for the treatment of nonresolving central serous chorioretinopathy. Retina. 2019 Jan;39(1):127-133. | CrossRef | PubMed | Falavarjani KG, Amirsardari A, Habibi A, Eshaghi A, Bakhti S, Aghdam KA. Visual and Anatomical Outcomes of Spironolactone Therapy in Patients with Chronic Central Serous Chorioretinopathy. J Ophthalmic Vis Res. 2017 Jul-Sep;12(3):281-289. | CrossRef | PubMed | PMC |
Falavarjani KG, Amirsardari A, Habibi A, Eshaghi A, Bakhti S, Aghdam KA. Visual and Anatomical Outcomes of Spironolactone Therapy in Patients with Chronic Central Serous Chorioretinopathy. J Ophthalmic Vis Res. 2017 Jul-Sep;12(3):281-289. | CrossRef | PubMed | PMC | Zhao M, Célérier I, Bousquet E, Jeanny JC, Jonet L, Savoldelli M, Offret O, Curan A, Farman N, Jaisser F, Behar-Cohen F. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest. 2012 Jul;122(7):2672-9. | CrossRef | PubMed | PMC |
Zhao M, Célérier I, Bousquet E, Jeanny JC, Jonet L, Savoldelli M, Offret O, Curan A, Farman N, Jaisser F, Behar-Cohen F. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest. 2012 Jul;122(7):2672-9. | CrossRef | PubMed | PMC | Herold TR, Prause K, Wolf A, Mayer WJ, Ulbig MW. Spironolactone in the treatment of central serous chorioretinopathy - a case series. Graefes Arch Clin Exp Ophthalmol. 2014 Dec;252(12):1985-91. | CrossRef | PubMed |
Herold TR, Prause K, Wolf A, Mayer WJ, Ulbig MW. Spironolactone in the treatment of central serous chorioretinopathy - a case series. Graefes Arch Clin Exp Ophthalmol. 2014 Dec;252(12):1985-91. | CrossRef | PubMed | Daruich A, Matet A, Dirani A, Gallice M, Nicholson L, Sivaprasad S, Behar-Cohen F. Oral Mineralocorticoid-Receptor Antagonists: Real-Life Experience in Clinical Subtypes of Nonresolving Central Serous Chorioretinopathy With Chronic Epitheliopathy. Transl Vis Sci Technol. 2016 Mar 4;5(2):2. eCollection 2016 Mar. | PubMed | PMC |
Daruich A, Matet A, Dirani A, Gallice M, Nicholson L, Sivaprasad S, Behar-Cohen F. Oral Mineralocorticoid-Receptor Antagonists: Real-Life Experience in Clinical Subtypes of Nonresolving Central Serous Chorioretinopathy With Chronic Epitheliopathy. Transl Vis Sci Technol. 2016 Mar 4;5(2):2. eCollection 2016 Mar. | PubMed | PMC | Van Rijssen TJ, Van Dijk EHC, Yzer S, Ohno-Matsui K, Keunen JEE, Schlingemann RO, Sivaprasad S, Querques G, Downes SM, Fauser S, Hoyng CB, Piccolino FC, Chhablani JK, Lai TYY, Lotery AJ, Larsen M, Holz FG, Freund KB, Yannuzzi LA, Boon CJF. Central serous chorioretinopathy: Towards an evidence-based treatment guideline. Prog Retin Eye Res. 2019 Jul 15. pii: S1350-9462(18)30094-6. | CrossRef | PubMed |
Van Rijssen TJ, Van Dijk EHC, Yzer S, Ohno-Matsui K, Keunen JEE, Schlingemann RO, Sivaprasad S, Querques G, Downes SM, Fauser S, Hoyng CB, Piccolino FC, Chhablani JK, Lai TYY, Lotery AJ, Larsen M, Holz FG, Freund KB, Yannuzzi LA, Boon CJF. Central serous chorioretinopathy: Towards an evidence-based treatment guideline. Prog Retin Eye Res. 2019 Jul 15. pii: S1350-9462(18)30094-6. | CrossRef | PubMed | Lotery A, Sivaprasad S, O'Connell A, et al. Eplerenone for chronic central serous chorioretinopathy in patients with active, previously untreated disease for more than 4 months (VICI): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;395(10220):294-303. | CrossRef | PubMed |
Lotery A, Sivaprasad S, O'Connell A, et al. Eplerenone for chronic central serous chorioretinopathy in patients with active, previously untreated disease for more than 4 months (VICI): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;395(10220):294-303. | CrossRef | PubMed | CFJ, Boon. Photodynamic Therapy Versus Eplerenone: Treatment Trial for Chronic Central Serous Chorioretinopathy. ClinicalTrials.gov Identifier: NCT03079141.
CFJ, Boon. Photodynamic Therapy Versus Eplerenone: Treatment Trial for Chronic Central Serous Chorioretinopathy. ClinicalTrials.gov Identifier: NCT03079141.  Ghasemi K. Spironolactone in the treatment of chronic central serous chorioretinopathy. Iranian Registry of Clinical Trials Identifier: IRCT2015092224140N1.
Ghasemi K. Spironolactone in the treatment of chronic central serous chorioretinopathy. Iranian Registry of Clinical Trials Identifier: IRCT2015092224140N1.  Civile V., Felipe C., Carvas N., Koike M., Serracarbassa P. Mineralocorticoid receptor antagonists for the treatment of chronic central serous chorioretinopathy: systematic review with meta-analysis. PROSPERO 2020 CRD42020182601. | Link |
Civile V., Felipe C., Carvas N., Koike M., Serracarbassa P. Mineralocorticoid receptor antagonists for the treatment of chronic central serous chorioretinopathy: systematic review with meta-analysis. PROSPERO 2020 CRD42020182601. | Link | Duan J. Effectiveness of mineralcorticoid receptor antagonist in central serous chorioretinopathy: a systematic review and meta-analysis. PROSPERO 2020 CRD42020173466. | Link |
Duan J. Effectiveness of mineralcorticoid receptor antagonist in central serous chorioretinopathy: a systematic review and meta-analysis. PROSPERO 2020 CRD42020173466. | Link |