 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Palabras clave: Meniere, tinnitus, vertigo, positive pressure, Epistemonikos, GRADE.
INTRODUCTION
Ménière's disease is a disorder of the inner ear characterized by episodes of spontaneous vertigo, fluctuating hearing loss and tinnitus. Positive pressure therapy has been used to reduce the intensity and frequency of episodes, but it is not clear whether it is actually effective.
METHODS
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified five systematic reviews including 22 studies overall, of which five were randomized trials. We concluded positive pressure therapy probably leads to slightly worse hearing and makes little or no difference in the intensity of vertigo. In addition, we are uncertain whether positive pressure therapy improves functionality or decreases vertigo attacks as the certainty of the evidence has been assessed as very low.
Ménière's disease is characterized by episodes of spontaneous vertigo, tinnitus and fluctuating hearing loss. The American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) guidelines has defined the diagnostic criteria as two episodes of vertigo lasting more than 20 minutes, sensorineural hearing loss confirmed by audiometry and tinnitus or aural fullness [1]. The proposed mechanism of Ménière's disease would be an increase in endolymphatic pressure in the inner ear, whose cause is idiopathic. This would increase the frequency of episodes that, even though may transiently remit, can last several months, with the consequent deterioration in the quality of life [2].
The treatment aims to reduce the number and severity of vertigo episodes, to revert hearing loss and tinnitus, to relieve chronic symptoms and to prevent the progression of the disease. However, there are no treatment alternatives that can achieve these objectives, so positive pressure therapy has been proposed as a minimally invasive intervention.
This therapy uses a device (Meniett) that is inserted into the external auditory canal and produces low intensity pressure pulses. It is believed that these pulses are transmitted to the inner ear, exerting pressure. Before using this device, it is necessary to install a ventilation tube (collar) in the tympanic membrane to allow the passage of waves. This treatment is considered second line and there is still controversy regarding its efficacy in Ménière's disease.
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
|
What is the evidence. |
We found five systematic reviews [3], [4], [5], [6], [7] including 22 primary studies [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29] of which five corresponded to randomized trials [14], [18], [20], [23], [27]. This table and the summary in general are based on the latter, since the observational studies did not increase the certainty of the existing evidence nor provide additional relevant information. |
|
What types of patients were included* |
The diagnostic criteria used in four of the trials [14], [18], [20], [27] were those of the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) of 1995 for definitive diagnosis of Ménière's disease, specifying certain special characteristics for each of them, while in one trial [23] the criteria were not specified. |
|
What types of interventions were included* |
All trials used the Meniett device or some similar device as intervention, which produced pressure to the middle and inner ear. All trials used a placebo device that was identical to the functioning device, but did not produce pressure. In four trials, the ventilation tube was installed two weeks to two months prior to the beginning of treatment [18], [20], [23], [27]. In one trial it was not reported. [14]. All trials compared against placebo [14], [18], [20], [23], [27]. |
|
What types of outcomes |
The trials evaluated multiple outcomes, which were grouped by the systematic reviews as follows:
The average follow-up of the trials was 10.5 weeks, with a range between 2 and 16 weeks. |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
The information on the effects of positive pressure therapy in Ménière’s disease is based on three randomized trials [18], [20], [27] that included 163 patients in total. The other two trials did not provide sufficient data to be incorporated into a meta-analysis. Two trials [18], [20] reported change in hearing (123 patients),one trial [27] evaluated functionality and frequency of vertigo episodes (40 patients) and one trial reported [20] the intensity of vertigo (68 patients).
The summary of findings is as follows:
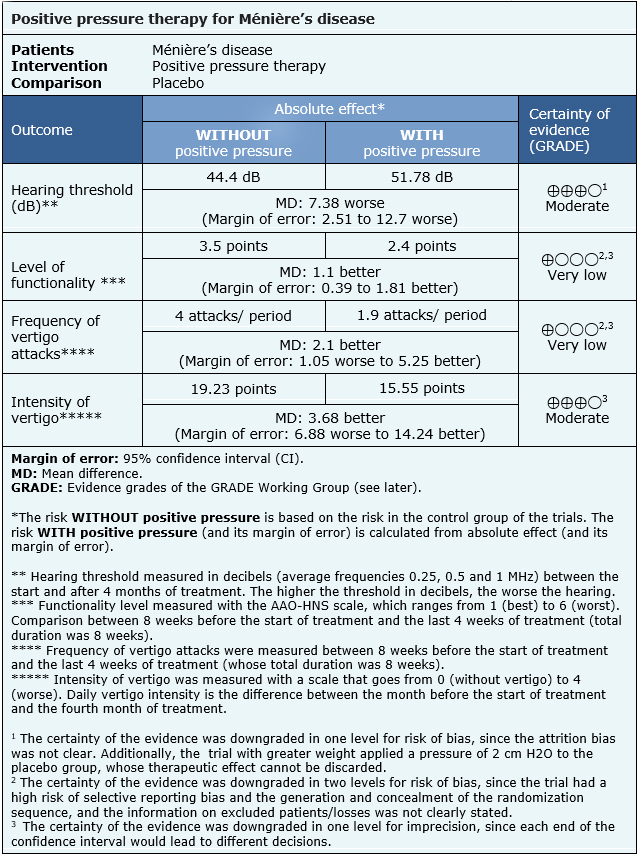
| Follow the link to access the interactive version of this table (Interactive Summary of Findings – iSoF) |

|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
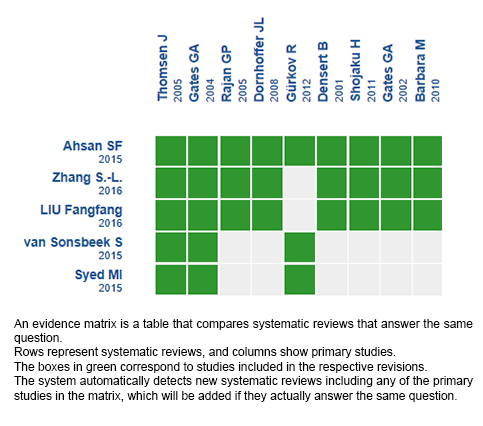
Follow the link to access the interactive version: Positive pressure therapy for Ménière's disease.
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCTION
Ménière's disease is a disorder of the inner ear characterized by episodes of spontaneous vertigo, fluctuating hearing loss and tinnitus. Positive pressure therapy has been used to reduce the intensity and frequency of episodes, but it is not clear whether it is actually effective.
METHODS
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified five systematic reviews including 22 studies overall, of which five were randomized trials. We concluded positive pressure therapy probably leads to slightly worse hearing and makes little or no difference in the intensity of vertigo. In addition, we are uncertain whether positive pressure therapy improves functionality or decreases vertigo attacks as the certainty of the evidence has been assessed as very low.
 Autores:
Jorge Holmberg[1,2], Martín de Amesti[1,2], Andrés Rosenbaum[2,3], Matías Winter[2,3]
Autores:
Jorge Holmberg[1,2], Martín de Amesti[1,2], Andrés Rosenbaum[2,3], Matías Winter[2,3]

Citación: Holmberg J, de Amesti M, Rosenbaum A, Winter M. Positive pressure therapy for Ménière’s disease. Medwave 2019;19(2):e7609 doi: 10.5867/medwave.2019.03.7609
Fecha de envío: 22/12/2018
Fecha de aceptación: 28/12/2018
Fecha de publicación: 8/4/2019
Origen: Este artículo es producto del Epistemonikos Evidence Synthesis Project de la Fundación Epistemonikos, en colaboración con Medwave para su publicación.
Tipo de revisión: Con revisión por pares sin ciego por parte del equipo metodológico del Epistemonikos Evidence Synthesis Project.

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Menière's disease. American Academy of Otolaryngology-Head and Neck Foundation, Inc. Otolaryngol Head Neck Surg. 1995 Sep;113(3):181-5.
Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Menière's disease. American Academy of Otolaryngology-Head and Neck Foundation, Inc. Otolaryngol Head Neck Surg. 1995 Sep;113(3):181-5.  Organización Panamericana de la Salud. Salud en las Américas, (2012). Volumen regional; Capítulo Determinantes e inequidades en salud, OPS; 2012:12- 59.
Organización Panamericana de la Salud. Salud en las Américas, (2012). Volumen regional; Capítulo Determinantes e inequidades en salud, OPS; 2012:12- 59.  Ahsan SF, Standring R, Wang Y. Systematic review and meta-analysis of Meniett therapy for Meniere's disease. Laryngoscope. 2015 Jan;125(1):203-8. | CrossRef | PubMed |
Ahsan SF, Standring R, Wang Y. Systematic review and meta-analysis of Meniett therapy for Meniere's disease. Laryngoscope. 2015 Jan;125(1):203-8. | CrossRef | PubMed | LIU Fangfang LI Lianhe. Systematic review of Meniett micropressure therapy for Meniere's disease. Chinese Archives of Otolaryngology-Head and Neck Surgery. 2016 Nov;7:650-656.
LIU Fangfang LI Lianhe. Systematic review of Meniett micropressure therapy for Meniere's disease. Chinese Archives of Otolaryngology-Head and Neck Surgery. 2016 Nov;7:650-656.  Syed MI, Rutka JA, Hendry J, Browning GG. Positive pressure therapy for Meniere's syndrome/disease with a Meniett device: a systematic review of randomised controlled trials. Clin Otolaryngol. 2015 Jun;40(3):197-207. | CrossRef | PubMed |
Syed MI, Rutka JA, Hendry J, Browning GG. Positive pressure therapy for Meniere's syndrome/disease with a Meniett device: a systematic review of randomised controlled trials. Clin Otolaryngol. 2015 Jun;40(3):197-207. | CrossRef | PubMed | van Sonsbeek S, Pullens B, van Benthem PP. Positive pressure therapy for Ménière's disease or syndrome. Cochrane Database Syst Rev. 2015 Mar 10;(3):CD008419. | CrossRef | PubMed |
van Sonsbeek S, Pullens B, van Benthem PP. Positive pressure therapy for Ménière's disease or syndrome. Cochrane Database Syst Rev. 2015 Mar 10;(3):CD008419. | CrossRef | PubMed | Zhang SL, Leng Y, Liu B, Shi H, Lu M, Kong WJ. Meniett Therapy for Ménière's Disease: An Updated Meta-analysis. Otol Neurotol. 2016 Mar;37(3):290-8. | CrossRef | PubMed |
Zhang SL, Leng Y, Liu B, Shi H, Lu M, Kong WJ. Meniett Therapy for Ménière's Disease: An Updated Meta-analysis. Otol Neurotol. 2016 Mar;37(3):290-8. | CrossRef | PubMed | Barbara M, Consagra C, Monini S, Nostro G, Harguindey A, Vestri A, Filipo R. Local pressure protocol, including Meniett, in the treatment of Ménière's disease: short-term results during the active stage. Acta Otolaryngol. 2001 Dec;121(8):939-44. | PubMed |
Barbara M, Consagra C, Monini S, Nostro G, Harguindey A, Vestri A, Filipo R. Local pressure protocol, including Meniett, in the treatment of Ménière's disease: short-term results during the active stage. Acta Otolaryngol. 2001 Dec;121(8):939-44. | PubMed | Barbara M, Lazzarino AI, Biagini M, Costa M, Monini S. Influence of Meniett® treatment on hearing. Acta Otolaryngol. 2010 Nov;130(11):1256-9. | CrossRef | PubMed |
Barbara M, Lazzarino AI, Biagini M, Costa M, Monini S. Influence of Meniett® treatment on hearing. Acta Otolaryngol. 2010 Nov;130(11):1256-9. | CrossRef | PubMed | Barbara M, Monini S, Chiappini I, Filipo R. Meniett therapy may avoid vestibular neurectomy in disabling Meniere's disease. Acta Otolaryngol. 2007 Nov;127(11):1136-41. | PubMed |
Barbara M, Monini S, Chiappini I, Filipo R. Meniett therapy may avoid vestibular neurectomy in disabling Meniere's disease. Acta Otolaryngol. 2007 Nov;127(11):1136-41. | PubMed | Boudewyns AN, Wuyts FL, Hoppenbrouwers M, Ketelslagers K, Vanspauwen R, Forton G, Van de Heyning PH. Meniett therapy: rescue treatment in severe drug-resistant Ménière's disease? Acta Otolaryngol. 2005 Dec;125(12):1283-9. | PubMed |
Boudewyns AN, Wuyts FL, Hoppenbrouwers M, Ketelslagers K, Vanspauwen R, Forton G, Van de Heyning PH. Meniett therapy: rescue treatment in severe drug-resistant Ménière's disease? Acta Otolaryngol. 2005 Dec;125(12):1283-9. | PubMed | Buchanan MA, Rai A, Prinsley PR. Initial UK experience of patient satisfaction with the Meniett® device for Ménière's disease treatment. J Laryngol Otol. 2010 Oct;124(10):1067-72. | CrossRef | PubMed |
Buchanan MA, Rai A, Prinsley PR. Initial UK experience of patient satisfaction with the Meniett® device for Ménière's disease treatment. J Laryngol Otol. 2010 Oct;124(10):1067-72. | CrossRef | PubMed | Chen JS, Rau KM, Chen YY, Huang JS, Yang TS, Lin YC, Liau CT, Lee KD, Su YC, Kao RH. A multiple-center phase II study of biweekly oxaliplatin and tegafur-uracil/leucovorin for chemonaive patients with advanced gastric cancer. Cancer Chemother Pharmacol. 2009 Apr;63(5):819-25. | CrossRef | PubMed |
Chen JS, Rau KM, Chen YY, Huang JS, Yang TS, Lin YC, Liau CT, Lee KD, Su YC, Kao RH. A multiple-center phase II study of biweekly oxaliplatin and tegafur-uracil/leucovorin for chemonaive patients with advanced gastric cancer. Cancer Chemother Pharmacol. 2009 Apr;63(5):819-25. | CrossRef | PubMed | Densert B, Densert O, Arlinger S, Sass K, Odkvist L. Immediate effects of middle ear pressure changes on the electrocochleographic recordings in patients with Menière's disease: a clinical placebo-controlled study. Am J Otol. 1997 Nov;18(6):726-33. | PubMed |
Densert B, Densert O, Arlinger S, Sass K, Odkvist L. Immediate effects of middle ear pressure changes on the electrocochleographic recordings in patients with Menière's disease: a clinical placebo-controlled study. Am J Otol. 1997 Nov;18(6):726-33. | PubMed | Densert B, Sass K. Control of symptoms in patients with Meniere's disease using middle ear pressure applications: two years follow-up. Acta Otolaryngol. 2001 Jul;121(5):616-21. | PubMed |
Densert B, Sass K. Control of symptoms in patients with Meniere's disease using middle ear pressure applications: two years follow-up. Acta Otolaryngol. 2001 Jul;121(5):616-21. | PubMed | Dornhoffer JL, King D. The effect of the Meniett device in patients with Ménière's disease: long-term results. Otol Neurotol. 2008 Sep;29(6):868-74. | CrossRef | PubMed |
Dornhoffer JL, King D. The effect of the Meniett device in patients with Ménière's disease: long-term results. Otol Neurotol. 2008 Sep;29(6):868-74. | CrossRef | PubMed | Gates GA, Verrall A, Green JD Jr, Tucci DL, Telian SA. Meniett clinical trial: long-term follow-up. Arch Otolaryngol Head Neck Surg. 2006 Dec;132(12):1311-6. | PubMed |
Gates GA, Verrall A, Green JD Jr, Tucci DL, Telian SA. Meniett clinical trial: long-term follow-up. Arch Otolaryngol Head Neck Surg. 2006 Dec;132(12):1311-6. | PubMed | Gates GA, Green JD Jr, Tucci DL, Telian SA. The effects of transtympanic micropressure treatment in people with unilateral Meniere's disease. Arch Otolaryngol Head Neck Surg. 2004 Jun;130(6):718-25. | PubMed |
Gates GA, Green JD Jr, Tucci DL, Telian SA. The effects of transtympanic micropressure treatment in people with unilateral Meniere's disease. Arch Otolaryngol Head Neck Surg. 2004 Jun;130(6):718-25. | PubMed | Gates GA, Green JD Jr. Intermittent pressure therapy of intractable Ménière's disease using the Meniett device: a preliminary report. Laryngoscope. 2002 Aug;112(8 Pt 1):1489-93. | PubMed |
Gates GA, Green JD Jr. Intermittent pressure therapy of intractable Ménière's disease using the Meniett device: a preliminary report. Laryngoscope. 2002 Aug;112(8 Pt 1):1489-93. | PubMed | Gürkov R, Filipe Mingas LB, Rader T, Louza J, Olzowy B, Krause E. Effect of transtympanic low-pressure therapy in patients with unilateral Menière's disease unresponsive to betahistine: a randomised, placebo-controlled, double-blinded, clinical trial. J Laryngol Otol. 2012 Apr;126(4):356-62. | CrossRef | PubMed |
Gürkov R, Filipe Mingas LB, Rader T, Louza J, Olzowy B, Krause E. Effect of transtympanic low-pressure therapy in patients with unilateral Menière's disease unresponsive to betahistine: a randomised, placebo-controlled, double-blinded, clinical trial. J Laryngol Otol. 2012 Apr;126(4):356-62. | CrossRef | PubMed | Huang W, Liu F, Gao B, Zhou J. Clinical long-term effects of Meniett pulse generator for Meniere's disease. Acta Otolaryngol. 2009 Aug;129(8):819-25. | CrossRef | PubMed |
Huang W, Liu F, Gao B, Zhou J. Clinical long-term effects of Meniett pulse generator for Meniere's disease. Acta Otolaryngol. 2009 Aug;129(8):819-25. | CrossRef | PubMed | Mattox DE, Reichert M. Meniett device for Ménière's disease: use and compliance at 3 to 5 years. Otol Neurotol. 2008 Jan;29(1):29-32. | CrossRef | PubMed |
Mattox DE, Reichert M. Meniett device for Ménière's disease: use and compliance at 3 to 5 years. Otol Neurotol. 2008 Jan;29(1):29-32. | CrossRef | PubMed | Odkvist LM, Arlinger S, Billermark E, Densert B, Lindholm S, Wallqvist J. Effects of middle ear pressure changes on clinical symptoms in patients with Ménière's disease--a clinical multicentre placebo-controlled study. Acta Otolaryngol Suppl. 2000;543:99-101. | PubMed |
Odkvist LM, Arlinger S, Billermark E, Densert B, Lindholm S, Wallqvist J. Effects of middle ear pressure changes on clinical symptoms in patients with Ménière's disease--a clinical multicentre placebo-controlled study. Acta Otolaryngol Suppl. 2000;543:99-101. | PubMed | Rajan GP, Din S, Atlas MD. Long-term effects of the Meniett device in Ménière's disease: the Western Australian experience. J Laryngol Otol. 2005 May;119(5):391-5. | PubMed |
Rajan GP, Din S, Atlas MD. Long-term effects of the Meniett device in Ménière's disease: the Western Australian experience. J Laryngol Otol. 2005 May;119(5):391-5. | PubMed | Shojaku H, Watanabe Y, Mineta H, Aoki M, Tsubota M, Watanabe K, Goto F, Shigeno K. Long-term effects of the Meniett device in Japanese patients with Meniere's disease and delayed endolymphatic hydrops reported by the Middle Ear Pressure Treatment Research Group of Japan. Acta Otolaryngol. 2011 Mar;131(3):277-83. | CrossRef | PubMed |
Shojaku H, Watanabe Y, Mineta H, Aoki M, Tsubota M, Watanabe K, Goto F, Shigeno K. Long-term effects of the Meniett device in Japanese patients with Meniere's disease and delayed endolymphatic hydrops reported by the Middle Ear Pressure Treatment Research Group of Japan. Acta Otolaryngol. 2011 Mar;131(3):277-83. | CrossRef | PubMed | Stokroos R, Olvink MK, Hendrice N, Kingma H. Functional outcome of treatment of Ménière's disease with the Meniett pressure generator. Acta Otolaryngol. 2006 Mar;126(3):254-8. | PubMed |
Stokroos R, Olvink MK, Hendrice N, Kingma H. Functional outcome of treatment of Ménière's disease with the Meniett pressure generator. Acta Otolaryngol. 2006 Mar;126(3):254-8. | PubMed | Thomsen J, Sass K, Odkvist L, Arlinger S. Local overpressure treatment reduces vestibular symptoms in patients with Meniere's disease: a clinical, randomized, multicenter, double-blind, placebo-controlled study. Otol Neurotol. 2005 Jan;26(1):68-73. | PubMed |
Thomsen J, Sass K, Odkvist L, Arlinger S. Local overpressure treatment reduces vestibular symptoms in patients with Meniere's disease: a clinical, randomized, multicenter, double-blind, placebo-controlled study. Otol Neurotol. 2005 Jan;26(1):68-73. | PubMed | Watanabe Y, Shojaku H, Junicho M, Asai M, Fujisaka M, Takakura H, Tsubota M, Yasumura S. Intermittent pressure therapy of intractable Meniere's disease and delayed endolymphatic hydrops using the transtympanic membrane massage device: a preliminary report. Acta Otolaryngol. 2011 Nov;131(11):1178-86. | CrossRef | PubMed |
Watanabe Y, Shojaku H, Junicho M, Asai M, Fujisaka M, Takakura H, Tsubota M, Yasumura S. Intermittent pressure therapy of intractable Meniere's disease and delayed endolymphatic hydrops using the transtympanic membrane massage device: a preliminary report. Acta Otolaryngol. 2011 Nov;131(11):1178-86. | CrossRef | PubMed | Yang Haidi, Zheng Yiqing, Liu Xiang, et al. Meniett Low Pressure Pulse Therapy for Meniere. Short-term efficacy observation of Angstrom disease. Journal of Audiology and Speech Disorders, 2007, 230-232.
Yang Haidi, Zheng Yiqing, Liu Xiang, et al. Meniett Low Pressure Pulse Therapy for Meniere. Short-term efficacy observation of Angstrom disease. Journal of Audiology and Speech Disorders, 2007, 230-232.  Nevoux J, Franco-Vidal V, Bouccara D, Parietti-Winkler C, Uziel A, Chays A, Dubernard X, Couloigner V, Darrouzet V, Mom T; Groupe de Travail de la SFORL. Diagnostic and therapeutic strategy in Menière's disease. Guidelines of the French Otorhinolaryngology-Head and Neck Surgery Society (SFORL). Eur Ann Otorhinolaryngol Head Neck Dis. 2017 Jan 3. pii: S1879-7296(16)30222-8.
Nevoux J, Franco-Vidal V, Bouccara D, Parietti-Winkler C, Uziel A, Chays A, Dubernard X, Couloigner V, Darrouzet V, Mom T; Groupe de Travail de la SFORL. Diagnostic and therapeutic strategy in Menière's disease. Guidelines of the French Otorhinolaryngology-Head and Neck Surgery Society (SFORL). Eur Ann Otorhinolaryngol Head Neck Dis. 2017 Jan 3. pii: S1879-7296(16)30222-8.  Diagnóstico y Tratamiento de la Enfermedad de Ménière en los tres niveles de atención México; Secretaría de Salud; 12 de diciembre de 2013.
Diagnóstico y Tratamiento de la Enfermedad de Ménière en los tres niveles de atención México; Secretaría de Salud; 12 de diciembre de 2013.  Espinosa-Sánchez JM, Lopez-Escamez JA. Menière's disease. Handb Clin Neurol. 2016;137:257-77.
Espinosa-Sánchez JM, Lopez-Escamez JA. Menière's disease. Handb Clin Neurol. 2016;137:257-77.  Russo FY, Nguyen Y, De Seta D, Bouccara D, Sterkers O, Ferrary E, Bernardeschi D. Meniett device in meniere disease: Randomized, double-blind, placebo-controlled multicenter trial. Laryngoscope. 2017 Feb;127(2):470-475. | CrossRef | PubMed |
Russo FY, Nguyen Y, De Seta D, Bouccara D, Sterkers O, Ferrary E, Bernardeschi D. Meniett device in meniere disease: Randomized, double-blind, placebo-controlled multicenter trial. Laryngoscope. 2017 Feb;127(2):470-475. | CrossRef | PubMed | Liu F, Huang WN. [Clinical short-term effect of Meniett pulse generator for Meniere disease]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007 Mar;42(3):169-72. | PubMed |
Liu F, Huang WN. [Clinical short-term effect of Meniett pulse generator for Meniere disease]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007 Mar;42(3):169-72. | PubMed | Krause E, Gürkov R. Efficacy of Local Overpressure Treatment for Meniere's Disease. Clinicaltrials.gov. NCT00831688, 2009. | Link |
Krause E, Gürkov R. Efficacy of Local Overpressure Treatment for Meniere's Disease. Clinicaltrials.gov. NCT00831688, 2009. | Link | van Esch BF, van der Zaag-Loonen HJ, Bruintjes TD, van Benthem PP. Interventions for Menière's disease: protocol for an umbrella systematic review and a network meta-analysis. BMJ Open. 2016 Jun 9;6(6):e010269.
van Esch BF, van der Zaag-Loonen HJ, Bruintjes TD, van Benthem PP. Interventions for Menière's disease: protocol for an umbrella systematic review and a network meta-analysis. BMJ Open. 2016 Jun 9;6(6):e010269.