 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Palabras clave: proliferative diabetic retinopathy, bevacizumab, vitrectomy, Epistemonikos, GRADE.
UPDATE
This Living FRISBEE (Living FRIendly Summary of the Body of Evidence using Epistemonikos) is an update of the summary published in December 2014.
INTRODUCTION
Proliferative diabetic retinopathy can cause severe vision loss and even blindness if left untreated. Vitrectomy is often required in the treatment of more severe cases. Preoperative administration of bevacizumab, a humanized anti-vascular endothelial growth factor would improve intraoperative variables that facilitate surgery and improve postoperative course.
METHODS
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified five systematic reviews including 16 studies overall, of which 14 were randomized trials. We concluded the preoperative use of intravitreal bevacizumab reduces the rate of vitreous hemorrhage in the early postoperative period, and probably also in the late postoperative period, but its effect on visual acuity is not clear. Furthermore, it probably decreases the surgical time and may decrease the incidence of iatrogenic retinal breaks. Although we are uncertain whether preoperative bevacizumab decreases intraoperative bleeding, it may reduce the need for endodiathermy.
This Living FRISBEE (Living Friendly Summary of the Body of Evidence using Epistemonikos) is an update of the summary published in December 2014 (doi: 10.5867/medwave. 2015.6160)[1], based on a new systematic review [2] and the update [3] of one of the reviews already included in the previous version [4]. These systematic reviews included four new trials [5], [6], [7], [8] compared to the previous version of this summary.
Considering the new evidence, we have redefined the relevant outcomes, updating and conducting new analyses with the available data. The evidence incorporated in this summary leads to changes related to the certainty of the evidence on the outcomes associated with intraoperative variables, the addition of postoperative outcomes and the reformulation of the adverse effects analysis previously published.
Proliferative diabetic retinopathy, neovascularization and fibrous proliferation lead to blindness if not treated appropriately. Patients with this condition may have complications such as vitreous hemorrhage, tractional retinal detachment or extensive fibrovascular proliferation requiring vitrectomy as part of their treatment. This procedure has the risk of intraoperative bleeding, which decreases the visibility during the surgery, with the subsequent risk of complications during the postoperative period.
Bevacizumab is a monoclonal antibody directed against vascular endothelial growth factor. Its antiangiogenic properties would be useful in patients with active neovascularization who undergo vitrectomy, facilitating surgery by decreasing intraoperative complications such as bleeding and decreasing the occurrence of vitreous hemorrhage during the postoperative period.
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
|
What is the evidence. |
We found five systematic reviews [2], [3], [9], [10], [11] including 16 primary studies [5], [6], [7], [8], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], of which 14 are randomized trials [4], [5], [6], [7], [12], [13], [15], [16], [17], [19], [20], [21], [22], [23]. This table and the summary in general are based on the latter, since the observational studies did not increase the certainty of the existing evidence, nor did they provide relevant additional information. |
|
What types of patients were included* |
Most trials included patients without distinction by sex or age. Only one trial excluded patients under 18 years of age [4]. The age reported by the different trials ranged from 44 [12] to 62 years [5]. All trials included patients with indication of pars plana vitrectomy for complications of proliferative diabetic retinopathy, mainly vitreous hemorrhage [5], [7], [8], [12], [13], [15], [19], [21], [22], [23] and tractional retinal detachment [5], [7], [8], [12], [13], [15], [16], [17], [19], [20], [21], [22], [23]. Only one trial excluded patients with vitreous hemorrhage of any grade [16], while another trial excluded cases of dense vitreous hemorrhage preventing preoperative grading of fibrovascular membranes [5]. One trial did not consider cases of tractional-rhegmatogenous retinal detachment [19] and one trial excluded retinal detachment with macular involvement [20]. There was great variability in the exclusion criteria reported. It should be noted that most trials excluded patients with previous intraocular surgery, especially vitreoretinal surgery [5], [6], [8], [13], [16], [17], [21], [22]; five trials excluded patients with history of myocardial infarction, stroke or thrombotic events [4], [7], [13], [16], [22]; four trials excluded patients with blood coagulopathy, alterations in coagulation tests or use of antithrombotics other than aspirin [13], [16], [21], [22]. Only two trials excluded pregnant women [5], [15] and two excluded previous intravitreal injection of bevacizumab [15], [17]. The trials included 674 eyes in 649 patients, all of them adults. Four trials included more than one eye per patient [5], [8], [19], [21]. |
|
What types of interventions were included* |
All trials compared intravitreal injection of bevacizumab before vitrectomy against vitrectomy alone. Only three trials used simulated injection in the control group [5], [15], [19]. Intravitreal bevacizumab was used in the intervention group in different administration protocols. The dose was mostly 1.25 mg in 0.05 ml [5], [6], [7], [12], [13], [15], [19], [20], [21], [22], [23]. Two trials used higher doses of bevacizumab in the same concentration: one of them used 2.5 mg [17] and another 1.5 mg [16]. The concentration of bevacizumab was the same in all trials except in the most recent one that used a significantly lower concentration with a dose of 0.16 mg in 0.05 ml [8]. The timing of the intervention ranged from 1 to 20 days before surgery. Most trials administered bevacizumab at least 7 days prior to vitrectomy [5], [6], [7], [8], [12], [13], [15], [17], [20], [22]. One trial administered bevacizumab 14 days before the procedure [16]. In two trials patients were randomized to one of three groups: control and two intervention groups, which varied in the preoperative time intervals [19], [21]: 7 or 20 days before vitrectomy respectively [19] and 1 to 14 days prior vitrectomy, with an average of 4.9 days [21]. Data obtained from the 37 eyes of this study [21] receiving intraoperative bevacizumab were excluded when meta-analyses were performed. |
|
What types of outcomes |
There is great variability in the outcomes measured by the trials. These can be classified into intraoperative variables, postoperative course and adverse effects:
|
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
The information about the effects of bevacizumab is based on 13 randomized trials including 674 eyes in 649 patients [5], [6], [7], [8], [12], [13], [15], [16], [17], [19], [20], [21], [22]. One of the trials [23] did not provide information about any outcome of interest.
Nine trials reported early postoperative vitreous hemorrhage (475 eyes) [6], [7], [8], [12], [15], [17], [20], [21], [22] and six reported late postoperative vitreous hemorrhage (269 eyes) [7], [12], [17], [20], [21], [22]. Six trials reported visual acuity during postoperative follow-up (270 eyes) [5], [12], [17], [19], [20], [21], six assessed total surgical time (246 eyes) [8], [12], [13], [17], [19], [20], six intraoperative bleeding (218 eyes) [5], [12], [13], [15], [19], [20], six intraoperative use of endodiathermy (276 eyes) [6], [8], [12], [17], [20], [22] and seven reported iatrogenic retinal tears (333 eyes) [8], [12], [13], [15], [19], [20], [22].
Eight trials assessed systemic adverse events including myocardial infarction, stroke or systemic thrombosis (366 eyes) [5], [8], [12], [13], [15], [16], [19], [21] and six endophthalmitis (318 eyes) [8], [13], [15], [16], [19], [21].
The summary of findings is as follows:
Postoperative course:
Intraoperative Variables:
Adverse effects:
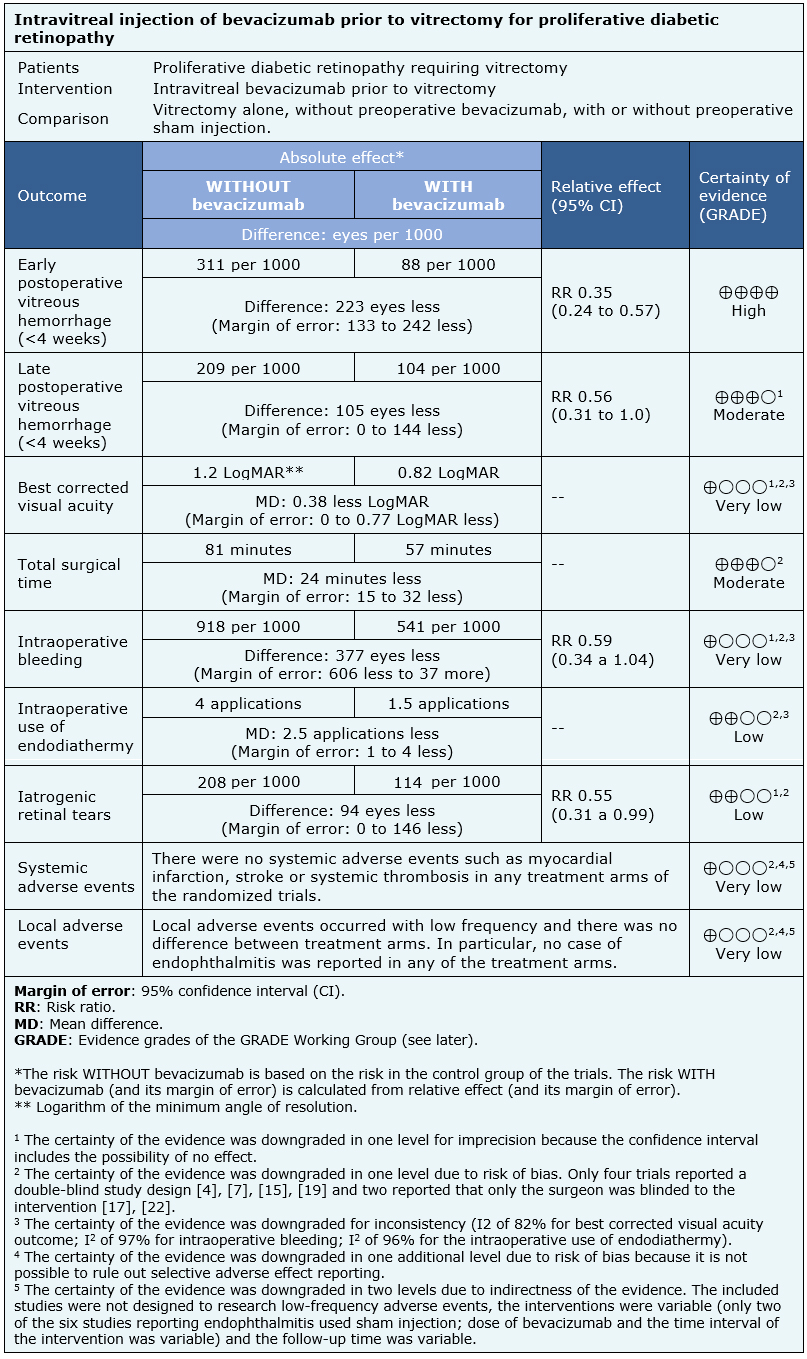
| Follow the link to access the interactive version of this table (Interactive Summary of Findings – iSoF) |

|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
Follow the link to access the interactive version: Preoperative bevacizumab for proliferative diabetic retinopathy.
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

UPDATE
This Living FRISBEE (Living FRIendly Summary of the Body of Evidence using Epistemonikos) is an update of the summary published in December 2014.
INTRODUCTION
Proliferative diabetic retinopathy can cause severe vision loss and even blindness if left untreated. Vitrectomy is often required in the treatment of more severe cases. Preoperative administration of bevacizumab, a humanized anti-vascular endothelial growth factor would improve intraoperative variables that facilitate surgery and improve postoperative course.
METHODS
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified five systematic reviews including 16 studies overall, of which 14 were randomized trials. We concluded the preoperative use of intravitreal bevacizumab reduces the rate of vitreous hemorrhage in the early postoperative period, and probably also in the late postoperative period, but its effect on visual acuity is not clear. Furthermore, it probably decreases the surgical time and may decrease the incidence of iatrogenic retinal breaks. Although we are uncertain whether preoperative bevacizumab decreases intraoperative bleeding, it may reduce the need for endodiathermy.
 Autores:
Efraín Pérez-Argandoña[1,2], Juan Verdaguer[1], Sergio Zacharías[1], Raúl González[2,3]
Autores:
Efraín Pérez-Argandoña[1,2], Juan Verdaguer[1], Sergio Zacharías[1], Raúl González[2,3]

Citación: Pérez-Argandoña E, Verdaguer J, Zacharías S, González R. Preoperative intravitreal bevacizumab for proliferative diabetic retinopathy patients undergoing vitrectomy - First update. Medwave 2019;19(1):e7511 doi: 10.5867/medwave.2019.01.7511
Fecha de envío: 24/5/2018
Fecha de aceptación: 11/12/2018
Fecha de publicación: 25/1/2019
Origen: Este artículo es producto del Epistemonikos Evidence Synthesis Project de la Fundación Epistemonikos, en colaboración con Medwave para su publicación.
Tipo de revisión: Con revisión por pares sin ciego por parte del equipo metodológico del Epistemonikos Evidence Synthesis Project.

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Véliz D, Rada G. [Does preoperative intravitreal bevacizumab reduce complications of vitrectomy for proliferative diabetic retinopathy?]. Medwave. 2014;14(11):e6052. | PubMed |
Véliz D, Rada G. [Does preoperative intravitreal bevacizumab reduce complications of vitrectomy for proliferative diabetic retinopathy?]. Medwave. 2014;14(11):e6052. | PubMed | Simunovic MP, Maberley DA. ANTI-VASCULAR ENDOTHELIAL GROWTH FACTOR THERAPY FOR PROLIFERATIVE DIABETIC RETINOPATHY: A Systematic Review and Meta-Analysis. Retina. 2015;35(10):1931-42. | CrossRef | PubMed |
Simunovic MP, Maberley DA. ANTI-VASCULAR ENDOTHELIAL GROWTH FACTOR THERAPY FOR PROLIFERATIVE DIABETIC RETINOPATHY: A Systematic Review and Meta-Analysis. Retina. 2015;35(10):1931-42. | CrossRef | PubMed | Smith JM, Steel DH. Anti-vascular endothelial growth factor for prevention of postoperative vitreous cavity haemorrhage after vitrectomy for proliferative diabetic retinopathy. Cochrane Database Syst Rev. 2015;8:CD008214. | CrossRef | PubMed |
Smith JM, Steel DH. Anti-vascular endothelial growth factor for prevention of postoperative vitreous cavity haemorrhage after vitrectomy for proliferative diabetic retinopathy. Cochrane Database Syst Rev. 2015;8:CD008214. | CrossRef | PubMed | Smith JM, Steel DH. Anti-vascular endothelial growth factor for prevention of postoperative vitreous cavity haemorrhage after vitrectomy for proliferative diabetic retinopathy. Cochrane Database Syst Rev. 2011(5):CD008214. | CrossRef | PubMed |
Smith JM, Steel DH. Anti-vascular endothelial growth factor for prevention of postoperative vitreous cavity haemorrhage after vitrectomy for proliferative diabetic retinopathy. Cochrane Database Syst Rev. 2011(5):CD008214. | CrossRef | PubMed | Sohn EH, He S, Kim LA, Salehi-Had H, Javaheri M, Spee C, et al. Angiofibrotic response to vascular endothelial growth factor inhibition in diabetic retinal detachment: report no. 1. Arch Ophthalmol. 2012;130(9):1127-34. | CrossRef | PubMed | PMC |
Sohn EH, He S, Kim LA, Salehi-Had H, Javaheri M, Spee C, et al. Angiofibrotic response to vascular endothelial growth factor inhibition in diabetic retinal detachment: report no. 1. Arch Ophthalmol. 2012;130(9):1127-34. | CrossRef | PubMed | PMC | Elwan MM, Ghanem AA, Abousamra WA. Outcome of a Single Intravitreal Bevacizumab Injection on the Visual Acuity and Course of Pars Plana Vitrectomy in Proliferative Diabetic Retinopathy. Curr Eye Res. 2013. | CrossRef | PubMed |
Elwan MM, Ghanem AA, Abousamra WA. Outcome of a Single Intravitreal Bevacizumab Injection on the Visual Acuity and Course of Pars Plana Vitrectomy in Proliferative Diabetic Retinopathy. Curr Eye Res. 2013. | CrossRef | PubMed | Zaman Y, Rehman AU, Memon AF. Intravitreal Avastin as an adjunct in patients with proliferative diabetic retinopathy undergoing pars plana vitrectomy. Pak J Med Sci. 2013;29(2):590-2. | PubMed | PMC |
Zaman Y, Rehman AU, Memon AF. Intravitreal Avastin as an adjunct in patients with proliferative diabetic retinopathy undergoing pars plana vitrectomy. Pak J Med Sci. 2013;29(2):590-2. | PubMed | PMC | Manabe A, Shimada H, Hattori T, Nakashizuka H, Yuzawa M. RANDOMIZED CONTROLLED STUDY OF INTRAVITREAL BEVACIZUMAB 0.16 MG INJECTED ONE DAY BEFORE SURGERY FOR PROLIFERATIVE DIABETIC RETINOPATHY. Retina. 2015;35(9):1800-7. | CrossRef | PubMed |
Manabe A, Shimada H, Hattori T, Nakashizuka H, Yuzawa M. RANDOMIZED CONTROLLED STUDY OF INTRAVITREAL BEVACIZUMAB 0.16 MG INJECTED ONE DAY BEFORE SURGERY FOR PROLIFERATIVE DIABETIC RETINOPATHY. Retina. 2015;35(9):1800-7. | CrossRef | PubMed | Zhao LQ, Zhu H, Zhao PQ, Hu YQ. A systematic review and meta-analysis of clinical outcomes of vitrectomy with or without intravitreal bevacizumab pretreatment for severe diabetic retinopathy. Br J Ophthalmol. 2011;95(9):1216-22. | CrossRef | PubMed | PMC |
Zhao LQ, Zhu H, Zhao PQ, Hu YQ. A systematic review and meta-analysis of clinical outcomes of vitrectomy with or without intravitreal bevacizumab pretreatment for severe diabetic retinopathy. Br J Ophthalmol. 2011;95(9):1216-22. | CrossRef | PubMed | PMC | Zhang ZH, Liu HY, Hernandez-Da Mota SE, Romano MR, Falavarjani KG, Ahmadieh H, et al. Vitrectomy with or without preoperative intravitreal bevacizumab for proliferative diabetic retinopathy: a meta-analysis of randomized controlled trials. Am J Ophthalmol. 2013;156(1):106-15.e2. | CrossRef | PubMed |
Zhang ZH, Liu HY, Hernandez-Da Mota SE, Romano MR, Falavarjani KG, Ahmadieh H, et al. Vitrectomy with or without preoperative intravitreal bevacizumab for proliferative diabetic retinopathy: a meta-analysis of randomized controlled trials. Am J Ophthalmol. 2013;156(1):106-15.e2. | CrossRef | PubMed | Martinez-Zapata MJ, Martí-Carvajal AJ, Solà I, Pijoán JI, Buil-Calvo JA, Cordero JA, et al. Anti-vascular endothelial growth factor for proliferative diabetic retinopathy. Cochrane Database Syst Rev. 2014;11:CD008721. | CrossRef | PubMed |
Martinez-Zapata MJ, Martí-Carvajal AJ, Solà I, Pijoán JI, Buil-Calvo JA, Cordero JA, et al. Anti-vascular endothelial growth factor for proliferative diabetic retinopathy. Cochrane Database Syst Rev. 2014;11:CD008721. | CrossRef | PubMed | El-Batarny AM. Intravitreal bevacizumab as an adjunctive therapy before diabetic vitrectomy. Clin Ophthalmol. 2008;2(4):709-16. | PubMed | PMC |
El-Batarny AM. Intravitreal bevacizumab as an adjunctive therapy before diabetic vitrectomy. Clin Ophthalmol. 2008;2(4):709-16. | PubMed | PMC | Rizzo S, Genovesi-Ebert F, Di Bartolo E, Vento A, Miniaci S, Williams G. Injection of intravitreal bevacizumab (Avastin) as a preoperative adjunct before vitrectomy surgery in the treatment of severe proliferative diabetic retinopathy (PDR). Graefes Arch Clin Exp Ophthalmol. 2008;246(6):837-42. | CrossRef | PubMed |
Rizzo S, Genovesi-Ebert F, Di Bartolo E, Vento A, Miniaci S, Williams G. Injection of intravitreal bevacizumab (Avastin) as a preoperative adjunct before vitrectomy surgery in the treatment of severe proliferative diabetic retinopathy (PDR). Graefes Arch Clin Exp Ophthalmol. 2008;246(6):837-42. | CrossRef | PubMed | Yang CM, Yeh PT, Yang CH, Chen MS. Bevacizumab pretreatment and long-acting gas infusion on vitreous clear-up after diabetic vitrectomy. Am J Ophthalmol. 2008;146(2):211-7 | CrossRef | PubMed |
Yang CM, Yeh PT, Yang CH, Chen MS. Bevacizumab pretreatment and long-acting gas infusion on vitreous clear-up after diabetic vitrectomy. Am J Ophthalmol. 2008;146(2):211-7 | CrossRef | PubMed | Ahmadieh H, Shoeibi N, Entezari M, Monshizadeh R. Intravitreal bevacizumab for prevention of early postvitrectomy hemorrhage in diabetic patients: a randomized clinical trial. Ophthalmology. 2009;116(10):1943-8. | CrossRef | PubMed |
Ahmadieh H, Shoeibi N, Entezari M, Monshizadeh R. Intravitreal bevacizumab for prevention of early postvitrectomy hemorrhage in diabetic patients: a randomized clinical trial. Ophthalmology. 2009;116(10):1943-8. | CrossRef | PubMed | da R Lucena D, Ribeiro JA, Costa RA, Barbosa JC, Scott IU, de Figueiredo-Pontes LL, et al. Intraoperative bleeding during vitrectomy for diabetic tractional retinal detachment with versus without preoperative intravitreal bevacizumab (IBeTra study). Br J Ophthalmol. 2009;93(5):688-91. | CrossRef | PubMed |
da R Lucena D, Ribeiro JA, Costa RA, Barbosa JC, Scott IU, de Figueiredo-Pontes LL, et al. Intraoperative bleeding during vitrectomy for diabetic tractional retinal detachment with versus without preoperative intravitreal bevacizumab (IBeTra study). Br J Ophthalmol. 2009;93(5):688-91. | CrossRef | PubMed | Modarres M, Nazari H, Falavarjani KG, Naseripour M, Hashemi M, Parvaresh MM. Intravitreal injection of bevacizumab before vitrectomy for proliferative diabetic retinopathy. Eur J Ophthalmol. 2009;19(5):848-52. | PubMed |
Modarres M, Nazari H, Falavarjani KG, Naseripour M, Hashemi M, Parvaresh MM. Intravitreal injection of bevacizumab before vitrectomy for proliferative diabetic retinopathy. Eur J Ophthalmol. 2009;19(5):848-52. | PubMed | Yeh PT, Yang CM, Lin YC, Chen MS, Yang CH. Bevacizumab pretreatment in vitrectomy with silicone oil for severe diabetic retinopathy. Retina. 2009;29(6):768-74. | CrossRef | PubMed |
Yeh PT, Yang CM, Lin YC, Chen MS, Yang CH. Bevacizumab pretreatment in vitrectomy with silicone oil for severe diabetic retinopathy. Retina. 2009;29(6):768-74. | CrossRef | PubMed | di Lauro R, De Ruggiero P, di Lauro MT, Romano MR. Intravitreal bevacizumab for surgical treatment of severe proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2010;248(6):785-91. | CrossRef | PubMed |
di Lauro R, De Ruggiero P, di Lauro MT, Romano MR. Intravitreal bevacizumab for surgical treatment of severe proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2010;248(6):785-91. | CrossRef | PubMed | Hernández-Da Mota SE, Nuñez-Solorio SM. Experience with intravitreal bevacizumab as a preoperative adjunct in 23-G vitrectomy for advanced proliferative diabetic retinopathy. Eur J Ophthalmol. 2010;20(6):1047-52. | PubMed |
Hernández-Da Mota SE, Nuñez-Solorio SM. Experience with intravitreal bevacizumab as a preoperative adjunct in 23-G vitrectomy for advanced proliferative diabetic retinopathy. Eur J Ophthalmol. 2010;20(6):1047-52. | PubMed | Ahn J, Woo SJ, Chung H, Park KH. The effect of adjunctive intravitreal bevacizumab for preventing postvitrectomy hemorrhage in proliferative diabetic retinopathy. Ophthalmology. 2011;118(11):2218-26. | CrossRef | PubMed |
Ahn J, Woo SJ, Chung H, Park KH. The effect of adjunctive intravitreal bevacizumab for preventing postvitrectomy hemorrhage in proliferative diabetic retinopathy. Ophthalmology. 2011;118(11):2218-26. | CrossRef | PubMed | Farahvash MS, Majidi AR, Roohipoor R, Ghassemi F. Preoperative injection of intravitreal bevacizumab in dense diabetic vitreous hemorrhage. Retina. 2011;31(7):1254-60. | CrossRef | PubMed |
Farahvash MS, Majidi AR, Roohipoor R, Ghassemi F. Preoperative injection of intravitreal bevacizumab in dense diabetic vitreous hemorrhage. Retina. 2011;31(7):1254-60. | CrossRef | PubMed | Han XX, Guo CM, Li Y, Hui YN. Effects of bevacizumab on the neovascular membrane of proliferative diabetic retinopathy: reduction of endothelial cells and expressions of VEGF and HIF-1α. Mol Vis. 2012;18:1-9. | PubMed | PMC |
Han XX, Guo CM, Li Y, Hui YN. Effects of bevacizumab on the neovascular membrane of proliferative diabetic retinopathy: reduction of endothelial cells and expressions of VEGF and HIF-1α. Mol Vis. 2012;18:1-9. | PubMed | PMC | Szabo SM, Beusterien KM, Pleil AM, Wirostko B, Potter MJ, Tildesley H, et al. Patient preferences for diabetic retinopathy health States. Invest Ophthalmol Vis Sci. 2010;51(7):3387-94. | CrossRef | PubMed |
Szabo SM, Beusterien KM, Pleil AM, Wirostko B, Potter MJ, Tildesley H, et al. Patient preferences for diabetic retinopathy health States. Invest Ophthalmol Vis Sci. 2010;51(7):3387-94. | CrossRef | PubMed | Wirostko B, Beusterien K, Grinspan J, Ciulla T, Gonder J, Barsdorf A, et al. Patient preferences in the treatment of diabetic retinopathy. Patient Prefer Adherence. 2011;5:229-37. | CrossRef | PubMed | PMC |
Wirostko B, Beusterien K, Grinspan J, Ciulla T, Gonder J, Barsdorf A, et al. Patient preferences in the treatment of diabetic retinopathy. Patient Prefer Adherence. 2011;5:229-37. | CrossRef | PubMed | PMC | Ranpura V, Hapani S, Wu S. Treatment-related mortality with bevacizumab in cancer patients: a meta-analysis. JAMA. 2011;305(5):487-94. | CrossRef | PubMed |
Ranpura V, Hapani S, Wu S. Treatment-related mortality with bevacizumab in cancer patients: a meta-analysis. JAMA. 2011;305(5):487-94. | CrossRef | PubMed | Zehetner C, Kirchmair R, Huber S, Kralinger MT, Kieselbach GF. Plasma levels of vascular endothelial growth factor before and after intravitreal injection of bevacizumab, ranibizumab and pegaptanib in patients with age-related macular degeneration, and in patients with diabetic macular oedema. Br J Ophthalmol. 2013;97(4):454-9. | CrossRef | PubMed |
Zehetner C, Kirchmair R, Huber S, Kralinger MT, Kieselbach GF. Plasma levels of vascular endothelial growth factor before and after intravitreal injection of bevacizumab, ranibizumab and pegaptanib in patients with age-related macular degeneration, and in patients with diabetic macular oedema. Br J Ophthalmol. 2013;97(4):454-9. | CrossRef | PubMed | Poku E, Rathbone J, Wong R, Everson-Hock E, Essat M, Pandor A, et al. The safety of intravitreal bevacizumab monotherapy in adult ophthalmic conditions: systematic review. BMJ Open. 2014;4(7):e005244. | CrossRef | PubMed | PMC |
Poku E, Rathbone J, Wong R, Everson-Hock E, Essat M, Pandor A, et al. The safety of intravitreal bevacizumab monotherapy in adult ophthalmic conditions: systematic review. BMJ Open. 2014;4(7):e005244. | CrossRef | PubMed | PMC | Thulliez M, Angoulvant D, Le Lez ML, Jonville-Bera AP, Pisella PJ, Gueyffier F, et al. Cardiovascular events and bleeding risk associated with intravitreal antivascular endothelial growth factor monoclonal antibodies: systematic review and meta-analysis. JAMA Ophthalmol. 2014;132(11):1317-26. | CrossRef | PubMed |
Thulliez M, Angoulvant D, Le Lez ML, Jonville-Bera AP, Pisella PJ, Gueyffier F, et al. Cardiovascular events and bleeding risk associated with intravitreal antivascular endothelial growth factor monoclonal antibodies: systematic review and meta-analysis. JAMA Ophthalmol. 2014;132(11):1317-26. | CrossRef | PubMed | Dedania VS, Bakri SJ. Systemic safety of intravitreal anti-vascular endothelial growth factor agents in age-related macular degeneration. Curr Opin Ophthalmol. 2016;27(3):224-43. | CrossRef | PubMed |
Dedania VS, Bakri SJ. Systemic safety of intravitreal anti-vascular endothelial growth factor agents in age-related macular degeneration. Curr Opin Ophthalmol. 2016;27(3):224-43. | CrossRef | PubMed | Mikačić I, Bosnar D. Intravitreal Bevacizumab and Cardiovascular Risk in Patients with Age-Related Macular Degeneration: Systematic Review and Meta-Analysis of Randomized Controlled Trials and Observational Studies. Drug Saf. 2016;39(6):517-41. | CrossRef | PubMed |
Mikačić I, Bosnar D. Intravitreal Bevacizumab and Cardiovascular Risk in Patients with Age-Related Macular Degeneration: Systematic Review and Meta-Analysis of Randomized Controlled Trials and Observational Studies. Drug Saf. 2016;39(6):517-41. | CrossRef | PubMed | R Deonandan SJ. Anti-Vascular Endothelial Growth Factor Drugs for the Treatment of Retinal Conditions: A Review of the Safety. CADTH Rapid Response Reports. 2017. | PubMed |
R Deonandan SJ. Anti-Vascular Endothelial Growth Factor Drugs for the Treatment of Retinal Conditions: A Review of the Safety. CADTH Rapid Response Reports. 2017. | PubMed | Rayess N, Rahimy E, Storey P, Shah CP, Wolfe JD, Chen E, DeCroos FC, Garg SJ, Hsu J, post-injection endophthalmitis rates and characteristics following intravitreal bevacizumab, ranibizumab and aflibercept, American Journal of Ophthalmology (2016). | CrossRef | PubMed |
Rayess N, Rahimy E, Storey P, Shah CP, Wolfe JD, Chen E, DeCroos FC, Garg SJ, Hsu J, post-injection endophthalmitis rates and characteristics following intravitreal bevacizumab, ranibizumab and aflibercept, American Journal of Ophthalmology (2016). | CrossRef | PubMed | CHEN, Guohai, et al. INCIDENCE OF ENDOPHTHALMITIS AFTER VITRECTOMY: A Systematic Review and Meta-analysis. Retina (Philadelphia, Pa.), 2018. [23 Jan 2018]. | CrossRef | PubMed |
CHEN, Guohai, et al. INCIDENCE OF ENDOPHTHALMITIS AFTER VITRECTOMY: A Systematic Review and Meta-analysis. Retina (Philadelphia, Pa.), 2018. [23 Jan 2018]. | CrossRef | PubMed | Bakri SJ, Snyder MR, Reid JM, Pulido JS, Ezzat MK, Singh RJ. Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology. 2007;114(12):2179-82. | CrossRef | PubMed |
Bakri SJ, Snyder MR, Reid JM, Pulido JS, Ezzat MK, Singh RJ. Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology. 2007;114(12):2179-82. | CrossRef | PubMed | Moisseiev E, Waisbourd M, Ben-Artsi E, Levinger E, Barak A, Daniels T, et al. Pharmacokinetics of bevacizumab after topical and intravitreal administration in human eyes. Graefes Arch Clin Exp Ophthalmol. 2014;252(2):331-7. | CrossRef | PubMed | PMC |
Moisseiev E, Waisbourd M, Ben-Artsi E, Levinger E, Barak A, Daniels T, et al. Pharmacokinetics of bevacizumab after topical and intravitreal administration in human eyes. Graefes Arch Clin Exp Ophthalmol. 2014;252(2):331-7. | CrossRef | PubMed | PMC | Ye Z, Ji YL, Ma X, Wen JG, Wei W, Huang SM. Pharmacokinetics and distributions of bevacizumab by intravitreal injection of bevacizumab-PLGA microspheres in rabbits. Int J Ophthalmol. 2015;8(4):653-8. | CrossRef | PubMed | PMC |
Ye Z, Ji YL, Ma X, Wen JG, Wei W, Huang SM. Pharmacokinetics and distributions of bevacizumab by intravitreal injection of bevacizumab-PLGA microspheres in rabbits. Int J Ophthalmol. 2015;8(4):653-8. | CrossRef | PubMed | PMC | Meyer CH, Krohne TU, Holz FG. Intraocular pharmacokinetics after a single intravitreal injection of 1.5 mg versus 3.0 mg of bevacizumab in humans. Retina. 2011;31(9):1877-84. | CrossRef | PubMed |
Meyer CH, Krohne TU, Holz FG. Intraocular pharmacokinetics after a single intravitreal injection of 1.5 mg versus 3.0 mg of bevacizumab in humans. Retina. 2011;31(9):1877-84. | CrossRef | PubMed | Canadian Ophthalmological Society Diabetic Retinopathy Clinical Practice Guideline Expert Committee, Hooper P, Boucher MC, Cruess A, Dawson KG, Delpero W, et al. Canadian Ophthalmological Society Evidence-based Clinical Practice Guidelines for the Management of Diabetic Retinopathy - executive summary. Can J Ophthalmol. 2012 Apr;47(2):91-6. | CrossRef | PubMed |
Canadian Ophthalmological Society Diabetic Retinopathy Clinical Practice Guideline Expert Committee, Hooper P, Boucher MC, Cruess A, Dawson KG, Delpero W, et al. Canadian Ophthalmological Society Evidence-based Clinical Practice Guidelines for the Management of Diabetic Retinopathy - executive summary. Can J Ophthalmol. 2012 Apr;47(2):91-6. | CrossRef | PubMed | Pareja-Ríos A, Serrano-García MA, Marrero-Saavedra MD, Abraldes-López VM, Reyes-Rodríguez MA, Cabrera-López F, et al. [Guidelines of clinical practice of the SERV (Spanish Retina and Vitreous Society): management of ocular complications of diabetes. Diabetic retinopathy and macular oedema]. Arch Soc Esp Oftalmol. 2009;84(9):429-50. | PubMed |
Pareja-Ríos A, Serrano-García MA, Marrero-Saavedra MD, Abraldes-López VM, Reyes-Rodríguez MA, Cabrera-López F, et al. [Guidelines of clinical practice of the SERV (Spanish Retina and Vitreous Society): management of ocular complications of diabetes. Diabetic retinopathy and macular oedema]. Arch Soc Esp Oftalmol. 2009;84(9):429-50. | PubMed | American Academy of Ophthalmology Retina/Vitreous Panel. Preferred Practice Pattern®Guidelines. Diabetic Retinopathy. San Francisco, CA: American Academy of Ophthalmology; 2017.
American Academy of Ophthalmology Retina/Vitreous Panel. Preferred Practice Pattern®Guidelines. Diabetic Retinopathy. San Francisco, CA: American Academy of Ophthalmology; 2017.  NCT01151722. Adjuvant intravitreal bevacizumab in pars plana vitrectomy for diabetic vitreous hemorrhage. | Link |
NCT01151722. Adjuvant intravitreal bevacizumab in pars plana vitrectomy for diabetic vitreous hemorrhage. | Link | NCT01091896. Adjuvant intravitreal bevacizumab in pars plana vitrectomy for vitreous hemorrhage secondary to diabetic retinopathy. | Link |
NCT01091896. Adjuvant intravitreal bevacizumab in pars plana vitrectomy for vitreous hemorrhage secondary to diabetic retinopathy. | Link | ISRCTN79120387. A randomised, single-masked, phase IV pilot study of the efficacy and safety of adjunctive intravitreal Avastin® (bevacizumab) in the prevention of early postoperative vitreous haemorrhage following diabetic vitrectomy. | Link |
ISRCTN79120387. A randomised, single-masked, phase IV pilot study of the efficacy and safety of adjunctive intravitreal Avastin® (bevacizumab) in the prevention of early postoperative vitreous haemorrhage following diabetic vitrectomy. | Link |