 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Palabras clave: Cannabinoids, cannabis abuse disorder, Epistemonikos, GRADE.
INTRODUCTION
Cannabis stands as the most used illegal drug in the world. Currently there are no pharmacologic alternatives to treat its addiction, so the use of Cannabinoids has been postulated as a therapeutic tool. They would act mainly through decrease in abstinence and craving symptoms but its effectiveness remains unclear.
METHODS
To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified seven systematic reviews including 15 studies, of which four were randomized trials. We concluded the use of cannabinoids might result in little or no increase in abstinence at the end of treatment, and it probably increases adverse effects.
Substance abuse disorder is an important epidemiological problem which is defined by the development of a maladaptive behavioral pattern in relation to the use of a substance and is usually accompanied by tolerance, one of the diagnostic elements of dependence. In this context, cannabis stands as one the most consumed illicit drugs, with addictive potential [1].
Even though there are no specific pharmacological alternatives to treat cannabis use disorders, diverse studies have postulated that the endocannabinoid system has a role in the modulation of many neurological pathways associated to drug addiction. In this context, the use of cannabinoids has been proposed as a therapeutic alternative for patients affected by cannabis use disorder. In a similar way nicotine replacement therapy is used as tobacco cessation strategy, it is postulated cannabinoids might help decrease abstinence and craving in cannabis abuse disorder.
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a section of other considerations for decision-making.
|
Key messages
|
|
What is the evidence. |
We found seven systematic reviews [2],[3],[4],[5], |
|
What types of patients were included* |
Three trials [9],[11],[12] included adult patients with cannabis dependence according to DSM-IV-TR diagnostic criteria and one [10] included patients described as cannabis dependent recruited from the community without specifying diagnostic criteria. Three trials [9],[11],[12] excluded patients that had significant psychiatric comorbidities or other substance dependence (except for nicotine and caffeine) and one [10] did not specify exclusion criteria. |
|
What types of interventions were included* |
Two trials evaluated nabiximol (Sativex) as intervention for 6 days [9] and for 9 weeks [10]. One trial [11] used oral dronabinol as monotherapy and another [12] used dronabinol associated with lofexidine (2-alpha adrenergic agonist). In one trial [9] both arms also received cognitive behavioral therapy. |
|
What types of outcomes |
The trials evaluated multiples outcomes, which were grouped by the different systematic reviews as follow:
|
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
The information about the effects of cannabinoids is based on four randomized controlled trials including 338 patients [9],[10],[11],[12].
One trial reported abstinence at the end of treatment (156 patients) [11], four trials reported abstinence and craving symptoms (338 patients) [9],[10],[11],[12] and one trial reported adverse effects (156 patients) [11]. Regarding abstinence and craving symptoms, no systematic review allowed the extraction of data in a way that could be included in a meta analysis, so the information of this outcome is presented as a narrative synthesis.
The summary of findings is as follows:
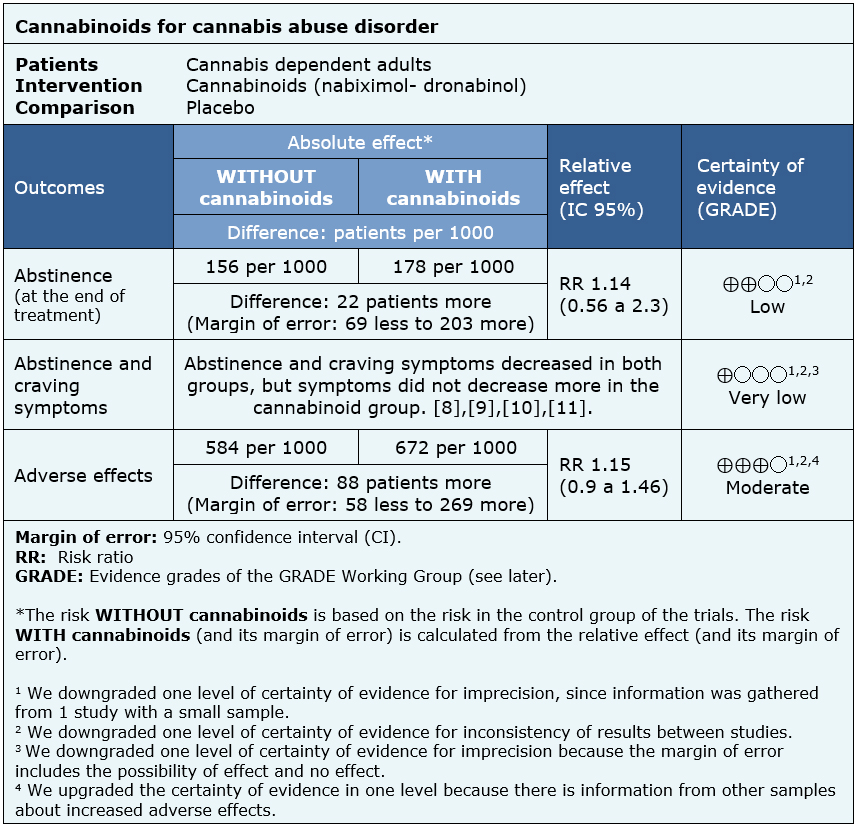
| Follow the link to access the interactive version of this table (Interactive Summary of Findings – iSoF) |

|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
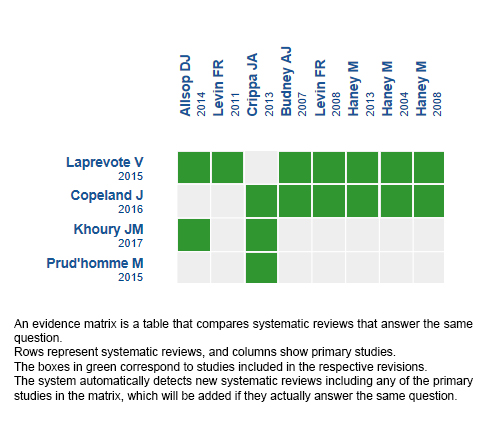
Follow the link to access the interactive version: Cannabinoids for cannabis use disorder
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCTION
Cannabis stands as the most used illegal drug in the world. Currently there are no pharmacologic alternatives to treat its addiction, so the use of Cannabinoids has been postulated as a therapeutic tool. They would act mainly through decrease in abstinence and craving symptoms but its effectiveness remains unclear.
METHODS
To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified seven systematic reviews including 15 studies, of which four were randomized trials. We concluded the use of cannabinoids might result in little or no increase in abstinence at the end of treatment, and it probably increases adverse effects.
 Autores:
Andrés Rodríguez[1,2], Cynthia Zavala[2,3]
Autores:
Andrés Rodríguez[1,2], Cynthia Zavala[2,3]

Citación: Rodríguez A, Zavala C. Cannabinoids for the treatment of cannabis abuse disorder. Medwave 2018;18(6):e7286 doi: 10.5867/medwave.2018.06.7286
Fecha de envío: 28/7/2018
Fecha de aceptación: 30/7/2018
Fecha de publicación: 11/10/2018
Origen: Este artículo es producto del Epistemonikos Evidence Synthesis Project de la Fundación Epistemonikos, en colaboración con Medwave para su publicación.
Tipo de revisión: Con revisión por pares sin ciego por parte del equipo metodológico del Epistemonikos Evidence Synthesis Project.

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Oficina de las Naciones Unidas contra la Droga y el Delito, Informe Mundial sobre las Drogas 2017 (ISBN: 978-92-1-148291-1, eISBN: 978-92-1-060623-3, publicación de las Naciones Unidas, núm. de venta S.17.XI.6).
Oficina de las Naciones Unidas contra la Droga y el Delito, Informe Mundial sobre las Drogas 2017 (ISBN: 978-92-1-148291-1, eISBN: 978-92-1-060623-3, publicación de las Naciones Unidas, núm. de venta S.17.XI.6).  Marshall K, Gowing L, Ali R, Le Foll B. Pharmacotherapies for cannabis dependence. Cochrane Database of Systematic Reviews. 2014;12(12):CD008940.
Marshall K, Gowing L, Ali R, Le Foll B. Pharmacotherapies for cannabis dependence. Cochrane Database of Systematic Reviews. 2014;12(12):CD008940.  Kowal MA, Hazekamp A, Grotenhermen F. Review on clinical studies with cannabis and cannabinoids 2010-2014. Cannabinoids. 2016;11(special issue):1-18.
Kowal MA, Hazekamp A, Grotenhermen F. Review on clinical studies with cannabis and cannabinoids 2010-2014. Cannabinoids. 2016;11(special issue):1-18.  Laprevote V, Schwan R, Schwitzer T, Rolland B, Thome J. Is there a place for off-label pharmacotherapy in cannabis use disorder? A review on efficacy and safety. Current pharmaceutical design. 2015;21(23):3298-305.
Laprevote V, Schwan R, Schwitzer T, Rolland B, Thome J. Is there a place for off-label pharmacotherapy in cannabis use disorder? A review on efficacy and safety. Current pharmaceutical design. 2015;21(23):3298-305.  Marshall K, Gowing L, Ali R, Le Foll B. Pharmacotherapies for cannabis dependence. Cochrane Database of Systematic Reviews. 2014;12(12):CD008940.
Marshall K, Gowing L, Ali R, Le Foll B. Pharmacotherapies for cannabis dependence. Cochrane Database of Systematic Reviews. 2014;12(12):CD008940.  Prud'homme M, Cata R, Jutras-Aswad D. Cannabidiol as an Intervention for Addictive Behaviors: A Systematic Review of the Evidence. Substance abuse : research and treatment. 2015;9:33-8.
Prud'homme M, Cata R, Jutras-Aswad D. Cannabidiol as an Intervention for Addictive Behaviors: A Systematic Review of the Evidence. Substance abuse : research and treatment. 2015;9:33-8.  Bahji A., Mazhar M.N.. Treatment of cannabis dependence with synthetic cannabinoids: A systematic review. Canadian Journal of Addiction. 2016;7(4):8-13.
Bahji A., Mazhar M.N.. Treatment of cannabis dependence with synthetic cannabinoids: A systematic review. Canadian Journal of Addiction. 2016;7(4):8-13.  Copeland J, Pokorski I. Progress toward pharmacotherapies for cannabis-use disorder: an evidence-based review. Subst Abuse Rehabil. 2016 May 3;7:41-53. | CrossRef | PubMed | PMC |
Copeland J, Pokorski I. Progress toward pharmacotherapies for cannabis-use disorder: an evidence-based review. Subst Abuse Rehabil. 2016 May 3;7:41-53. | CrossRef | PubMed | PMC | Allsop DJ, Copeland J, Lintzeris N, Dunlop AJ, Montebello M, Sadler C, Rivas GR, Holland RM, Muhleisen P, Norberg MM, Booth J, McGregor IS. Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA psychiatry. 2014;71(3):281-91.
Allsop DJ, Copeland J, Lintzeris N, Dunlop AJ, Montebello M, Sadler C, Rivas GR, Holland RM, Muhleisen P, Norberg MM, Booth J, McGregor IS. Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA psychiatry. 2014;71(3):281-91.  Trigo JM, Lagzdins D, Rehm J, Selby P, Gamaleddin I, Fischer B, Barnes AJ, Huestis MA, Le Foll B. Effects of fixed or self-titrated dosages of Sativex on cannabis withdrawal and cravings. Drug and alcohol dependence. 2016;161:298-306.
Trigo JM, Lagzdins D, Rehm J, Selby P, Gamaleddin I, Fischer B, Barnes AJ, Huestis MA, Le Foll B. Effects of fixed or self-titrated dosages of Sativex on cannabis withdrawal and cravings. Drug and alcohol dependence. 2016;161:298-306.  Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes EV. Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug and alcohol dependence. 2011;116(1-3):142-50.
Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes EV. Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug and alcohol dependence. 2011;116(1-3):142-50.  Levin FR, Mariani JJ, Pavlicova M, Brooks D, Glass A, Mahony A, Nunes EV, Bisaga A, Dakwar E, Carpenter KM, Sullivan MA, Choi JC. Dronabinol and lofexidine for cannabis use disorder: A randomized, double-blind, placebo-controlled trial. Drug and alcohol dependence. 2016;159:53-60.
Levin FR, Mariani JJ, Pavlicova M, Brooks D, Glass A, Mahony A, Nunes EV, Bisaga A, Dakwar E, Carpenter KM, Sullivan MA, Choi JC. Dronabinol and lofexidine for cannabis use disorder: A randomized, double-blind, placebo-controlled trial. Drug and alcohol dependence. 2016;159:53-60.  Trigo JM, Soliman A, Staios G, Quilty L, Fischer B, George TP, Rehm J, Selby P, Barnes AJ, Huestis MA, Le Foll B. Sativex Associated With Behavioral-Relapse Prevention Strategy as Treatment for Cannabis Dependence: A Case Series. Journal of addiction medicine. 2016;10(4):274-9.
Trigo JM, Soliman A, Staios G, Quilty L, Fischer B, George TP, Rehm J, Selby P, Barnes AJ, Huestis MA, Le Foll B. Sativex Associated With Behavioral-Relapse Prevention Strategy as Treatment for Cannabis Dependence: A Case Series. Journal of addiction medicine. 2016;10(4):274-9.  Shannon S, Opila-Lehman J. Cannabidiol Oil for Decreasing Addictive Use of Marijuana: A Case Report. Integrative medicine (Encinitas, Calif.). 2015;14(6):31-5.
Shannon S, Opila-Lehman J. Cannabidiol Oil for Decreasing Addictive Use of Marijuana: A Case Report. Integrative medicine (Encinitas, Calif.). 2015;14(6):31-5.  Crippa JA, Hallak JE, Machado-de-Sousa JP, Queiroz RH, Bergamaschi M, Chagas MH, Zuardi AW. Cannabidiol for the treatment of cannabis withdrawal syndrome: a case report. Journal of clinical pharmacy and therapeutics. 2013;38(2):162-4.
Crippa JA, Hallak JE, Machado-de-Sousa JP, Queiroz RH, Bergamaschi M, Chagas MH, Zuardi AW. Cannabidiol for the treatment of cannabis withdrawal syndrome: a case report. Journal of clinical pharmacy and therapeutics. 2013;38(2):162-4.  Budney AJ, Vandrey RG, Hughes JR, Moore BA, Bahrenburg B. Oral delta-9-tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug and alcohol dependence. 2007;86(1):22-9.
Budney AJ, Vandrey RG, Hughes JR, Moore BA, Bahrenburg B. Oral delta-9-tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug and alcohol dependence. 2007;86(1):22-9.  Levin FR, Kleber HD. Use of dronabinol for cannabis dependence: two case reports and review. The American journal on addictions. 2008;17(2):161-4.
Levin FR, Kleber HD. Use of dronabinol for cannabis dependence: two case reports and review. The American journal on addictions. 2008;17(2):161-4.  Hart CL, Haney M, Ward AS, Fischman MW, Foltin RW. Effects of oral THC maintenance on smoked marijuana self-administration. Drug and alcohol dependence. 2002;67(3):301-9.
Hart CL, Haney M, Ward AS, Fischman MW, Foltin RW. Effects of oral THC maintenance on smoked marijuana self-administration. Drug and alcohol dependence. 2002;67(3):301-9.  Haney M, Cooper ZD, Bedi G, Vosburg SK, Comer SD, Foltin RW. Nabilone decreases marijuana withdrawal and a laboratory measure of marijuana relapse. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38(8):1557-65.
Haney M, Cooper ZD, Bedi G, Vosburg SK, Comer SD, Foltin RW. Nabilone decreases marijuana withdrawal and a laboratory measure of marijuana relapse. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38(8):1557-65.  Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, Foltin RW. Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29(1):158-70.
Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, Foltin RW. Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29(1):158-70.  Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology. 2008;197(1):157-68.
Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology. 2008;197(1):157-68.  Morgan CJ, Freeman TP, Schafer GL, Curran HV. Cannabidiol attenuates the appetitive effects of Delta 9-tetrahydrocannabinol in humans smoking their chosen cannabis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(9):1879-85.
Morgan CJ, Freeman TP, Schafer GL, Curran HV. Cannabidiol attenuates the appetitive effects of Delta 9-tetrahydrocannabinol in humans smoking their chosen cannabis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(9):1879-85.  Morgan CJ, Schafer G, Freeman TP, Curran HV. Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study: naturalistic study [corrected]. The British journal of psychiatry : the journal of mental science. 2010;197(4):285-90.
Morgan CJ, Schafer G, Freeman TP, Curran HV. Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study: naturalistic study [corrected]. The British journal of psychiatry : the journal of mental science. 2010;197(4):285-90.  Gabriel Rada, David Aceituno, Rubén Allende, Gonzalo Bravo, Rocío Bravo, Oscar Corsi, Juan Franco, Evelyn Gómez, Rami Guinguis, Ariel Izcovich, Valentina Llovet, Diego Lobos, Eva Madrid, Macarena Morel, Luis Ortiz, Javier Pérez-Bracchiglione, Matías Rocco, Jana Stojanova, Gerard Urrútia, Cynthia Zavala. Therapeutic use of cannabis, cannabis-derived products and synthetic cannabinoids: a protocol for multiple systematic reviews. PROSPERO 2018 CRD42018097382. | Link |
Gabriel Rada, David Aceituno, Rubén Allende, Gonzalo Bravo, Rocío Bravo, Oscar Corsi, Juan Franco, Evelyn Gómez, Rami Guinguis, Ariel Izcovich, Valentina Llovet, Diego Lobos, Eva Madrid, Macarena Morel, Luis Ortiz, Javier Pérez-Bracchiglione, Matías Rocco, Jana Stojanova, Gerard Urrútia, Cynthia Zavala. Therapeutic use of cannabis, cannabis-derived products and synthetic cannabinoids: a protocol for multiple systematic reviews. PROSPERO 2018 CRD42018097382. | Link |