 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Palabras clave: Células madre, enfermedad de Parkinson, Epistemonikos, GRADE.
INTRODUCTION
There are many patients with Parkinson's disease who have a limited response to conventional pharmacological treatment. The use of stem cells has been postulated as an alternative, although its effectiveness remains a matter of controversy.
METHODS
To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified two systematic reviews including 21 studies overall, of which three were randomized trials. We concluded it is not clear whether stem cells have any effect on the symptoms of Parkinson's disease because the certainty of the available evidence is very low.
Parkinson’s disease is a progressive and degenerative condition characterized by the destruction of dopaminergic neurons located in the substantia nigra, specifically in the nigrostriatal pathway, which leads to a progressive decrease in dopamine levels. As the disease advances there is also a loss in other neuronal groups (e.g. serotonergic, noradrenergic), which leads to a decrease in the number of functional dopaminergic and non-dopaminergic neurons, and the subsequent loss in the capacity to respond to treatment [1].
Despite improvements in pharmacological treatment which makes possible to control symptoms in a large proportion of patients, many patients remain symptomatic, or experience treatment-related complications, such as dyskinesias, akinesia and end-of-dose wearing off. In addition, Parkinson’s disease also leads to non-motor manifestations such as cognitive impairment, which limits the therapeutic alternatives [2].
Consequently, new alternatives are actively investigated, such as the implantation of stem cells. Stem cells are precursor cells with the ability to self-renew and generate multiple mature cell types. Embryonic stem cells result from the isolation and culture of blastocyst cells, which are formed 5 days after fertilization, and are pluripotent, which means they can grow into any type of adult cellular lineage. Adult or somatic stem cells remain quiescent with limited self-renewal and differentiation capacity. Numerous types of precursor cells have been isolated from adult tissues, leading to the concept that all tissues have their own compartment of stem cells, responsible for replenishing cells that die within human organs. Mesenchymal cells, for instance, are stromal in origin and can be isolated virtually from any tissue in the body. The most obvious therapeutic potential of adult stem cells is to restore or replace tissues that have been damaged by disease or injury and to avoid the ethical problem of using embryonic derived cells. However, the actual efficacy of stem cells derived from non-hematological tissue remains a matter of debate [3].
Stem cell transplantation is a novel alternative in Parkinson’s disease. Through implantation in the affected regions, it seeks to develop new synapses and restore the levels of dopamine in the nigrostriatal pathway. This technique requires stereotactic neurosurgery to graft them directly into required areas, which is not exempt of risks, generating controversy about the role of this intervention in Parkinson’s disease.
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
|
What is the evidence. |
We found two systematic reviews [1], [4], including 21 primary studies [5],[6],[7],[8],[9],[10],[11],[12],[13], |
|
What types of patients were included* |
Both trials included participants with Parkinson's disease with at least two of the following symptoms: bradykinesia, rigidity, tremor at rest and stability in their medication.The age range was 34 to 75 years. One trial included patients with Parkinson's disease for more than 7 years (average duration of 14 years) [5], while the other trial included patients with advanced Parkinson's disease, but did not mention the duration of the disease [7]. Both trials excluded patients with previous neurosurgery or a history of psychiatric illness. |
|
What types of interventions were included* |
All trials compared grafting of mesencephalic tissue cells of embryonic origin by stereotactic surgery. No trial reported the dose of levodopa that was administered if necessary. |
|
What types of outcomes |
All trials evaluated:
One trial [5] reported outcomes at the first year after surgery, while the second [7] reported them at two years after the procedure. |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
The information about the effects of stem cells for patients with Parkinson’s disease is based on two randomized trials [5],[7], including 74 participants.
None of the systematic reviews presented a meta-analysis, or information that allows to reanalyse the trials, so the information presented below corresponds to a narrative synthesis of the information as reported by the reviews.
The summary of findings is as follows:
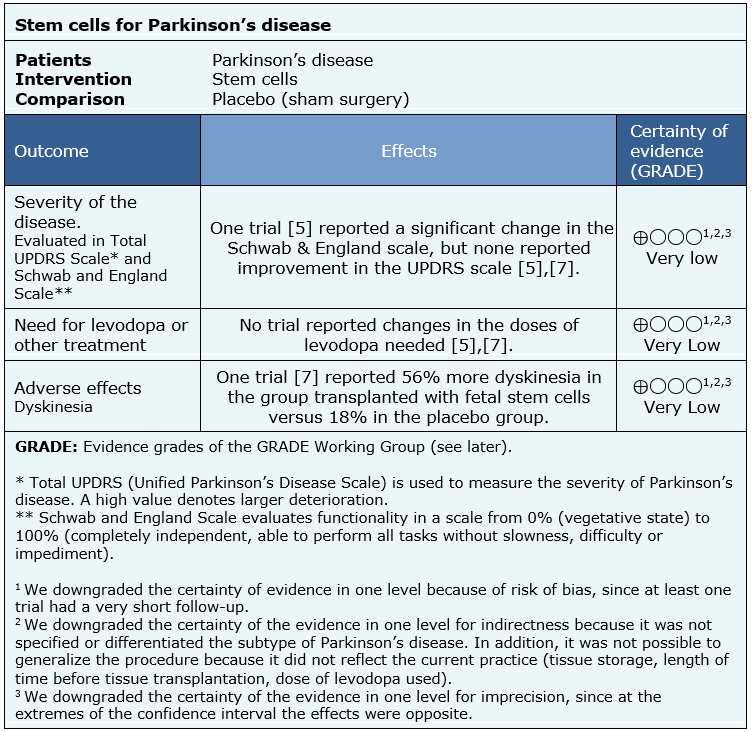

|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
| Feasibility |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
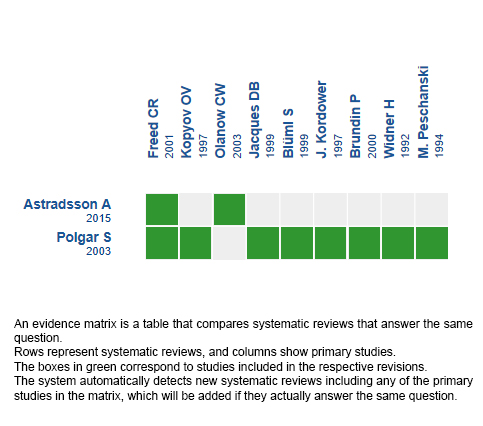
Follow the link to access the interactive version: Stem cells for Parkinson’s disease
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCTION
There are many patients with Parkinson's disease who have a limited response to conventional pharmacological treatment. The use of stem cells has been postulated as an alternative, although its effectiveness remains a matter of controversy.
METHODS
To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified two systematic reviews including 21 studies overall, of which three were randomized trials. We concluded it is not clear whether stem cells have any effect on the symptoms of Parkinson's disease because the certainty of the available evidence is very low.
 Autores:
Matías Rocco[1,2], Carlos Juri[2,3]
Autores:
Matías Rocco[1,2], Carlos Juri[2,3]

Citación: Rocco M, Juri C. Is treatment with stem cells effective in Parkinson's disease?. Medwave 2018;18(4):e7241 doi: 10.5867/medwave.2018.05.7241
Fecha de envío: 1/4/2018
Fecha de aceptación: 14/8/2018
Fecha de publicación: 12/9/2018
Origen: Este artículo es producto del Epistemonikos Evidence Synthesis Project de la Fundación Epistemonikos, en colaboración con Medwave para su publicación.
Tipo de revisión: Con revisión por pares sin ciego por parte del equipo metodológico del Epistemonikos Evidence Synthesis Project.

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Polgar S, Morris ME, Reilly S, Bilney B, Sanberg PR. Reconstructive neurosurgery for Parkinson's disease: a systematic review and preliminary
meta-analysis. Brain Res Bull. 2003 Apr 15;60(1-2):1-24. Review.
| PubMed |
Polgar S, Morris ME, Reilly S, Bilney B, Sanberg PR. Reconstructive neurosurgery for Parkinson's disease: a systematic review and preliminary
meta-analysis. Brain Res Bull. 2003 Apr 15;60(1-2):1-24. Review.
| PubMed | Jankovic J, Aguilar LG. Current approaches to the treatment of Parkinson's disease. Neuropsychiatr Dis Treat. 2008 Aug;4(4):743-57. | PubMed | PMC |
Jankovic J, Aguilar LG. Current approaches to the treatment of Parkinson's disease. Neuropsychiatr Dis Treat. 2008 Aug;4(4):743-57. | PubMed | PMC | Chagastelles PC, Nardi NB. Biology of stem cells: an overview. Kidney Int Suppl (2011). 2011 Sep;1(3):63-67. | PubMed | PMC |
Chagastelles PC, Nardi NB. Biology of stem cells: an overview. Kidney Int Suppl (2011). 2011 Sep;1(3):63-67. | PubMed | PMC | Astradsson A, Aziz TZ. Parkinson's disease: fetal cell or stem cell-derived treatments. BMJ Clin Evid. 2015 Apr 21;2015. pii: 1203. | PubMed | PMC |
Astradsson A, Aziz TZ. Parkinson's disease: fetal cell or stem cell-derived treatments. BMJ Clin Evid. 2015 Apr 21;2015. pii: 1203. | PubMed | PMC | Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, Eidelberg D, Fahn S. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001 Mar 8;344(10):710-9. | PubMed |
Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, Eidelberg D, Fahn S. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001 Mar 8;344(10):710-9. | PubMed | Kopyov OV, Jacques DS, Lieberman A, Duma CM, Rogers RL. Outcome following intrastriatal fetal mesencephalic grafts for Parkinson's patients is directly related to the volume of grafted tissue. Exp Neurol. 1997 Aug;146(2):536-45. | PubMed |
Kopyov OV, Jacques DS, Lieberman A, Duma CM, Rogers RL. Outcome following intrastriatal fetal mesencephalic grafts for Parkinson's patients is directly related to the volume of grafted tissue. Exp Neurol. 1997 Aug;146(2):536-45. | PubMed | Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, Shannon KM, Nauert GM, Perl DP, Godbold J, Freeman TB. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol. 2003 Sep;54(3):403-14. | PubMed |
Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, Shannon KM, Nauert GM, Perl DP, Godbold J, Freeman TB. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol. 2003 Sep;54(3):403-14. | PubMed | Jacques DB, Kopyov OV, Eagle KS, Carter T, Lieberman A. Outcomes and complications of fetal tissue transplantation in Parkinson's disease. Stereotact Funct Neurosurg. 1999;72(2-4):219-24. | PubMed |
Jacques DB, Kopyov OV, Eagle KS, Carter T, Lieberman A. Outcomes and complications of fetal tissue transplantation in Parkinson's disease. Stereotact Funct Neurosurg. 1999;72(2-4):219-24. | PubMed | Blüml S, Kopyov O, Jacques S, Ross BD. Activation of neurotransplants in humans. Exp Neurol. 1999 Jul;158(1):121-5. | PubMed |
Blüml S, Kopyov O, Jacques S, Ross BD. Activation of neurotransplants in humans. Exp Neurol. 1999 Jul;158(1):121-5. | PubMed | Kordower J., Goetz C., Freeman T., Olanow C. Dopaminergic transplants in patients with Parkinson’s disease: neuroanatomical correlates of clinical recovery. Exp. Neurol. 1997;144:41–46. | Link |
Kordower J., Goetz C., Freeman T., Olanow C. Dopaminergic transplants in patients with Parkinson’s disease: neuroanatomical correlates of clinical recovery. Exp. Neurol. 1997;144:41–46. | Link | Brundin P, Pogarell O, Hagell P, Piccini P, Widner H, Schrag A, Kupsch A, Crabb L, Odin P, Gustavii B, Björklund A, Brooks DJ, Marsden CD, Oertel WH, Quinn NP, Rehncrona S, Lindvall O. Bilateral caudate and putamen grafts of embryonic
mesencephalic tissue treated with lazaroids in Parkinson's disease. Brain. 2000 Jul;123 ( Pt 7):1380-90. | PubMed |
Brundin P, Pogarell O, Hagell P, Piccini P, Widner H, Schrag A, Kupsch A, Crabb L, Odin P, Gustavii B, Björklund A, Brooks DJ, Marsden CD, Oertel WH, Quinn NP, Rehncrona S, Lindvall O. Bilateral caudate and putamen grafts of embryonic
mesencephalic tissue treated with lazaroids in Parkinson's disease. Brain. 2000 Jul;123 ( Pt 7):1380-90. | PubMed | Widner H, Tetrud J, Rehncrona S, Snow B, Brundin P, Gustavii B, Björklund A, Lindvall O, Langston JW. Bilateral fetal mesencephalic grafting in two patients with parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). N Engl J Med. 1992 Nov 26;327(22):1556-63. | PubMed |
Widner H, Tetrud J, Rehncrona S, Snow B, Brundin P, Gustavii B, Björklund A, Lindvall O, Langston JW. Bilateral fetal mesencephalic grafting in two patients with parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). N Engl J Med. 1992 Nov 26;327(22):1556-63. | PubMed | Peschanski M, Defer G,N’Guyen J, Ricolfi F, Monfort J, Remy P. Bilateral motor improvement and alteration of l-Dopa effect in two patients with Parkinson’s disease following intrastriatal transplantation of foetal vental mesencephalon. Brain. 1994;117:487–499. | Link |
Peschanski M, Defer G,N’Guyen J, Ricolfi F, Monfort J, Remy P. Bilateral motor improvement and alteration of l-Dopa effect in two patients with Parkinson’s disease following intrastriatal transplantation of foetal vental mesencephalon. Brain. 1994;117:487–499. | Link | Kordower J, Rosenstein J, Collier T, Burke M, Chen E, Li J, Martel L, Levey A, Mufson E, Freeman T, Olanow C. Functional fetal nigral grafts in a patient with Parkinson’s disease: chemoanatomic, ultrastructural, and metabolic studies. J. Comp. Neurol. 1996;37:203–230. | Link |
Kordower J, Rosenstein J, Collier T, Burke M, Chen E, Li J, Martel L, Levey A, Mufson E, Freeman T, Olanow C. Functional fetal nigral grafts in a patient with Parkinson’s disease: chemoanatomic, ultrastructural, and metabolic studies. J. Comp. Neurol. 1996;37:203–230. | Link | Kopyov O, Jacques D, Lieberman A, Duma C, Rogers R. Clinical study of fetal mesencephalic intracerebral transplants for the treatment of Parkinson’s disease. Cell Transplant. 1996;5(2):327–337. | Link |
Kopyov O, Jacques D, Lieberman A, Duma C, Rogers R. Clinical study of fetal mesencephalic intracerebral transplants for the treatment of Parkinson’s disease. Cell Transplant. 1996;5(2):327–337. | Link | Hagell P, Schrag A, Piccini P, Jahanshahi M, Brown R, Rehncrona S, Widner H, Brundin P, Rothwell JC, Odin P, Wenning GK, Morrish P, Gustavii B, Björklund A,
Brooks DJ, Marsden CD, Quinn NP, Lindvall O. Sequential bilateral transplantation in Parkinson's disease: effects of the second graft. Brain. 1999 Jun;122 (Pt 6):1121-32. | PubMed |
Hagell P, Schrag A, Piccini P, Jahanshahi M, Brown R, Rehncrona S, Widner H, Brundin P, Rothwell JC, Odin P, Wenning GK, Morrish P, Gustavii B, Björklund A,
Brooks DJ, Marsden CD, Quinn NP, Lindvall O. Sequential bilateral transplantation in Parkinson's disease: effects of the second graft. Brain. 1999 Jun;122 (Pt 6):1121-32. | PubMed | Freed CR, Breeze RE, Schneck SA. Transplantation of fetal mesencephalic tissue in Parkinson's disease. N Engl J Med. 1995 Sep 14;333(11):730-1. | PubMed |
Freed CR, Breeze RE, Schneck SA. Transplantation of fetal mesencephalic tissue in Parkinson's disease. N Engl J Med. 1995 Sep 14;333(11):730-1. | PubMed | Schumacher JM, Ellias SA, Palmer EP, Kott HS, Dinsmore J, Dempsey PK, Fischman AJ, Thomas C, Feldman RG, Kassissieh S, Raineri R, Manhart C, Penney D, Fink JS, Isacson O. Transplantation of embryonic porcine mesencephalic tissue in patients with PD. Neurology. 2000 Mar 14;54(5):1042-50. | PubMed |
Schumacher JM, Ellias SA, Palmer EP, Kott HS, Dinsmore J, Dempsey PK, Fischman AJ, Thomas C, Feldman RG, Kassissieh S, Raineri R, Manhart C, Penney D, Fink JS, Isacson O. Transplantation of embryonic porcine mesencephalic tissue in patients with PD. Neurology. 2000 Mar 14;54(5):1042-50. | PubMed | Freeman T, Watts R, Hauser R, Bakay R, Elias S, Stoessl A, Eidelberg D, Dinsmore J, Fink S. A prospective, randomized, double-blind, surgical placebo-controlled trial of intrastriatal transplantation of fetal porcine ventral mesencephalic tissue (neurocellPD) in subjects with Parkinson’s disease. Experimental Neurology. 2002;175(2):426. | Link |
Freeman T, Watts R, Hauser R, Bakay R, Elias S, Stoessl A, Eidelberg D, Dinsmore J, Fink S. A prospective, randomized, double-blind, surgical placebo-controlled trial of intrastriatal transplantation of fetal porcine ventral mesencephalic tissue (neurocellPD) in subjects with Parkinson’s disease. Experimental Neurology. 2002;175(2):426. | Link | Defer GL, Geny C, Ricolfi F, Fenelon G, Monfort JC, Remy P, Villafane G, Jeny R, Samson Y, Keravel Y, Gaston A, Degos JD, Peschanski M, Cesaro P, Nguyen JP. Long-term outcome of unilaterally transplanted parkinsonian patients. I. Clinical approach. Brain. 1996 Feb;119 ( Pt 1):41-50. | PubMed |
Defer GL, Geny C, Ricolfi F, Fenelon G, Monfort JC, Remy P, Villafane G, Jeny R, Samson Y, Keravel Y, Gaston A, Degos JD, Peschanski M, Cesaro P, Nguyen JP. Long-term outcome of unilaterally transplanted parkinsonian patients. I. Clinical approach. Brain. 1996 Feb;119 ( Pt 1):41-50. | PubMed | Remy P, Samson Y, Hantraye P, Fontaine A, Defer G, Mangin J. Neural grafting in five parkinsonian patients: correlations between PET and clinical evolution. Ann. Neurol. 1995;38:580–588. | Link |
Remy P, Samson Y, Hantraye P, Fontaine A, Defer G, Mangin J. Neural grafting in five parkinsonian patients: correlations between PET and clinical evolution. Ann. Neurol. 1995;38:580–588. | Link | Wenning GK, Odin P, Morrish P, Rehncrona S, Widner H, Brundin P, Rothwell JC, Brown R, Gustavii B, Hagell P, Jahanshahi M, Sawle G, Björklund A, Brooks DJ,
Marsden CD, Quinn NP, Lindvall O. Short- and long-term survival and function of unilateral intrastriatal dopaminergic grafts in Parkinson's disease. Ann Neurol. 1997 Jul;42(1):95-107. | PubMed |
Wenning GK, Odin P, Morrish P, Rehncrona S, Widner H, Brundin P, Rothwell JC, Brown R, Gustavii B, Hagell P, Jahanshahi M, Sawle G, Björklund A, Brooks DJ,
Marsden CD, Quinn NP, Lindvall O. Short- and long-term survival and function of unilateral intrastriatal dopaminergic grafts in Parkinson's disease. Ann Neurol. 1997 Jul;42(1):95-107. | PubMed | Freeman TB, Olanow CW, Hauser RA, Nauert GM, Smith DA, Borlongan CV, Sanberg PR, Holt DA, Kordower JH, Vingerhoets FJ, et al. Bilateral fetal nigral transplantation into the postcommissural putamen in Parkinson's disease. Ann Neurol. 1995 Sep;38(3):379-88. | PubMed |
Freeman TB, Olanow CW, Hauser RA, Nauert GM, Smith DA, Borlongan CV, Sanberg PR, Holt DA, Kordower JH, Vingerhoets FJ, et al. Bilateral fetal nigral transplantation into the postcommissural putamen in Parkinson's disease. Ann Neurol. 1995 Sep;38(3):379-88. | PubMed | Lindvall O, Rehncrona S, Brundin P, Gustavii B, Astedt B, Widner H, Lindholm T, Björklund A, Leenders K, Rothwell J, Frackowiak R, Marsden C, Johnels B, Steg G, Freedman R, Hoffer B, Seiger A, Bygdeman M, Stromberg I, Olson L. Human fetal dopamine neurons grafted into the striatum in two patients with severe Parkinson’s disease: a detailed account of methodology and a 6-month follow-up. Arch. Neurol. 1990;46:615–631. | Link |
Lindvall O, Rehncrona S, Brundin P, Gustavii B, Astedt B, Widner H, Lindholm T, Björklund A, Leenders K, Rothwell J, Frackowiak R, Marsden C, Johnels B, Steg G, Freedman R, Hoffer B, Seiger A, Bygdeman M, Stromberg I, Olson L. Human fetal dopamine neurons grafted into the striatum in two patients with severe Parkinson’s disease: a detailed account of methodology and a 6-month follow-up. Arch. Neurol. 1990;46:615–631. | Link | Hauser RA, Freeman TB, Snow BJ, Nauert M, Gauger L, Kordower JH, Olanow CW. Long-term evaluation of bilateral fetal nigral transplantation in Parkinson disease. Arch Neurol. 1999 Feb;56(2):179-87. | PubMed |
Hauser RA, Freeman TB, Snow BJ, Nauert M, Gauger L, Kordower JH, Olanow CW. Long-term evaluation of bilateral fetal nigral transplantation in Parkinson disease. Arch Neurol. 1999 Feb;56(2):179-87. | PubMed | Fox SH, Katzenschlager R, Lim SY, Ravina B, Seppi K, Coelho M et al. (2011). The movement disorder society evidence‐based medicine review update: Treatments for the motor symptoms of Parkinson's disease. Movement Disorders, 26(S3), S2-S41.
Fox SH, Katzenschlager R, Lim SY, Ravina B, Seppi K, Coelho M et al. (2011). The movement disorder society evidence‐based medicine review update: Treatments for the motor symptoms of Parkinson's disease. Movement Disorders, 26(S3), S2-S41.  Grimes D, Gordon J, Snelgrove B, Lim-Carter I, Fon E, Martin W, et al. Canadian Guidelines on Parkinson's Disease. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 2012. Jul;39 (4 Suppl 4):S1-30. | PubMed |
Grimes D, Gordon J, Snelgrove B, Lim-Carter I, Fon E, Martin W, et al. Canadian Guidelines on Parkinson's Disease. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 2012. Jul;39 (4 Suppl 4):S1-30. | PubMed | National Collaborating Centre for Chronic Conditions (UK). Parkinson's Disease: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care. London: Royal College of Physicians (UK); 2006. | PubMed |
National Collaborating Centre for Chronic Conditions (UK). Parkinson's Disease: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care. London: Royal College of Physicians (UK); 2006. | PubMed | Hort J, O'Brien JT, Gainotti G, Pirttila T, Popescu BO, Rektorova I, et al; EFNS Scientist Panel on Dementia. EFNS guidelines for the diagnosis and management of Alzheimer's disease. Eur J Neurol. 2010 Oct;17(10):1236-48. | PubMed |
Hort J, O'Brien JT, Gainotti G, Pirttila T, Popescu BO, Rektorova I, et al; EFNS Scientist Panel on Dementia. EFNS guidelines for the diagnosis and management of Alzheimer's disease. Eur J Neurol. 2010 Oct;17(10):1236-48. | PubMed | Lige L, Zengmin T. Transplantation of Neural Precursor Cells in the Treatment of Parkinson Disease: An Efficacy and Safety Analysis. Turk Neurosurg. 2016;26(3):378-83. | CrossRef | PubMed |
Lige L, Zengmin T. Transplantation of Neural Precursor Cells in the Treatment of Parkinson Disease: An Efficacy and Safety Analysis. Turk Neurosurg. 2016;26(3):378-83. | CrossRef | PubMed | Yin F, Tian ZM, Liu S, Zhao QJ, Wang RM, Shen L, Wieman J, Yan Y. Transplantation of human retinal pigment epithelium cells in the treatment for Parkinson disease. CNS Neurosci Ther. 2012 Dec;18(12):1012-20. | CrossRef | PubMed |
Yin F, Tian ZM, Liu S, Zhao QJ, Wang RM, Shen L, Wieman J, Yan Y. Transplantation of human retinal pigment epithelium cells in the treatment for Parkinson disease. CNS Neurosci Ther. 2012 Dec;18(12):1012-20. | CrossRef | PubMed | Brazzini A, Cantella R, De la Cruz A, Yupanqui J, León C, Jorquiera T, Brazzini M, Ortega M, Saenz LN. Intraarterial autologous implantation of adult stem cells for patients with Parkinson disease. J Vasc Interv Radiol. 2010
Apr;21(4):443-51. Erratum in: J Vasc Interv Radiol. 2010 Jul;21(7):1141. | CrossRef | PubMed |
Brazzini A, Cantella R, De la Cruz A, Yupanqui J, León C, Jorquiera T, Brazzini M, Ortega M, Saenz LN. Intraarterial autologous implantation of adult stem cells for patients with Parkinson disease. J Vasc Interv Radiol. 2010
Apr;21(4):443-51. Erratum in: J Vasc Interv Radiol. 2010 Jul;21(7):1141. | CrossRef | PubMed | Venkataramana NK, Kumar SK, Balaraju S, Radhakrishnan RC, Bansal A, Dixit A, Rao DK, Das M, Jan M, Gupta PK, Totey SM. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson's disease. Transl Res. 2010 Feb;155(2):62-70. | CrossRef | PubMed |
Venkataramana NK, Kumar SK, Balaraju S, Radhakrishnan RC, Bansal A, Dixit A, Rao DK, Das M, Jan M, Gupta PK, Totey SM. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson's disease. Transl Res. 2010 Feb;155(2):62-70. | CrossRef | PubMed | Hayek M, A Study to Evaluate the Safety of Neural Stem Cells in Patients With Parkinson's Disease, NCT02452723. | Link |
Hayek M, A Study to Evaluate the Safety of Neural Stem Cells in Patients With Parkinson's Disease, NCT02452723. | Link | Lie J, A Study To Evaluate the Safety and Efficacy of Human Neural Stem Cells for Parkinson's Disease Patient hNSCPD, NCT03128450. | Link |
Lie J, A Study To Evaluate the Safety and Efficacy of Human Neural Stem Cells for Parkinson's Disease Patient hNSCPD, NCT03128450. | Link | Santos V, Pacheco C, Ferreira P. Clinical trials of stem-cell based therapy used in central nervous system disease a systematic review. | Link |
Santos V, Pacheco C, Ferreira P. Clinical trials of stem-cell based therapy used in central nervous system disease a systematic review. | Link |