 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
INTRODUCTION
Bronchiolitis consists of an acute small airways inflammation secondary to a viral infection and is a frequent pathology among children under 2 years. The use of inhaled corticosteroids during bronchiolitis has been proposed to reduce recurrent wheeze or asthma, however there is controversy about it.
METHODS
To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified three systematic reviews including 11 randomized trials. We concluded that inhaled corticosteroids do not reduce recurrent wheeze or asthma in patients with bronchiolitis.
Bronchiolitis consists of an acute small airways inflammation commonly caused by a viral infection, predominantly respiratory syncytial virus. It is frequent in children under 2 years and is associated with high hospitalization rates and even mortality. Considering the inflammatory component and the reduction of recurrent wheeze or asthma in other respiratory pathologies with the use of inhaled corticosteroids, they have been proposed to be useful in bronchiolitis.
This article aims to review if inhaled corticosteroids are an alternative to reduce recurrent wheeze or asthma in patients with bronchiolitis.
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
|
What is the evidence. |
Three systematic reviews were identified [1],[2],[3], including 11 primary studies [4],[5],[6],[7],[8],[9],[10],[11],[12],[13],[14]. All primary studies were randomized trials. |
|
What types of patients were included* |
All trials consider only patients with bronchiolitis. Regarding age, all trials included patients under 24 months. Specifically, one trial included patients aged 9 months or less [8], one trial 42 weeks or less [7] and five trials included patients under 12 months [4],[5],[6],[11],[12],[13]. Mean age was five months, with a range from two to 11 months. Five trials included only patients with confirmed respiratory syncytial virus [4],[6],[8],[13],[14]. In remaining trials, respiratory syncytial virus confirmation varied from 26 to 83% [5],[7],[10],[11],[12]. One trial did not report viral etiology [9]. The main exclusion criteria were: previous wheeze in six trials [6],[10],[11],[12],[13],[14], prematurity in four trials [4],[8],[10],[12], immunodeficiency in five trials [4],[5],[8],[11],[12], previous use of systemic corticosteroids in five trials [4],[5],[6],[12],[14], and assisted ventilation in five trials [5],[7],[8],[11],[12]. All of the trials excluded patients with chronic respiratory or cardiac illness. Seven trials included inpatients [4],[6],[8],[9],[10],[11],[14]. Four trials included outpatients [5],[7],[12],[13]. |
|
What types of interventions were included* |
Seven trials compared inhaled corticosteroids versus placebo [4],[6],[7],[10],[11],[12],[14]. Remaining four trials compared them versus no treatment [5],[8],[9],[13]. Particularly, seven trials used budesonide [4],[7],[8],[9],[10],[11],[13], two used beclomethasone [5],[6], one used fluticasone [12] and one used dexamethasone [14]. In six trials corticosteroids were administered via metered dose inhaler [5],[6],[7],[8],[12],[13]. In remaining five trials, they were nebulized [4],[9],[10],[11],[14]. Regarding intervention length, in two trials it lasted less than 21 days [4],[8], in two it lasted up to 8 weeks [7],[11], in four it lasted three months [5],[6],[12],[13] and in two trials it lasted 4 months [9],[10]. One trial did not report exact intervention length [14]. |
|
What types of outcomes |
Systematic reviews reported the following outcomes: recurrent wheeze, asthma, hospital readmission, need of bronchodilator, length of oxygen supplementation, oxygen supplementation at night, symptoms frequency and adverse effects. Follow-up was three months in one trial [14], six months in one trial [11], a year in six trials [4],[5],[6],[7],[9],[12], two years in two trials [8],[13] and three years in one trial [10]. |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
Information regarding the effects of inhaled corticosteroids is based on ten randomized trials including 927 patients [4],[5],[6],[7],[8],[10],[11],[12],[13],[14]. One included trial had no extractable data from systematic reviews, therefore it was not added to a meta-analysis [9]. Seven trials reported recurrent wheeze [4],[5],[6],[7],[11],[12],[14], and three reported asthma [8],[10],[13]. Five trials reported hospital readmission [4],[7],[11],[12],[14]. Three trials mentioned adverse effects [7],[11],[12], but only oral candidiasis could be analyzed from one trial [12].
The summary of findings is the following:
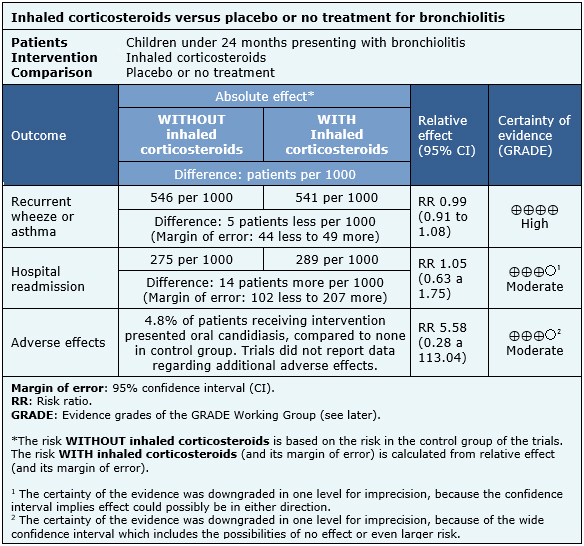
| Follow the link to access the interactive version of this table (Interactive Summary of Findings – iSoF) |

|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
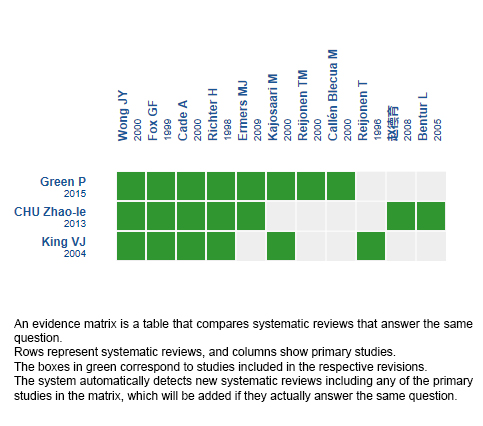
Follow the link to access the interactive version: Inhaled corticosteroids for bronchiolitis
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCTION
Bronchiolitis consists of an acute small airways inflammation secondary to a viral infection and is a frequent pathology among children under 2 years. The use of inhaled corticosteroids during bronchiolitis has been proposed to reduce recurrent wheeze or asthma, however there is controversy about it.
METHODS
To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified three systematic reviews including 11 randomized trials. We concluded that inhaled corticosteroids do not reduce recurrent wheeze or asthma in patients with bronchiolitis.
 Autores:
Gonzalo Alarcón-Andrade[1,2], Lorena Cifuentes[2,3,4]
Autores:
Gonzalo Alarcón-Andrade[1,2], Lorena Cifuentes[2,3,4]

Citación: Alarcón-Andrade G, Cifuentes L. Do inhaled corticosteroids have a role for bronchiolitis?. Medwave 2018 Mar-Abr;18(2):e7182 doi: 10.5867/medwave.2018.02.7182
Fecha de envío: 24/11/2017
Fecha de aceptación: 29/12/2017
Fecha de publicación: 17/4/2018
Origen: Este artículo es producto del Epistemonikos Evidence Synthesis Project de la Fundación Epistemonikos, en colaboración con Medwave para su publicación.
Tipo de revisión: Con revisión por pares sin ciego por parte del equipo metodológico del Epistemonikos Evidence Synthesis Project.

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Green P, Aronoff SC, DelVecchio M. The Effects of Inhaled Steroids on Recurrent Wheeze After Acute Bronchiolitis: A Systematic Review and Meta-Analysis of 748 Patients. Glob Pediatr Health. 2015 Jul 6;2:2333794X15595964. | CrossRef | PubMed |
Green P, Aronoff SC, DelVecchio M. The Effects of Inhaled Steroids on Recurrent Wheeze After Acute Bronchiolitis: A Systematic Review and Meta-Analysis of 748 Patients. Glob Pediatr Health. 2015 Jul 6;2:2333794X15595964. | CrossRef | PubMed | King VJ, Viswanathan M, Bordley WC, Jackman AM, Sutton SF, Lohr KN, et al. Pharmacologic treatment of bronchiolitis in infants and children: a systematic review. Arch Pediatr Adolesc Med. 2004 Feb;158(2):127-37. | PubMed |
King VJ, Viswanathan M, Bordley WC, Jackman AM, Sutton SF, Lohr KN, et al. Pharmacologic treatment of bronchiolitis in infants and children: a systematic review. Arch Pediatr Adolesc Med. 2004 Feb;158(2):127-37. | PubMed | Chu Z, Peng M, Li Z. Effect of inhaled corticosteroids on preventing post-bronchiolitic wheezing with bronchiolitis: a Meta-analysis of randomized controlled trials. Chin J Clinicians. 2003 Oct;7(19):8814-20. | CrossRef | Link |
Chu Z, Peng M, Li Z. Effect of inhaled corticosteroids on preventing post-bronchiolitic wheezing with bronchiolitis: a Meta-analysis of randomized controlled trials. Chin J Clinicians. 2003 Oct;7(19):8814-20. | CrossRef | Link | Cade A, Brownlee KG, Conway SP, Haigh D, Short A, Brown J, et al. Randomised placebo controlled trial of nebulised corticosteroids in acute respiratory syncytial viral bronchiolitis. Arch Dis Child. 2000 Feb;82(2):126-30. | PubMed |
Cade A, Brownlee KG, Conway SP, Haigh D, Short A, Brown J, et al. Randomised placebo controlled trial of nebulised corticosteroids in acute respiratory syncytial viral bronchiolitis. Arch Dis Child. 2000 Feb;82(2):126-30. | PubMed | Callén Blecua M, Aizpurua Galdeano P, Ozcoidi Erro I, Mancisidor Aguinagalde L, Guedea Adiego C, Busselo Ortega E, et al. [Inhaled corticosteroids and wheezing post-bronchiolitis]. An Esp Pediatr. 2000 Apr;52(4):351-5. | PubMed |
Callén Blecua M, Aizpurua Galdeano P, Ozcoidi Erro I, Mancisidor Aguinagalde L, Guedea Adiego C, Busselo Ortega E, et al. [Inhaled corticosteroids and wheezing post-bronchiolitis]. An Esp Pediatr. 2000 Apr;52(4):351-5. | PubMed | Ermers MJ, Rovers MM, van Woensel JB, Kimpen JL, Bont LJ; RSV Corticosteroid Study Group. The effect of high dose inhaled corticosteroids on wheeze in infants after respiratory syncytial virus infection: randomised double blind placebo controlled trial. BMJ. 2009 Mar 31;338:b897. | CrossRef | PubMed |
Ermers MJ, Rovers MM, van Woensel JB, Kimpen JL, Bont LJ; RSV Corticosteroid Study Group. The effect of high dose inhaled corticosteroids on wheeze in infants after respiratory syncytial virus infection: randomised double blind placebo controlled trial. BMJ. 2009 Mar 31;338:b897. | CrossRef | PubMed | Fox GF, Everard ML, Marsh MJ, Milner AD. Randomised controlled trial of budesonide for the prevention of post-bronchiolitis wheezing. Arch Dis Child. 1999 Apr;80(4):343-7. | PubMed |
Fox GF, Everard ML, Marsh MJ, Milner AD. Randomised controlled trial of budesonide for the prevention of post-bronchiolitis wheezing. Arch Dis Child. 1999 Apr;80(4):343-7. | PubMed | Kajosaari M, Syvänen P, Förars M, Juntunen-Backman K. Inhaled corticosteroids during and after respiratory syncytial virus-bronchiolitis may decrease subsequent asthma. Pediatr Allergy Immunol. 2000 Aug;11(3):198-202. | PubMed |
Kajosaari M, Syvänen P, Förars M, Juntunen-Backman K. Inhaled corticosteroids during and after respiratory syncytial virus-bronchiolitis may decrease subsequent asthma. Pediatr Allergy Immunol. 2000 Aug;11(3):198-202. | PubMed | Reijonen T, Korppi M, Kuikka L, Remes K. Anti-inflammatory therapy reduces wheezing after bronchiolitis. Arch Pediatr Adolesc Med. 1996 May;150(5):512-7. | PubMed |
Reijonen T, Korppi M, Kuikka L, Remes K. Anti-inflammatory therapy reduces wheezing after bronchiolitis. Arch Pediatr Adolesc Med. 1996 May;150(5):512-7. | PubMed | Reijonen TM, Kotaniemi-Syrjänen A, Korhonen K, Korppi M. Predictors of asthma three years after hospital admission for wheezing in infancy. Pediatrics. 2000 Dec;106(6):1406-12. | PubMed |
Reijonen TM, Kotaniemi-Syrjänen A, Korhonen K, Korppi M. Predictors of asthma three years after hospital admission for wheezing in infancy. Pediatrics. 2000 Dec;106(6):1406-12. | PubMed | Richter H, Seddon P. Early nebulized budesonide in the treatment of bronchiolitis and the prevention of postbronchiolitic wheezing. J Pediatr. 1998 May;132(5):849-53. | PubMed |
Richter H, Seddon P. Early nebulized budesonide in the treatment of bronchiolitis and the prevention of postbronchiolitic wheezing. J Pediatr. 1998 May;132(5):849-53. | PubMed | Wong JY, Moon S, Beardsmore C, O'Callaghan C, Simpson H. No objective benefit from steroids inhaled via a spacer in infants recovering from bronchiolitis. Eur Respir J. 2000 Feb;15(2):388-94. | PubMed |
Wong JY, Moon S, Beardsmore C, O'Callaghan C, Simpson H. No objective benefit from steroids inhaled via a spacer in infants recovering from bronchiolitis. Eur Respir J. 2000 Feb;15(2):388-94. | PubMed | 赵德育, 田曼, 陈荣华, 等. Influence of inhaled glucocorticosteroid on prognosis of infant respiratory syncytial virus bronchiolitis. 临床儿科杂志 (Journal of Clinical Pediatrics). 2008;26(1):24-27. | CrossRef | Link |
赵德育, 田曼, 陈荣华, 等. Influence of inhaled glucocorticosteroid on prognosis of infant respiratory syncytial virus bronchiolitis. 临床儿科杂志 (Journal of Clinical Pediatrics). 2008;26(1):24-27. | CrossRef | Link | Bentur L, Shoseyov D, Feigenbaum D, Gorichovsky Y, Bibi H. Dexamethasone inhalations in RSV bronchiolitis: a double-blind, placebo-controlled study. Acta Paediatr. 2005 Jul;94(7):866-71. | PubMed |
Bentur L, Shoseyov D, Feigenbaum D, Gorichovsky Y, Bibi H. Dexamethasone inhalations in RSV bronchiolitis: a double-blind, placebo-controlled study. Acta Paediatr. 2005 Jul;94(7):866-71. | PubMed | Pedersen S. Clinical safety of inhaled corticosteroids for asthma in children: an update of long-term trials. Drug Saf. 2006;29(7):599-612. Review. | PubMed |
Pedersen S. Clinical safety of inhaled corticosteroids for asthma in children: an update of long-term trials. Drug Saf. 2006;29(7):599-612. Review. | PubMed | Ralston SL, Lieberthal AS, Meissner HC, Alverson BK, Baley JE, Gadomski AM, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014 Nov;134(5):e1474-502. | CrossRef | PubMed |
Ralston SL, Lieberthal AS, Meissner HC, Alverson BK, Baley JE, Gadomski AM, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014 Nov;134(5):e1474-502. | CrossRef | PubMed | Ricci V, Delgado Nunes V, Murphy MS, Cunningham S; Guideline Development Group and Technical Team. Bronchiolitis in children: summary of NICE guidance. BMJ. 2015 Jun 2;350:h2305. | CrossRef | PubMed |
Ricci V, Delgado Nunes V, Murphy MS, Cunningham S; Guideline Development Group and Technical Team. Bronchiolitis in children: summary of NICE guidance. BMJ. 2015 Jun 2;350:h2305. | CrossRef | PubMed | Fernandes RM, Bialy LM, Vandermeer B, Tjosvold L, Plint AC, Patel H, et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2013 Jun 4;(6):CD004878. | CrossRef | PubMed |
Fernandes RM, Bialy LM, Vandermeer B, Tjosvold L, Plint AC, Patel H, et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2013 Jun 4;(6):CD004878. | CrossRef | PubMed | Hartling L, Fernandes RM, Bialy L, Milne A, Johnson D, Plint A, et al. Steroids and bronchodilators for acute bronchiolitis in the first two years of life: systematic review and meta-analysis. BMJ. 2011 Apr 6;342:d1714. | CrossRef | PubMed |
Hartling L, Fernandes RM, Bialy L, Milne A, Johnson D, Plint A, et al. Steroids and bronchodilators for acute bronchiolitis in the first two years of life: systematic review and meta-analysis. BMJ. 2011 Apr 6;342:d1714. | CrossRef | PubMed | Gómez-y-López RE, Hernández-Sierra JF, Torres-Ruvalcaba BA, Martínez-Puente E, del Carmen Martínez-Garcia M. [Comparative clinical study of dexamethasone vs. nebulized salbutamol in acute bronchiolitis]. Gac Med Mex. 2007 May-Jun;143(3):189-92. | PubMed |
Gómez-y-López RE, Hernández-Sierra JF, Torres-Ruvalcaba BA, Martínez-Puente E, del Carmen Martínez-Garcia M. [Comparative clinical study of dexamethasone vs. nebulized salbutamol in acute bronchiolitis]. Gac Med Mex. 2007 May-Jun;143(3):189-92. | PubMed | Barlas C, Kiper N, Göçmen A, Ozçelik U, Dilber E, Anadol D, et al. Racemic adrenaline and other treatment regimens in mild and moderate bronchiolitis. Cocuk Sagligi Ve Hastaliklari Dergisi 1998;41(2):155–66.
Barlas C, Kiper N, Göçmen A, Ozçelik U, Dilber E, Anadol D, et al. Racemic adrenaline and other treatment regimens in mild and moderate bronchiolitis. Cocuk Sagligi Ve Hastaliklari Dergisi 1998;41(2):155–66.  Lisa Hartlin, Terry Klassen, Dominic Allain, Ricardo Fernandes, Stephen Freedman, David Johnson, et al. Interventions for bronchiolitis in the acute care setting: a systematic review and network meta-analysis. PROSPERO 2016:CRD42016048625 Available from crd.york.ac.uk [online] | Link |
Lisa Hartlin, Terry Klassen, Dominic Allain, Ricardo Fernandes, Stephen Freedman, David Johnson, et al. Interventions for bronchiolitis in the acute care setting: a systematic review and network meta-analysis. PROSPERO 2016:CRD42016048625 Available from crd.york.ac.uk [online] | Link |