 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
INTRODUCTION
Psoriatic arthritis is an inflammatory arthritis without a clear etiology. Biological therapy has become key for its treatment, especially in more severe cases. There are several alternatives for biological treatment, including secukinumab. However, it is not clear how effective and safe it is, which is particularly relevant considering its high cost.
METHODS
To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified eight systematic reviews including three randomized trials overall. We concluded secukinumab in psoriatic arthritis leads to an improvement of disease activity and symptoms, and it is probably not associated to severe adverse events.
Psoriatic arthritis is an inflammatory arthritis without a clear etiology. It is associated with psoriasis, and the majority of patients are seronegative for rheumatoid factor. Currently, there are some alternatives for its treatment, mainly based on drugs proven for rheumatoid arthritis. In recent years, the efficacy of new alternatives for psoriatic arthritis has been studied in greater depth, especially for those who are resistant to initial treatment, either with non-steroidal anti-inflammatories, disease modifying drugs (e.g. methotrexate) or biological drugs (e.g. TNF-inhibitors). Due to its high cost, it is important to evaluate their real effectiveness to achieve a good outcome. Among these new alternatives is secukinumab, an IL-17a inhibitor.
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
|
What is the evidence. |
We found eight systematic reviews [1],[2],[3],[4],[5],[6],[7],[8], that include three primary studies, reported in five references [9],[10],[11],[12],[13]. All of them correspond to randomized controlled trials. |
|
What types of patients were included* |
All of the trials included adult patients with clinical psoriatic arthritis diagnosed according to CASPAR criteria, with active disease and inadequate response with non-steroidal anti-inflammatories, disease modifying drugs or anti-TNF biological medication. |
|
What types of interventions were included* |
One trial [10] included subcutaneous secukinumab 75 mg, 150 mg or 300 mg once a week for four weeks. Then, the same dosage was repeated every four weeks. |
|
What types of outcomes |
The outcomes were pooled by the different systematic reviews as follows:
|
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
The information on the effects of secukinumab is based on three randomized trials that included 1045 patients overall [9],[10],[11]. All of these trials reported ACR 20, HAQ-DI and serious adverse events. Two trials reported PASI 75 and DAS-28. The summary of findings is the following:
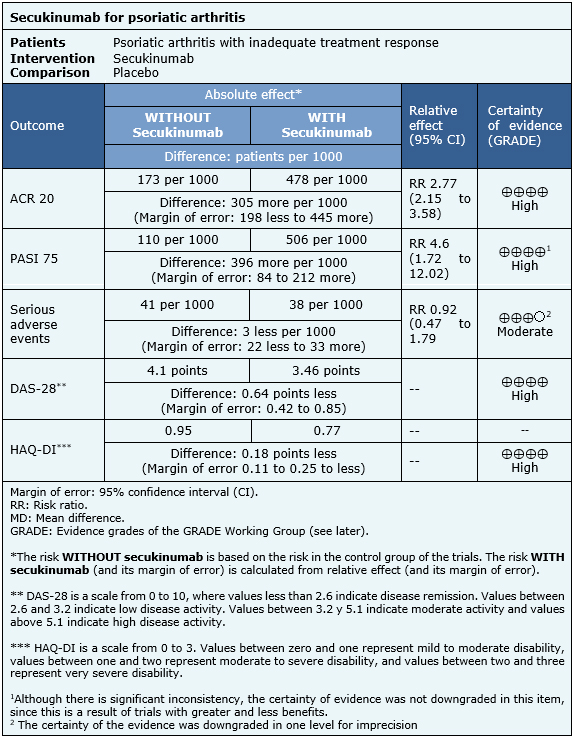

|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
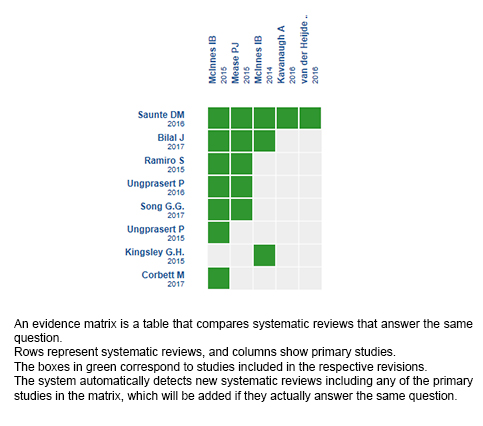
Follow the link to access the interactive version: Secukinumab for psoriatic arthritis
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCTION
Psoriatic arthritis is an inflammatory arthritis without a clear etiology. Biological therapy has become key for its treatment, especially in more severe cases. There are several alternatives for biological treatment, including secukinumab. However, it is not clear how effective and safe it is, which is particularly relevant considering its high cost.
METHODS
To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified eight systematic reviews including three randomized trials overall. We concluded secukinumab in psoriatic arthritis leads to an improvement of disease activity and symptoms, and it is probably not associated to severe adverse events.
 Autores:
Soledad Venegas-Iribarren[1,2], Romina Andino-Navarrete[2,3]
Autores:
Soledad Venegas-Iribarren[1,2], Romina Andino-Navarrete[2,3]

Citación: Venegas-Iribarren S, Andino-Navarrete R. Is secukinumab effective for psoriatic arthritis with insufficient response to initial treatment?. Medwave 2017 Nov-Dic;17(9):e7101 doi: 10.5867/medwave.2017.09.7101
Fecha de envío: 15/11/2017
Fecha de aceptación: 5/12/2017
Fecha de publicación: 20/12/2017
Origen: Este artículo es producto del Epistemonikos Evidence Synthesis Project de la Fundación Epistemonikos, en colaboración con Medwave para su publicación.
Tipo de revisión: Con revisión por pares sin ciego por parte del equipo metodológico del Epistemonikos Evidence Synthesis Project.

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Corbett M, Chehadah F, Biswas M, Moe-Byrne T, Palmer S, Soares M, Walton M, Harden M, Ho P, Woolacott N, Bojke L. Certolizumab pegol and secukinumab for treating active psoriatic arthritis following inadequate response to disease-modifying antirheumatic drugs: a systematic review and economic evaluation. Health technology assessment (Winchester, England). 2017;21(56):1-326.
Corbett M, Chehadah F, Biswas M, Moe-Byrne T, Palmer S, Soares M, Walton M, Harden M, Ho P, Woolacott N, Bojke L. Certolizumab pegol and secukinumab for treating active psoriatic arthritis following inadequate response to disease-modifying antirheumatic drugs: a systematic review and economic evaluation. Health technology assessment (Winchester, England). 2017;21(56):1-326.  Paccou J, Wendling D. Current treatment of psoriatic arthritis: update based
on a systematic literature review to establish French Society for Rheumatology
(SFR) recommendations for managing spondyloarthritis. Joint Bone Spine. 2015
Mar;82(2):80-5. | CrossRef | PubMed |
Paccou J, Wendling D. Current treatment of psoriatic arthritis: update based
on a systematic literature review to establish French Society for Rheumatology
(SFR) recommendations for managing spondyloarthritis. Joint Bone Spine. 2015
Mar;82(2):80-5. | CrossRef | PubMed | Ramiro S, Smolen JS, Landewé R, van der Heijde D, Dougados M, Emery P, de Wit M, Cutolo M, Oliver S, Gossec L. Pharmacological treatment of psoriatic arthritis: a systematic literature review for the 2015 update of the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis. 2016 Mar;75(3):490-8. | CrossRef | PubMed |
Ramiro S, Smolen JS, Landewé R, van der Heijde D, Dougados M, Emery P, de Wit M, Cutolo M, Oliver S, Gossec L. Pharmacological treatment of psoriatic arthritis: a systematic literature review for the 2015 update of the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis. 2016 Mar;75(3):490-8. | CrossRef | PubMed | Saunte DM, Mrowietz U, Puig L, Zachariae C. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017 Jul;177(1):47-62. | CrossRef | PubMed |
Saunte DM, Mrowietz U, Puig L, Zachariae C. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017 Jul;177(1):47-62. | CrossRef | PubMed | Ungprasert P, Thongprayoon C, Davis JM 3rd. Indirect comparisons of the efficacy of subsequent biological agents in patients with psoriatic arthritis with an inadequate response to tumor necrosis factor inhibitors: a meta-analysis. Clin Rheumatol. 2016 Jul;35(7):1795-803. | CrossRef | PubMed |
Ungprasert P, Thongprayoon C, Davis JM 3rd. Indirect comparisons of the efficacy of subsequent biological agents in patients with psoriatic arthritis with an inadequate response to tumor necrosis factor inhibitors: a meta-analysis. Clin Rheumatol. 2016 Jul;35(7):1795-803. | CrossRef | PubMed | Ungprasert P, Thongprayoon C, Davis JM 3rd. Indirect comparisons of the efficacy of biological agents in patients with psoriatic arthritis with an inadequate response to traditional disease-modifying anti-rheumatic drugs or to non-steroidal anti-inflammatory drugs: A meta-analysis. Semin Arthritis Rheum. 2016 Feb;45(4):428-38. | CrossRef | PubMed |
Ungprasert P, Thongprayoon C, Davis JM 3rd. Indirect comparisons of the efficacy of biological agents in patients with psoriatic arthritis with an inadequate response to traditional disease-modifying anti-rheumatic drugs or to non-steroidal anti-inflammatory drugs: A meta-analysis. Semin Arthritis Rheum. 2016 Feb;45(4):428-38. | CrossRef | PubMed | Kingsley G.H., Scott D. L. Assessing the effectiveness of synthetic and biologic disease-modifying antirheumatic drugs in psoriatic arthritis- A systematic review. Psoriasis: Targets and Therapy. 2015;5:71-81.
Kingsley G.H., Scott D. L. Assessing the effectiveness of synthetic and biologic disease-modifying antirheumatic drugs in psoriatic arthritis- A systematic review. Psoriasis: Targets and Therapy. 2015;5:71-81.  Bilal J, Riaz IB, Kamal MU, Elyan M, Sudano D, Khan MA. A Systematic Review and Meta-analysis of Efficacy and Safety of Novel Interleukin Inhibitors in the Management of Psoriatic Arthritis. Journal of clinical rheumatology: practical reports on rheumatic & musculoskeletal diseases. 2017.
Bilal J, Riaz IB, Kamal MU, Elyan M, Sudano D, Khan MA. A Systematic Review and Meta-analysis of Efficacy and Safety of Novel Interleukin Inhibitors in the Management of Psoriatic Arthritis. Journal of clinical rheumatology: practical reports on rheumatic & musculoskeletal diseases. 2017.  Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, van der Heijde D, Landewé R, Nash P, Pricop L, Yuan J, Richards HB, Mpofu S; FUTURE 1 Study Group. Secukinumab Inhibition of Interleukin-17A in Patients with Psoriatic Arthritis. N Engl J Med. 2015 Oct;373(14):1329-39. | CrossRef | PubMed |
Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, van der Heijde D, Landewé R, Nash P, Pricop L, Yuan J, Richards HB, Mpofu S; FUTURE 1 Study Group. Secukinumab Inhibition of Interleukin-17A in Patients with Psoriatic Arthritis. N Engl J Med. 2015 Oct;373(14):1329-39. | CrossRef | PubMed | McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, van der Heijde D, Landewé R, Conaghan PG, Gottlieb AB, Richards H, Pricop L, Ligozio G, Patekar M, Mpofu S; FUTURE 2 Study Group. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015 Sep 19;386(9999):1137-46. | CrossRef | PubMed |
McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, van der Heijde D, Landewé R, Conaghan PG, Gottlieb AB, Richards H, Pricop L, Ligozio G, Patekar M, Mpofu S; FUTURE 2 Study Group. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015 Sep 19;386(9999):1137-46. | CrossRef | PubMed | McInnes IB, Sieper J, Braun J, Emery P, van der Heijde D, Isaacs JD, Dahmen G, Wollenhaupt J, Schulze-Koops H, Kogan J, Ma S, Schumacher MM, Bertolino AP, Hueber W, Tak PP. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis. 2014 Feb;73(2):349-56. | CrossRef | PubMed |
McInnes IB, Sieper J, Braun J, Emery P, van der Heijde D, Isaacs JD, Dahmen G, Wollenhaupt J, Schulze-Koops H, Kogan J, Ma S, Schumacher MM, Bertolino AP, Hueber W, Tak PP. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis. 2014 Feb;73(2):349-56. | CrossRef | PubMed | Kavanaugh A, McInnes IB, Mease PJ, Hall S, Chinoy H, Kivitz AJ, Wang Z, Mpofu S. Efficacy of Subcutaneous Secukinumab in Patients with Active Psoriatic Arthritis Stratified by Prior Tumor Necrosis Factor Inhibitor Use: Results from the Randomized Placebo-controlled FUTURE 2 Study. J Rheumatol. 2016 Sep;43(9):1713-7. | CrossRef | PubMed |
Kavanaugh A, McInnes IB, Mease PJ, Hall S, Chinoy H, Kivitz AJ, Wang Z, Mpofu S. Efficacy of Subcutaneous Secukinumab in Patients with Active Psoriatic Arthritis Stratified by Prior Tumor Necrosis Factor Inhibitor Use: Results from the Randomized Placebo-controlled FUTURE 2 Study. J Rheumatol. 2016 Sep;43(9):1713-7. | CrossRef | PubMed | van der Heijde D, Landewé RB, Mease PJ, McInnes IB, Conaghan PG, Pricop L, Ligozio G, Richards HB, Mpofu S. Brief Report: Secukinumab Provides Significant and Sustained Inhibition of Joint Structural Damage in a Phase III Study of Active Psoriatic Arthritis. Arthritis Rheumatol. 2016 Aug;68(8):1914-21. | CrossRef | PubMed | PMC |
van der Heijde D, Landewé RB, Mease PJ, McInnes IB, Conaghan PG, Pricop L, Ligozio G, Richards HB, Mpofu S. Brief Report: Secukinumab Provides Significant and Sustained Inhibition of Joint Structural Damage in a Phase III Study of Active Psoriatic Arthritis. Arthritis Rheumatol. 2016 Aug;68(8):1914-21. | CrossRef | PubMed | PMC | American Academy of Dermatology Work Group, Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, Gottlieb A, Koo JY, Lebwohl M, Leonardi CL, Lim HW, Van Voorhees AS, Beutner KR, Ryan C, Bhushan R. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol. 2011 Jul;65(1):137-74. | CrossRef | PubMed |
American Academy of Dermatology Work Group, Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, Gottlieb A, Koo JY, Lebwohl M, Leonardi CL, Lim HW, Van Voorhees AS, Beutner KR, Ryan C, Bhushan R. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol. 2011 Jul;65(1):137-74. | CrossRef | PubMed | Smith CH, Anstey AV, Barker JN, Burden AD, Chalmers RJ, Chandler DA, Finlay AY, Griffiths CE, Jackson K, McHugh NJ, McKenna KE, Reynolds NJ, Ormerod AD; (Chair of Guideline Group). British Association of Dermatologists' guidelines for biologic interventions for psoriasis 2009. Br J Dermatol. 2009 Nov;161(5):987-1019. | CrossRef | PubMed |
Smith CH, Anstey AV, Barker JN, Burden AD, Chalmers RJ, Chandler DA, Finlay AY, Griffiths CE, Jackson K, McHugh NJ, McKenna KE, Reynolds NJ, Ormerod AD; (Chair of Guideline Group). British Association of Dermatologists' guidelines for biologic interventions for psoriasis 2009. Br J Dermatol. 2009 Nov;161(5):987-1019. | CrossRef | PubMed | Daudén E, Puig L, Ferrándiz C, Sánchez-Carazo JL, Hernanz-Hermosa JM; Spanish Psoriasis Group of the Spanish Academy of Dermatology and Venereology. Consensus document on the evaluation and treatment of moderate-to-severe psoriasis: Psoriasis Group of the Spanish Academy of Dermatology and Venereology. J Eur Acad Dermatol Venereol. 2016 Mar;30 Suppl 2:1-18. | CrossRef | PubMed |
Daudén E, Puig L, Ferrándiz C, Sánchez-Carazo JL, Hernanz-Hermosa JM; Spanish Psoriasis Group of the Spanish Academy of Dermatology and Venereology. Consensus document on the evaluation and treatment of moderate-to-severe psoriasis: Psoriasis Group of the Spanish Academy of Dermatology and Venereology. J Eur Acad Dermatol Venereol. 2016 Mar;30 Suppl 2:1-18. | CrossRef | PubMed | Novartis Pharmaceuticals. Efficacy of Secukinumab Compared to Adalimumab in Patients With Psoriatic Arthritis. clinicaltrials.gov. 2017.
Novartis Pharmaceuticals. Efficacy of Secukinumab Compared to Adalimumab in Patients With Psoriatic Arthritis. clinicaltrials.gov. 2017.  Mark Corbett, Laura Bojke, Fadi Chehadah, Thirimon Moe Byrne, Matthew Walton, Stephen Palmer, Nerys Woolacott, Melissa Harden. Certolizumab pegol and Secukinumab for treating active psoriatic arthritis following inadequate response to disease modifying anti-rheumatic drugs. PROSPERO 2016: CRD42016033357. | Link |
Mark Corbett, Laura Bojke, Fadi Chehadah, Thirimon Moe Byrne, Matthew Walton, Stephen Palmer, Nerys Woolacott, Melissa Harden. Certolizumab pegol and Secukinumab for treating active psoriatic arthritis following inadequate response to disease modifying anti-rheumatic drugs. PROSPERO 2016: CRD42016033357. | Link |