 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
PROBLEM
Meniere’s disease is an inner ear disorder characterized by episodes of spontaneous vertigo, fluctuating hearing loss and tinnitus. Betahistine has been used to reduce intensity and frecuency of vertigo attacks, but there is controversy regarding its effectiveness.
METHODS
To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified four systematic reviews including 12 trials overall. We concluded betahistine might reduce the number of attacks, vertigo intensity and lead to a symptomatic improvement according to global judgement in patients with Meniere’s disease, but the certainty of evidence is low. On the other hand, it probably does not have significant adverse effects.
Ménière’s disease is an inner ear disorder characterized by episodes of spontaneous vertigo, fluctuating hearing loss and tinnitus. One of the most used diagnostic criteria, although not universally accepted, includes the presence of two vertigo episodes with a duration grater than 20 minutes, audiometric confirmation of sensorineural hearing loss, plus tinnitus or aural fullness perception [1].
Ménière’s disease is caused by an increase in endolymphatic pressure in the inner ear, from unknown etiology. This leads to recurrent and frequent vertigo attacks with substantive impact on quality of life, followed by periods of remission that can last several months [2].
Betahistine has long been used to reduce intensity and frecuency of vertigo attacks and tinnitus, since it would delay progression of hearing loss in Ménière’s disease. The alleged mechanism is endolymph pressure reduction secondary to microcirculation improvement at the stria vascularis of the cochlea. Another proposed mechanism relates to vestibular nucleus activity inhibition. However, there is controversy regarding its effectiveness.
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
|
What is the evidence. |
We found four systematic reviews [3],[4],[5],[6] including 12 primary studies relevant for our question [7],[8],[9],[10],[11],[12],[13],[14],[15],[16],[17],[18], all of them randomized controlled trials. |
|
What types of patients were included* |
Some trials did not use the American Academy of Otolaryngology –Head and Neck Surgery guidelines’ criteria for Ménière’s disease [1]. In consequence, some systematic reviews did not include them into their analysis. In order to present direct evidence, these are presented in this table, but were not used to estimate the effect of benefits in the summary of findings table. Six trials specified the inclusion of patients with clinical Ménière’s disease [8],[10],[11],[12],[14],[17]. One of the trials included patients with progressive episodic vertigo [9], two included patients with two or three months of peripheral vertigo [7],[13] and one with recurrent vertigo defined as two or more vertigo attacks in the last month [15]. Two trials did not specify the inclusion criteria [16],[18]. |
|
What types of interventions were included* |
All trials compared betahistine against placebo. |
|
What types of outcomes |
Outcomes, according to how they were grouped in the identified systematic reviews were:
|
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
The information about the effects of betahistine on Ménière’s disease is based on 12 randomized trials. Only six trials included patients with clinical disease [8],[10],[11],[12],[14],[17] including 327 patients. Only one trial reported the number of vertigo attacks and intensity of symptoms [17] and three trials measured improvement according to global judgement of patients. Adverse effects were assessed in seven trials [7],[8],[10],[11],[13],[14],[17].
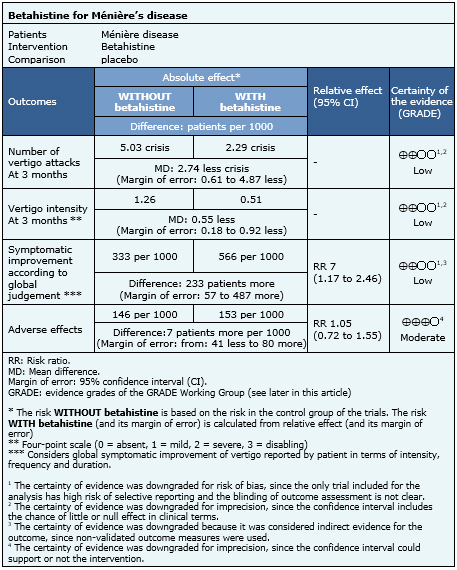

|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
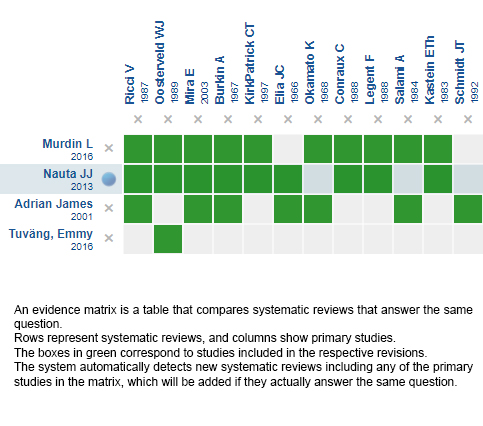
Follow the link to access the interactive version: Betahistine for Ménière's disease
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

PROBLEM
Meniere’s disease is an inner ear disorder characterized by episodes of spontaneous vertigo, fluctuating hearing loss and tinnitus. Betahistine has been used to reduce intensity and frecuency of vertigo attacks, but there is controversy regarding its effectiveness.
METHODS
To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified four systematic reviews including 12 trials overall. We concluded betahistine might reduce the number of attacks, vertigo intensity and lead to a symptomatic improvement according to global judgement in patients with Meniere’s disease, but the certainty of evidence is low. On the other hand, it probably does not have significant adverse effects.
 Autores:
Andrés Rosenbaum[1,2], Matías Winter[2,3]
Autores:
Andrés Rosenbaum[1,2], Matías Winter[2,3]

Citación: Rosenbaum A, Winter M. Is betahistine effective for Ménière’s disease?. Medwave 2017 Sep-Oct; 17(8):e7068 doi: 10.5867/medwave.2017.08.7068
Fecha de envío: 21/8/2017
Fecha de aceptación: 12/10/2017
Fecha de publicación: 31/10/2017
Origen: Este artículo es producto del Epistemonikos Evidence Synthesis Project de la Fundación Epistemonikos, en colaboración con Medwave para su publicación.
Tipo de revisión: Con revisión por pares sin ciego por parte del equipo metodológico del Epistemonikos Evidence Synthesis Project.

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Menière's disease. American Academy of Otolaryngology-Head and Neck Foundation, Inc. Otolaryngol Head Neck Surg. 1995 Sep;113(3):181-5. | PubMed |
Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Menière's disease. American Academy of Otolaryngology-Head and Neck Foundation, Inc. Otolaryngol Head Neck Surg. 1995 Sep;113(3):181-5. | PubMed | Friberg U, Stahle J, Svedberg A. The natural course of Meniere's disease. Acta Otolaryngol Suppl. 1984;406:72-7. | PubMed |
Friberg U, Stahle J, Svedberg A. The natural course of Meniere's disease. Acta Otolaryngol Suppl. 1984;406:72-7. | PubMed | Nauta JJ. Meta-analysis of clinical studies with betahistine in Ménière's disease and vestibular vertigo. Eur Arch Otorhinolaryngol. 2014 May;271(5):887-97. | CrossRef | PubMed |
Nauta JJ. Meta-analysis of clinical studies with betahistine in Ménière's disease and vestibular vertigo. Eur Arch Otorhinolaryngol. 2014 May;271(5):887-97. | CrossRef | PubMed | James AL, Burton MJ. Betahistine for Menière's disease or syndrome. Cochrane Database Syst Rev. 2001;(1):CD001873. | PubMed |
James AL, Burton MJ. Betahistine for Menière's disease or syndrome. Cochrane Database Syst Rev. 2001;(1):CD001873. | PubMed | Murdin L, Hussain K, Schilder AG. Betahistine for symptoms of vertigo.Cochrane Database Syst Rev. 2016 Jun 21;(6):CD010696. | CrossRef | PubMed |
Murdin L, Hussain K, Schilder AG. Betahistine for symptoms of vertigo.Cochrane Database Syst Rev. 2016 Jun 21;(6):CD010696. | CrossRef | PubMed | Tuväng, Emmy. Pharmacological options for the treatment of dizziness in Menieres disease. Umeå University, Sweden. 2016.
Tuväng, Emmy. Pharmacological options for the treatment of dizziness in Menieres disease. Umeå University, Sweden. 2016.  Kastein ETh. Betahistine dihydrochloride and peripheral vertigo, a double blind placebo-controlled trial in outpatients. Duphar report. 1983;77.054.
Kastein ETh. Betahistine dihydrochloride and peripheral vertigo, a double blind placebo-controlled trial in outpatients. Duphar report. 1983;77.054.  Okamato K, Hazeyama F, Taira T, Yoshida A.. Therapeutic results of betahistine in Meniere's disease with statistical analysis. Iryo. 1968;(22):650-66.
Okamato K, Hazeyama F, Taira T, Yoshida A.. Therapeutic results of betahistine in Meniere's disease with statistical analysis. Iryo. 1968;(22):650-66.  Legent F, Calais C, Celleir D. Recurrent paroxysmal vertigo and Serc: a controlled clinical study. Conc Méd. 1988;110:2539–2543.
Legent F, Calais C, Celleir D. Recurrent paroxysmal vertigo and Serc: a controlled clinical study. Conc Méd. 1988;110:2539–2543.  KirkPatrick CT, Saunders HF. Double-blind, multi-centre, randomised, comparative study of Serc® 24 mg versus placebo, in two groups of patients with recurrent vertigo. Solvay Pharmaceuticals report H. 1997;108.035.92/F.
KirkPatrick CT, Saunders HF. Double-blind, multi-centre, randomised, comparative study of Serc® 24 mg versus placebo, in two groups of patients with recurrent vertigo. Solvay Pharmaceuticals report H. 1997;108.035.92/F.  Burkin A. Betahistine treatment of Ménière’s syndrome. Clinical Medicine. 1967;74:41–48.
Burkin A. Betahistine treatment of Ménière’s syndrome. Clinical Medicine. 1967;74:41–48.  Ricci V, Sittoni V, Nicora M.. Efficacy and safety of betahistine hydrochloride versus placebo in Menière's disease. Rivista Italiana di Ornitologia Audiologia e Foniatria. 1987;7(3):347-50.
Ricci V, Sittoni V, Nicora M.. Efficacy and safety of betahistine hydrochloride versus placebo in Menière's disease. Rivista Italiana di Ornitologia Audiologia e Foniatria. 1987;7(3):347-50.  Conraux C, Cellier D. Chronic vertiginous sensations: a comparative double-blind randomized multicenter study of serc 8 mg versus placebo in patients suffering from chronic vertiginous sensations. Impact Médicin. 1988;260:63–65.
Conraux C, Cellier D. Chronic vertiginous sensations: a comparative double-blind randomized multicenter study of serc 8 mg versus placebo in patients suffering from chronic vertiginous sensations. Impact Médicin. 1988;260:63–65.  Salami A, Dellepiane M, Tinelle E, Jankowska B. Double blind study of betahistine and placebo in the treatment of Menière's Syndrome [Studio a doppia cecita' tra cloridrato di betaistina e placebo nel trattamento delle sindromi Menieriformi]. Il Valsalva. 1984;60:302-12.
Salami A, Dellepiane M, Tinelle E, Jankowska B. Double blind study of betahistine and placebo in the treatment of Menière's Syndrome [Studio a doppia cecita' tra cloridrato di betaistina e placebo nel trattamento delle sindromi Menieriformi]. Il Valsalva. 1984;60:302-12.  Oosterveld WJ, Blijleven W, Van Elferen LWM. Betahistine versus placebo in paroxysmal vertigo: a double-blind trial. J Drug Ther Res. 1989;14:122–126.
Oosterveld WJ, Blijleven W, Van Elferen LWM. Betahistine versus placebo in paroxysmal vertigo: a double-blind trial. J Drug Ther Res. 1989;14:122–126.  Elia JC. Double-blind evaluation of a new treatment for Ménière's syndrome. JAMA. 1966 Apr 11;196(2):187-9. | PubMed |
Elia JC. Double-blind evaluation of a new treatment for Ménière's syndrome. JAMA. 1966 Apr 11;196(2):187-9. | PubMed | Mira E, Guidetti G, Ghilardi L, Fattori B, Malannino N, Maiolino L, Mora R, Ottoboni S, Pagnini P, Leprini M, Pallestrini E, Passali D, Nuti D, Russolo M, Tirelli G, Simoncelli C, Brizi S, Vicini C, Frasconi P. Betahistine dihydrochloride in the treatment of peripheral vestibular vertigo. Eur Arch Otorhinolaryngol. 2003 Feb;260(2):73-7. | PubMed |
Mira E, Guidetti G, Ghilardi L, Fattori B, Malannino N, Maiolino L, Mora R, Ottoboni S, Pagnini P, Leprini M, Pallestrini E, Passali D, Nuti D, Russolo M, Tirelli G, Simoncelli C, Brizi S, Vicini C, Frasconi P. Betahistine dihydrochloride in the treatment of peripheral vestibular vertigo. Eur Arch Otorhinolaryngol. 2003 Feb;260(2):73-7. | PubMed | Schmidt JT, Huizing EH. The clinical drug trial in Menière's disease with emphasis on the effect of betahistine SR. Acta Otolaryngol Suppl. 1992;497:1-189. | PubMed |
Schmidt JT, Huizing EH. The clinical drug trial in Menière's disease with emphasis on the effect of betahistine SR. Acta Otolaryngol Suppl. 1992;497:1-189. | PubMed | Espinosa-Sanchez JM, Lopez-Escamez JA. Menière's disease. Handb Clin Neurol. 2016;137:257-77. | CrossRef | PubMed |
Espinosa-Sanchez JM, Lopez-Escamez JA. Menière's disease. Handb Clin Neurol. 2016;137:257-77. | CrossRef | PubMed | Diagnóstico y Tratamiento de la Enfermedad de Ménière en los tres niveles de atención México; Secretaría de Salud; 12 de diciembre de 2013. | Link |
Diagnóstico y Tratamiento de la Enfermedad de Ménière en los tres niveles de atención México; Secretaría de Salud; 12 de diciembre de 2013. | Link | Nevoux J, Franco-Vidal V, Bouccara D, Parietti-Winkler C, Uziel A, Chays A, Dubernard X, Couloigner V, Darrouzet V, Mom T; Groupe de Travail de la SFORL. Diagnostic and therapeutic strategy in Menière's disease. Guidelines of the French Otorhinolaryngology-Head and Neck Surgery Society (SFORL). Eur Ann Otorhinolaryngol Head Neck Dis. 2017 Jan 3. pii: S1879-7296(16)30222-8. | CrossRef | PubMed |
Nevoux J, Franco-Vidal V, Bouccara D, Parietti-Winkler C, Uziel A, Chays A, Dubernard X, Couloigner V, Darrouzet V, Mom T; Groupe de Travail de la SFORL. Diagnostic and therapeutic strategy in Menière's disease. Guidelines of the French Otorhinolaryngology-Head and Neck Surgery Society (SFORL). Eur Ann Otorhinolaryngol Head Neck Dis. 2017 Jan 3. pii: S1879-7296(16)30222-8. | CrossRef | PubMed | CPG working group. Clinical Practice Guidelines Vertigo in Adults – 2nd Edition. PhiliPPine Journal of otolaryngology-head and neck Surgery. 2014;29(1) | Link |
CPG working group. Clinical Practice Guidelines Vertigo in Adults – 2nd Edition. PhiliPPine Journal of otolaryngology-head and neck Surgery. 2014;29(1) | Link | Adrion C, Fischer CS, Wagner J, Gürkov R, Mansmann U, Strupp M; BEMED Study Group. Efficacy and safety of betahistine treatment in patients with Meniere's disease: primary results of a long term, multicentre, double blind, randomised, placebo controlled, dose defining trial (BEMED trial). BMJ. 2016 Jan 21;352:h6816. | CrossRef | PubMed | PMC |
Adrion C, Fischer CS, Wagner J, Gürkov R, Mansmann U, Strupp M; BEMED Study Group. Efficacy and safety of betahistine treatment in patients with Meniere's disease: primary results of a long term, multicentre, double blind, randomised, placebo controlled, dose defining trial (BEMED trial). BMJ. 2016 Jan 21;352:h6816. | CrossRef | PubMed | PMC | Seyed Tootoonchi SJ, Ghiasi S, Shadara P, Samani SM, Fouladi DF. Hearing function after betahistine therapy in patients with Ménière's disease. Braz J Otorhinolaryngol. 2016 Sep- Oct;82(5):500-6. | CrossRef | PubMed |
Seyed Tootoonchi SJ, Ghiasi S, Shadara P, Samani SM, Fouladi DF. Hearing function after betahistine therapy in patients with Ménière's disease. Braz J Otorhinolaryngol. 2016 Sep- Oct;82(5):500-6. | CrossRef | PubMed | Solvay Pharmaceuticals. Effects of Betaserc on Vestibular Compensation in Patients Suffering From Disabling Meniere's Disease and Having Undergone Vestibular Neurotomy. clinicaltrials.gov. 2003.
Solvay Pharmaceuticals. Effects of Betaserc on Vestibular Compensation in Patients Suffering From Disabling Meniere's Disease and Having Undergone Vestibular Neurotomy. clinicaltrials.gov. 2003.