 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Cannabidiol has recently been proposed as an antipsychotic for schizophrenia. However, its clinical use and safety is controversial. To answer this question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We identified six systematic reviews incorporating four primary studies overall, including two randomized trials. We extracted data from the systematic reviews, reanalyzed data from primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach. We concluded cannabidiol probably does not improve symptoms in schizophrenia and leads to frequent side effects.
Research has shown the endocannabinoid system plays an important role in the pathophysiology of schizophrenia. Several studies have concluded the use of cannabis is a risk factor for development and early onset of the illness. Moreover, tetrahydrocannabinol has been associated to psychotic symptoms typical of schizophrenia like hallucinations, delusions, depersonalization, and others [1],[3],[15],[16].
Nonetheless, cannabidiol might exert an antipsychotic effect opposing tetrahydrocannabinol in the brain and having an inverse relation with the positive symptoms of schizophrenia. Its mechanism of action is not clear, but it is suggested it would involve the cannabinoid receptors CB1/CB2 which inhibit anandamide reuptake, increasing endocannabinoid levels.
However, its efficacy and safety for the treatment of schizophrenia are not certain.
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
|
What is the evidence. |
We found six systematic reviews [1],[2],[3],[4],[5],[6], including four primary studies reported in seven references [7],[8],[9],[10],[11],[12],[13], including two randomized trials [7],[12]. This table and the summary in general are based on the latter. |
|
What types of patients were included* |
All the trials included patients with the diagnosis of schizophrenia, but only one specified the type, including patients with acute paranoid schizophrenia or schizophreniform disorder. |
|
What types of interventions were included* |
One trial used 600 mg of cannabidiol orally [12] and one trial used 300 mg or 600 mg of cannabidiol orally [7]. It could not be retrieved information related to the baseline treatment of patients. Both trials compared against placebo. |
|
What types of outcomes |
The different systematic reviews used the following psychiatric scales as outcome measure of the improvement of psychotic symptoms:
In addition, one trial evaluated the cognitive deficit through the SCWT scale (Stroop Color Word Test) Other outcomes evaluated were the presence of side effects associated to the traditional treatment of schizophrenia like extrapyramidal symptoms, levels of prolactine and weight gain. One trial lasted four weeks [12]. No information about the duration of the second trial could be retrieved. |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
The information about the effects of cannabidiol in schizophrenia is based on two randomized trials that include 57 patients [7],[12].
Both trials measured the improvement of symptoms through the PANSS and BPRS scale. One trial measured the cognitive deficit through the SCWT. None of the reviews identified were able to extract data in a way that could be incorporated in a meta-analysis, so the information presented below corresponds to a narrative synthesis of the information obtained from the reviews.
The summary of findings is the following:
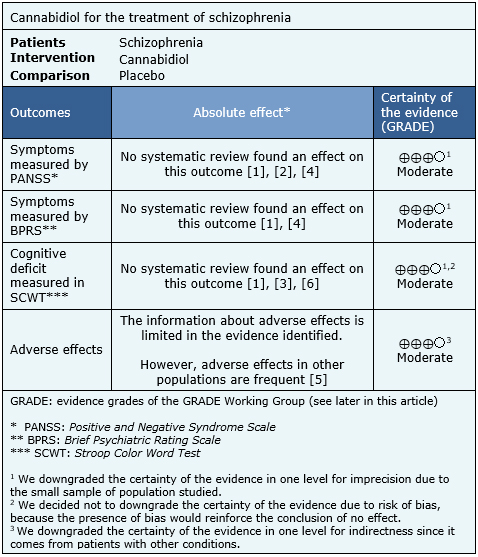
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
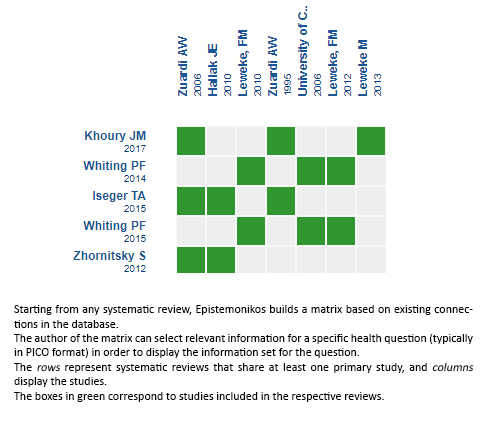
Follow the link to access the interactive version: Cannabidiol versus placebo for the treatment of schizophrenia
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrices and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Cannabidiol has recently been proposed as an antipsychotic for schizophrenia. However, its clinical use and safety is controversial. To answer this question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We identified six systematic reviews incorporating four primary studies overall, including two randomized trials. We extracted data from the systematic reviews, reanalyzed data from primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach. We concluded cannabidiol probably does not improve symptoms in schizophrenia and leads to frequent side effects.
 Autores:
Rami Guinguis[1,2], María Isabel Ruiz[2,3], Gabriel Rada[2,4,5,6,7]
Autores:
Rami Guinguis[1,2], María Isabel Ruiz[2,3], Gabriel Rada[2,4,5,6,7]

Citación: Guinguis R, Ruiz MI, Rada G. Is cannabidiol an effective treatment for schizophrenia?. Medwave 2017;17(7):e7010 doi: 10.5867/medwave.2017.07.7010
Fecha de envío: 26/7/2017
Fecha de aceptación: 29/7/2017
Fecha de publicación: 9/8/2017

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Iseger TA, Bossong MG. A systematic review of the antipsychotic properties of cannabidiol in humans. Schizophr Res. 2015 Mar;162(1-3):153-61 | CrossRef | PubMed |
Iseger TA, Bossong MG. A systematic review of the antipsychotic properties of cannabidiol in humans. Schizophr Res. 2015 Mar;162(1-3):153-61 | CrossRef | PubMed | Khoury JM, Neves MCLD, Roque MAV, Queiroz DAB, Corrêa de Freitas AA, de Fátima Â, et al. Is there a role for cannabidiol in psychiatry? World J Biol Psychiatry. 2017 Feb 20:1-16 | CrossRef | PubMed |
Khoury JM, Neves MCLD, Roque MAV, Queiroz DAB, Corrêa de Freitas AA, de Fátima Â, et al. Is there a role for cannabidiol in psychiatry? World J Biol Psychiatry. 2017 Feb 20:1-16 | CrossRef | PubMed | Osborne AL, Solowij N, Weston-Green K. A systematic review of the effect of cannabidiol on cognitive function: Relevance to schizophrenia. Neurosci Biobehav Rev. 2017 Jan;72:310-324 | CrossRef | PubMed |
Osborne AL, Solowij N, Weston-Green K. A systematic review of the effect of cannabidiol on cognitive function: Relevance to schizophrenia. Neurosci Biobehav Rev. 2017 Jan;72:310-324 | CrossRef | PubMed | Whiting P, Wolff R, Westwood M, Duffy S, Misso K, Keurentjes C, et al. Systematic review of cannabis for medical use. Kleijnen Systematic Reviews Ltd. 2014 | Link |
Whiting P, Wolff R, Westwood M, Duffy S, Misso K, Keurentjes C, et al. Systematic review of cannabis for medical use. Kleijnen Systematic Reviews Ltd. 2014 | Link | Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015 Jun 23-30;313(24):2456-73 | CrossRef | PubMed |
Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015 Jun 23-30;313(24):2456-73 | CrossRef | PubMed | Zhornitsky S, Potvin S. Cannabidiol in humans-the quest for therapeutic targets. Pharmaceuticals (Basel). 2012 May 21;5(5):529-52 | CrossRef | PubMed |
Zhornitsky S, Potvin S. Cannabidiol in humans-the quest for therapeutic targets. Pharmaceuticals (Basel). 2012 May 21;5(5):529-52 | CrossRef | PubMed | Hallak JE, Machado-de-Sousa JP, Crippa JA, Sanches RF, Trzesniak C, Chaves C, et al. Performance of schizophrenic patients in the Stroop Color Word Test and electrodermal responsiveness after acute administration of cannabidiol (CBD). Rev Bras Psiquiatr. 2010 Mar;32(1):56-61. | PubMed |
Hallak JE, Machado-de-Sousa JP, Crippa JA, Sanches RF, Trzesniak C, Chaves C, et al. Performance of schizophrenic patients in the Stroop Color Word Test and electrodermal responsiveness after acute administration of cannabidiol (CBD). Rev Bras Psiquiatr. 2010 Mar;32(1):56-61. | PubMed | Leweke FM, Kranaster L, Hellmich M, Koethe D. Cannabidiol as a new type of an antipsychotic: results from a placebo-controlled clinical trial. Paper presented at: 49th Annual Conference of the American College of Neuropsychopharmacology, ACNP 2010; December 5-9, 2010; Miami Beach, FL. Neuropsychopharmacology. 2010;35(suppl 1):S280 | CrossRef |
Leweke FM, Kranaster L, Hellmich M, Koethe D. Cannabidiol as a new type of an antipsychotic: results from a placebo-controlled clinical trial. Paper presented at: 49th Annual Conference of the American College of Neuropsychopharmacology, ACNP 2010; December 5-9, 2010; Miami Beach, FL. Neuropsychopharmacology. 2010;35(suppl 1):S280 | CrossRef | Leweke FM, Hellmich M, Kranaster L, Koethe D. Cannabidiol as a new type of an antipsychotic: results from a placebo-controlled clinical trial. Paper presented at: 67th Annual Scientific Convention and Meeting of the Society of Biological Psychiatry; May 3-5, 2012; Philadelphia, PA. Biol Psychiatry. 2012;78(8)(suppl 1):63S | Link |
Leweke FM, Hellmich M, Kranaster L, Koethe D. Cannabidiol as a new type of an antipsychotic: results from a placebo-controlled clinical trial. Paper presented at: 67th Annual Scientific Convention and Meeting of the Society of Biological Psychiatry; May 3-5, 2012; Philadelphia, PA. Biol Psychiatry. 2012;78(8)(suppl 1):63S | Link | Leweke M. 2013. The Endocannabinoid System in Schizophrenia - a Mechanistically New Approach to its Pathophysiology and Treatment. . Schizophr Bull.39 (Suppl 1):S341. | Link |
Leweke M. 2013. The Endocannabinoid System in Schizophrenia - a Mechanistically New Approach to its Pathophysiology and Treatment. . Schizophr Bull.39 (Suppl 1):S341. | Link | Markus F, Leweke M, Kranaster L, et al. The efficacy of cannabidiol in the treatment of schizophrenia—A translational approach. Paper presented at: 13th International Congress on Schizophrenia Research, ICOSR; April 2-6, 2011; Colorado Springs, CO. Schizophr Bull. 2011;37(suppl 1):313 | Link |
Markus F, Leweke M, Kranaster L, et al. The efficacy of cannabidiol in the treatment of schizophrenia—A translational approach. Paper presented at: 13th International Congress on Schizophrenia Research, ICOSR; April 2-6, 2011; Colorado Springs, CO. Schizophr Bull. 2011;37(suppl 1):313 | Link | Rohleder C, Pahlisch F, Schaefer C, et al. The endocannabinoid system as a pharmacological target for antipsychotic treatment and more? Paper presented at: 8th International Conference on Early Psychosis: From Neurobiology to Public Policy; October 11-13, 2012; San Francisco, CA. Early Interv Psychiatry. 2012;6(suppl 1):7 | CrossRef |
Rohleder C, Pahlisch F, Schaefer C, et al. The endocannabinoid system as a pharmacological target for antipsychotic treatment and more? Paper presented at: 8th International Conference on Early Psychosis: From Neurobiology to Public Policy; October 11-13, 2012; San Francisco, CA. Early Interv Psychiatry. 2012;6(suppl 1):7 | CrossRef | Stanley Medical Research Institute, Coordinating Centre for Clinical Trials Cologne. University of Cologne. A clinical trial on the antipsychotic properties of cannabidiol. ClinicalTrials.gov | Link |
Stanley Medical Research Institute, Coordinating Centre for Clinical Trials Cologne. University of Cologne. A clinical trial on the antipsychotic properties of cannabidiol. ClinicalTrials.gov | Link | Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007 Jul 28;370(9584):319-28 | PubMed |
Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007 Jul 28;370(9584):319-28 | PubMed | Arseneault L, Cannon M, Witton J, Murray RM. Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry. 2004 Feb;184:110-7 | PubMed |
Arseneault L, Cannon M, Witton J, Murray RM. Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry. 2004 Feb;184:110-7 | PubMed | Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004 Feb;161(2 Suppl):1-56 | PubMed |
Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004 Feb;161(2 Suppl):1-56 | PubMed | Leweke FM. A Placebo-Controlled Randomized Cross-Over Clinical Trial on the Antipsychotic Properties of the Endocannabinoid Modulator Cannabidiol. NCT00309413. | Link |
Leweke FM. A Placebo-Controlled Randomized Cross-Over Clinical Trial on the Antipsychotic Properties of the Endocannabinoid Modulator Cannabidiol. NCT00309413. | Link | Leweke FM. A four-week, multicentre, double-blinded, randomised, active- and placebo-controlled, parallel-group trial investigating efficacy and safety of cannabidiol in acute, early-stage schizophrenic patients. NCT02088060. | Link |
Leweke FM. A four-week, multicentre, double-blinded, randomised, active- and placebo-controlled, parallel-group trial investigating efficacy and safety of cannabidiol in acute, early-stage schizophrenic patients. NCT02088060. | Link | Leweke FM. Cannabidiol as a Different Type of an Antipsychotic: Drug Delivery and Interaction Study With Approved Antipsychotics in Vivo. NCT02051387 | Link |
Leweke FM. Cannabidiol as a Different Type of an Antipsychotic: Drug Delivery and Interaction Study With Approved Antipsychotics in Vivo. NCT02051387 | Link |