 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Systemic corticosteroids constitute standard treatment in children with acute obstructive laryngitis (croup). However, there is some uncertainty in relation with the magnitude of the benefits and risks associated with their use. To answer this question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We identified six systematic reviews including 25 randomized trials relevant for the question of interest. We extracted data from the systematic reviews, reananalysed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach. We concluded the use of systemic corticosteroids increases the number of patients with clinical improvement at 12 hours and reduces the risk of readmission.
Acute obstructive laryngitis is one of the most frequent respiratory diseases in childhood, predominantly in children between 6 months and 6 years, with a higher incidence in late autumn and early winter [1],[2]. They are usually mild and self-limiting, although occasionally they can cause severe respiratory obstruction. Systemic corticosteroids reduce laryngeal edema by reducing the local inflammatory reaction, contracting lymphoid inflammation and impairing capillary permeability [1],[3]. Due to their potent anti-inflammatory effect, they have been one of the pillars of treatment in patients with croup. However, there is some degree of uncertainty regarding the magnitude of the benefits.
On the other hand, although the use of corticosteroids in high doses, or for long time, is associated to multiple adverse effects, the use of a single dose in acute obstructive laryngitis would constitute a safe therapy [4].
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
|
What is the evidence. |
We found six systematic reviews [4],[5],[6],[7],[8],[9] including twenty-five randomized controlled trials [10], [11],[12],[13],[14],[15],[16],[17],[18],[19],[20],[21], [22],[23],[24],[25],[26],[27],[28],[29],[30],[31],[32], [33],[34] evaluating the use of corticosteroids compared to placebo. |
|
What types of patients were included* |
Eight trials included patients who consulted in an emergency department [12],[15],[16],[19],[21],[22],[25], [31] and sixteen included patients in the hospital setting [10],[11],[13],[14],[17],[18],[20],[23],[24],[26],[28], [29],[30],[33],[34]. One trial considered a group evaluated in emergency services that was then admitted to the hospital [32].The age range ranged from 3 months to 12 years. Three trials included patients with mild laryngitis [12], [16],[31], three with moderate laryngitis [21],[22],[25] and one mild to moderate [15]. Eighteen trials did not classify the severity of laryngitis [10],[11],[13],[14],[17], [18],[19],[20],[23],[24],[26],[27],[28],[29],[30],[32], |
|
What types of interventions were included* |
Seventeen trials considered only the use of systemic corticosteroids (oral, nasogastric, intravenous, subcutaneous and intramuscular) [12],[13],[14],[17],[18], The corticosteroids used were prednisone, dexamethasone, methylprednisolone, prednisolone and budesonide. The dosages used had great variation between the different trials. From the eighteen trials that considered the use of systemic corticosteroids, fourteen used dexamethasone in different single doses (0.6 mg/kg [12], [16],[17],[22],[25],[32],[33], 0.15 mg/kg [31], 0.3 mg/kg [19], 0.4 mg/kg [29], 0.5 mg/kg [24], between 4 and 12 mg total [20],[23],[28]). Two trials used 4 mg/kg methylprednisolone once or twice [14],[26] (equivalent to 0.8 mg/kg dexamethasone dose). Two trials used prednisolone, one at a dose of 2 mg/kg up to 24 hours post-extubation [13] (equivalent to 0.3 mg/kg dexamethasone), and another 2.5 mg at 6 hours without specifying time of treatment [18]. One trial used prednisone 2.5 to 5 mg three doses in one day (equivalent to 0.4 to 0.8 mg dexamethasone dose) [34] and one trial used dexamethasone used 0.04 mg/kg/day in 4 doses [30]. The rest of the trials used the inhalation route with budesonide from 1 to 4 mg per dose up to 4 doses, and nebulized dexamethasone from 10 to 20 mg. |
|
What types of outcomes |
The trials measured multiple outcomes, however, the different systematic reviews grouped them as follows:
|
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
The information on the effects of systemic corticosteroids in children with acute obstructive laryngitis is based on 13 randomized trials. The rest of the trials were not analyzed because they did not consider systemic steroids, or did not report the outcomes of interest. Six trials measured the outcome readmissions/reconsultations [12],[16],[22],[25],[28],[31], six endotracheal intubation/tracheostomy [19], [20],[22],[23],[28],[29] and five clinical improvement at 12 hours [14],[23],[26],[29],[33]. The summary of findings is as follows:
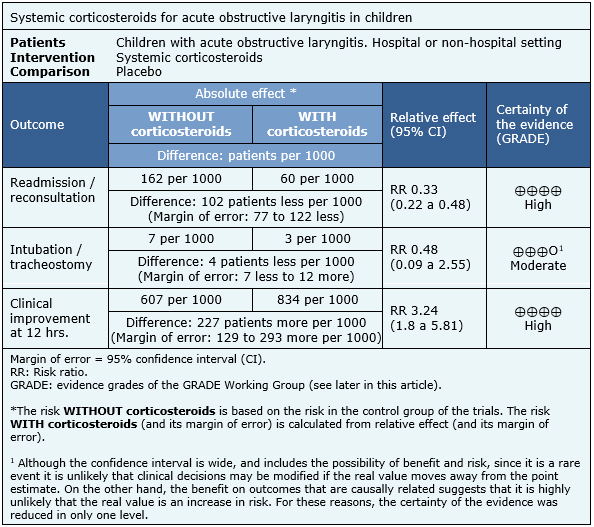
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
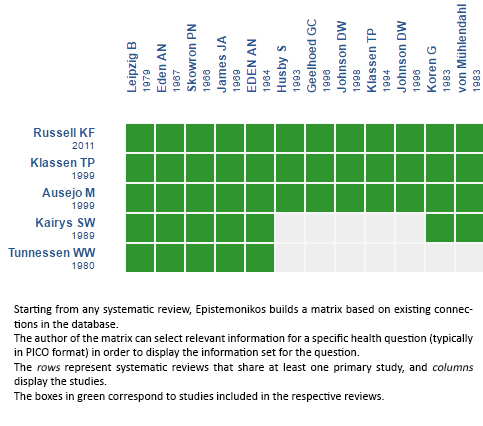
Follow the link to access the interactive version: Systemic corticosteroids versus placebo for croup
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrices and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Systemic corticosteroids constitute standard treatment in children with acute obstructive laryngitis (croup). However, there is some uncertainty in relation with the magnitude of the benefits and risks associated with their use. To answer this question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We identified six systematic reviews including 25 randomized trials relevant for the question of interest. We extracted data from the systematic reviews, reananalysed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach. We concluded the use of systemic corticosteroids increases the number of patients with clinical improvement at 12 hours and reduces the risk of readmission.
 Autores:
Elizabeth Muñoz-Osores [1,2], Deidyland Arenas[1,2]
Autores:
Elizabeth Muñoz-Osores [1,2], Deidyland Arenas[1,2]

Citación: Muñoz-Osores E, Arenas . What is the effectiveness of systemic corticosteroids in children with croup?. Medwave 2017;17(Suppl2):e6965 doi: 10.5867/medwave.2017.6965
Fecha de envío: 16/5/2017
Fecha de aceptación: 27/5/2017
Fecha de publicación: 6/6/2017

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Bustos M, Guzman M, Galeno C. Laringitis aguda obstructiva o crup viral. Rev Hosp Clín Univ Chile 2013; 25: 253 – 7 | Link |
Bustos M, Guzman M, Galeno C. Laringitis aguda obstructiva o crup viral. Rev Hosp Clín Univ Chile 2013; 25: 253 – 7 | Link | Ministerio de Salud. Guía Clínica Infección Respiratoria Aguda Baja de Manejo Ambulatorio en Menores de 5 años. MINSAL, 2013. | Link |
Ministerio de Salud. Guía Clínica Infección Respiratoria Aguda Baja de Manejo Ambulatorio en Menores de 5 años. MINSAL, 2013. | Link | NSW Kids and Families. Basic clinical practice guidelines for the acute treatment of infants and children with croup. 2010. | Link |
NSW Kids and Families. Basic clinical practice guidelines for the acute treatment of infants and children with croup. 2010. | Link | Russell KF, Liang Y, O'Gorman K, Johnson DW, Klassen TP. Glucocorticoids for croup. Cochrane Database Syst Rev. 2011 Jan 19;(1):CD001955 | CrossRef | PubMed |
Russell KF, Liang Y, O'Gorman K, Johnson DW, Klassen TP. Glucocorticoids for croup. Cochrane Database Syst Rev. 2011 Jan 19;(1):CD001955 | CrossRef | PubMed | Griffin S, Ellis S, Fitzgerald-Barron A, Rose J, Egger M. Nebulised steroid in the treatment of croup: a systematic review of randomised controlled trials. Br J Gen Pract. 2000 Feb;50(451):135-41 | PubMed |
Griffin S, Ellis S, Fitzgerald-Barron A, Rose J, Egger M. Nebulised steroid in the treatment of croup: a systematic review of randomised controlled trials. Br J Gen Pract. 2000 Feb;50(451):135-41 | PubMed | Ausejo M, Saenz A, Pham B, Kellner JD, Johnson DW, Moher D, et al. The effectiveness of glucocorticoids in treating croup: meta-analysis. BMJ. 1999 Sep 4;319(7210):595-600 | PubMed |
Ausejo M, Saenz A, Pham B, Kellner JD, Johnson DW, Moher D, et al. The effectiveness of glucocorticoids in treating croup: meta-analysis. BMJ. 1999 Sep 4;319(7210):595-600 | PubMed | Tunnessen WW Jr, Feinstein AR. The steroid-croup controversy: an analytic review of methodologic problems. J Pediatr. 1980 Apr;96(4):751-6 | PubMed |
Tunnessen WW Jr, Feinstein AR. The steroid-croup controversy: an analytic review of methodologic problems. J Pediatr. 1980 Apr;96(4):751-6 | PubMed | Kairys SW, Olmstead EM, O'Connor GT. Steroid treatment of laryngotracheitis: a meta-analysis of the evidence from randomized trials. Pediatrics. 1989 May;83(5):683-93 | PubMed |
Kairys SW, Olmstead EM, O'Connor GT. Steroid treatment of laryngotracheitis: a meta-analysis of the evidence from randomized trials. Pediatrics. 1989 May;83(5):683-93 | PubMed | Husby S, Agertoft L, Mortensen S, Pedersen S. Treatment of croup with nebulised steroid (budesonide): a double blind, placebo controlled study. Arch Dis Child. 1993 Mar;68(3):352-5 | PubMed |
Husby S, Agertoft L, Mortensen S, Pedersen S. Treatment of croup with nebulised steroid (budesonide): a double blind, placebo controlled study. Arch Dis Child. 1993 Mar;68(3):352-5 | PubMed | Godden CW, Campbell MJ, Hussey M, Cogswell JJ. Double blind placebo controlled trial of nebulised budesonide for croup. Arch Dis Child. 1997 Feb;76(2):155-8 | PubMed |
Godden CW, Campbell MJ, Hussey M, Cogswell JJ. Double blind placebo controlled trial of nebulised budesonide for croup. Arch Dis Child. 1997 Feb;76(2):155-8 | PubMed | Bjornson CL, Klassen TP, Williamson J, Brant R, Mitton C, Plint A, et al. A randomized trial of a single dose of oral dexamethasone for mild croup. N Engl J Med. 2004 Sep 23;351(13):1306-13 | PubMed |
Bjornson CL, Klassen TP, Williamson J, Brant R, Mitton C, Plint A, et al. A randomized trial of a single dose of oral dexamethasone for mild croup. N Engl J Med. 2004 Sep 23;351(13):1306-13 | PubMed | Tibballs J, Shann FA, Landau LI. Placebo-controlled trial of prednisolone in children intubated for croup. Lancet. 1992 Sep 26;340(8822):745-8 | PubMed |
Tibballs J, Shann FA, Landau LI. Placebo-controlled trial of prednisolone in children intubated for croup. Lancet. 1992 Sep 26;340(8822):745-8 | PubMed | Klassen TP, Feldman ME, Watters LK, Sutcliffe T, Rowe PC. Nebulized budesonide for children with mild-to-moderate croup. N Engl J Med. 1994 Aug 4;331(5):285-9 | PubMed |
Klassen TP, Feldman ME, Watters LK, Sutcliffe T, Rowe PC. Nebulized budesonide for children with mild-to-moderate croup. N Engl J Med. 1994 Aug 4;331(5):285-9 | PubMed | Luria JW, Gonzalez-del-Rey JA, DiGiulio GA, McAneney CM, Olson JJ, Ruddy RM. Effectiveness of oral or nebulized dexamethasone for children with mild croup. Arch Pediatr Adolesc Med. 2001 Dec;155(12):1340-5 | PubMed |
Luria JW, Gonzalez-del-Rey JA, DiGiulio GA, McAneney CM, Olson JJ, Ruddy RM. Effectiveness of oral or nebulized dexamethasone for children with mild croup. Arch Pediatr Adolesc Med. 2001 Dec;155(12):1340-5 | PubMed | Koren G, Frand M, Barzilay Z, MacLeod SM. Corticosteroid treatment of laryngotracheitis v spasmodic croup in children. Am J Dis Child. 1983 Oct;137(10):941-4 | PubMed |
Koren G, Frand M, Barzilay Z, MacLeod SM. Corticosteroid treatment of laryngotracheitis v spasmodic croup in children. Am J Dis Child. 1983 Oct;137(10):941-4 | PubMed | Martensson B, Nilsson G, Torbjar JE. The effect of corticosteroids in the treatment of pseudo-croup. Acta Otolaryngol Suppl. 1960;158:62-9 | PubMed |
Martensson B, Nilsson G, Torbjar JE. The effect of corticosteroids in the treatment of pseudo-croup. Acta Otolaryngol Suppl. 1960;158:62-9 | PubMed | Leipzig B, Oski FA, Cummings CW, Stockman JA, Swender P. A prospective randomized study to determine the efficacy of steroids in treatment of croup. J Pediatr. 1979 Feb;94(2):194-6 | PubMed |
Leipzig B, Oski FA, Cummings CW, Stockman JA, Swender P. A prospective randomized study to determine the efficacy of steroids in treatment of croup. J Pediatr. 1979 Feb;94(2):194-6 | PubMed | von Mühlendahl KE, Kahn D, Spohr HL, Dressler F. Steroid treatment of pseudo-croup. Helv Paediatr Acta. 1982;37(5):431-6 | PubMed |
von Mühlendahl KE, Kahn D, Spohr HL, Dressler F. Steroid treatment of pseudo-croup. Helv Paediatr Acta. 1982;37(5):431-6 | PubMed | Johnson DW, Schuh S, Koren G, Jaffee DM. Outpatient treatment of croup with nebulized dexamethasone. Arch Pediatr Adolesc Med. 1996 Apr;150(4):349-55 | PubMed |
Johnson DW, Schuh S, Koren G, Jaffee DM. Outpatient treatment of croup with nebulized dexamethasone. Arch Pediatr Adolesc Med. 1996 Apr;150(4):349-55 | PubMed | Johnson DW, Jacobson S, Edney PC, Hadfield P, Mundy ME, Schuh S. A comparison of nebulized budesonide, intramuscular dexamethasone, and placebo for moderately severe croup. N Engl J Med. 1998 Aug 20;339(8):498-503 | PubMed |
Johnson DW, Jacobson S, Edney PC, Hadfield P, Mundy ME, Schuh S. A comparison of nebulized budesonide, intramuscular dexamethasone, and placebo for moderately severe croup. N Engl J Med. 1998 Aug 20;339(8):498-503 | PubMed | James JA. Dexamethasone in croup. A controlled study. Am J Dis Child. 1969 May;117(5):511-6. | PubMed |
James JA. Dexamethasone in croup. A controlled study. Am J Dis Child. 1969 May;117(5):511-6. | PubMed | Martínez Fernández A, Sánchez González E, Rica Etxebarría I, Echaniz Urcelay I, Alonso Díez M, Vilella Ciriza M, et all. [Randomized double-blind study of treatment of croup with adrenaline and/or dexamethasone in children]. An Esp Pediatr. 1993 Jan;38(1):29-32 | PubMed |
Martínez Fernández A, Sánchez González E, Rica Etxebarría I, Echaniz Urcelay I, Alonso Díez M, Vilella Ciriza M, et all. [Randomized double-blind study of treatment of croup with adrenaline and/or dexamethasone in children]. An Esp Pediatr. 1993 Jan;38(1):29-32 | PubMed | Cruz MN, Stewart G, Rosenberg N. Use of dexamethasone in the outpatient management of acute laryngotracheitis. Pediatrics. 1995 Aug;96(2 Pt 1):220-3 | PubMed |
Cruz MN, Stewart G, Rosenberg N. Use of dexamethasone in the outpatient management of acute laryngotracheitis. Pediatrics. 1995 Aug;96(2 Pt 1):220-3 | PubMed | Massicotte P, Tétreault L. [Evaluation of methyl-prednisolone in the treatment of acute laryngitis in children]. Union Med Can. 1973 Oct;102(10):2064-72. | PubMed |
Massicotte P, Tétreault L. [Evaluation of methyl-prednisolone in the treatment of acute laryngitis in children]. Union Med Can. 1973 Oct;102(10):2064-72. | PubMed | Roberts GW, Master VV, Staugas RE, Raftos JV, Parsons DW, Coulthard KP, et al. Repeated dose inhaled budesonide versus placebo in the treatment of croup. J Paediatr Child Health. 1999 Apr;35(2):170-4 | PubMed |
Roberts GW, Master VV, Staugas RE, Raftos JV, Parsons DW, Coulthard KP, et al. Repeated dose inhaled budesonide versus placebo in the treatment of croup. J Paediatr Child Health. 1999 Apr;35(2):170-4 | PubMed | Skowron PN, Turner JA, McNaughton GA. The use of corticosteroid (dexamethasone) in the treatment of acute laryngotracheitis. Can Med Assoc J. 1966 Mar 12;94(11):528-31 | PubMed |
Skowron PN, Turner JA, McNaughton GA. The use of corticosteroid (dexamethasone) in the treatment of acute laryngotracheitis. Can Med Assoc J. 1966 Mar 12;94(11):528-31 | PubMed | Eden AN, Kaufman A, Yu R. Corticosteroids and croup. Controlled double-blind study. JAMA. 1967 May 1;200(5):403-4 | PubMed |
Eden AN, Kaufman A, Yu R. Corticosteroids and croup. Controlled double-blind study. JAMA. 1967 May 1;200(5):403-4 | PubMed | Sussman S, Grossman M, Magoffin R, Schieble J. Dexamethasone (16-alpha-methyl, 9-alpha-fluoroprednisolone) in obstructive respiratory tract infections in children. A controlled study. Pediatrics. 1964 Dec;34:851-5 | PubMed |
Sussman S, Grossman M, Magoffin R, Schieble J. Dexamethasone (16-alpha-methyl, 9-alpha-fluoroprednisolone) in obstructive respiratory tract infections in children. A controlled study. Pediatrics. 1964 Dec;34:851-5 | PubMed | Geelhoed GC, Turner J, Macdonald WB. Efficacy of a small single dose of oral dexamethasone for outpatient croup: a double blind placebo controlled clinical trial. BMJ. 1996 Jul 20;313(7050):140-2 | PubMed |
Geelhoed GC, Turner J, Macdonald WB. Efficacy of a small single dose of oral dexamethasone for outpatient croup: a double blind placebo controlled clinical trial. BMJ. 1996 Jul 20;313(7050):140-2 | PubMed | Geelhoed GC, Macdonald WB. Oral and inhaled steroids in croup: a randomized, placebo-controlled trial. Pediatr Pulmonol. 1995 Dec;20(6):355-61 | PubMed |
Geelhoed GC, Macdonald WB. Oral and inhaled steroids in croup: a randomized, placebo-controlled trial. Pediatr Pulmonol. 1995 Dec;20(6):355-61 | PubMed | Super DM, Cartelli NA, Brooks LJ, Lembo RM, Kumar ML. A prospective randomized double-blind study to evaluate the effect of dexamethasone in acute laryngotracheitis. J Pediatr. 1989 Aug;115(2):323-9 | PubMed |
Super DM, Cartelli NA, Brooks LJ, Lembo RM, Kumar ML. A prospective randomized double-blind study to evaluate the effect of dexamethasone in acute laryngotracheitis. J Pediatr. 1989 Aug;115(2):323-9 | PubMed | Novik A. Corticosteroid treatment of non-diphtheritic croup. Acta Otolaryngol Suppl. 1960;158:20-2 | PubMed |
Novik A. Corticosteroid treatment of non-diphtheritic croup. Acta Otolaryngol Suppl. 1960;158:20-2 | PubMed |