 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Idiopathic pulmonary fibrosis has poor prognosis and effective therapies are scarce. In the search for treatments that can modify the course of the disease, nintedanib (BIBF 1120), a tyrosine kinase inhibitor, has emerged as an alternative. However, its role is still unclear. To answer this question, we searched in Epistemonikos database, which is maintained by screening multiple sources of information. We identified seven systematic reviews including seven randomized trials overall. We extracted data, conducted a meta-analysis and generated a summary of findings table using the GRADE approach. We concluded nintedanib probably decreases the risk of acute exacerbations, and might reduce mortality in idiopathic pulmonary fibrosis. On the other hand, it is probably not associated with serious adverse events.
Idiopathic pulmonary fibrosis is a rare condition of unknown etiology [1]. The prognosis of this disease is poor and until the last decade, there were no interventions with a proven benefit on survival [2]. After diagnosis, survival decreases rapidly and factors such as acute exacerbations, time to disease progression and deterioration in respiratory functions are associated with a poorer prognosis [3],[4].
Nintedanib (BIBF 1120) is an inhibitor of multiple tyrosine kinase related signals through its binding to growth factor receptors. Among the most important are vascular endothelial growth factor receptor, fibroblast growth factor receptor and platelet-derived growth factor receptor [5]. An initial trial reported efficacy of this drug [6], which was later replicated, so the FDA approved this drug for idiopathic pulmonary fibrosis in 2015 [7],[8].
We used Epistemonikos database, which is maintained by screening multiple databases, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
|
What is the evidence. |
We found seven systematic reviews, reported in eight references [9],[10],[11],[12],[13],[14],[15],[16] including seven randomized controlled trials reported in 10 references [6],[7],[17],[18],[19],[20],[21],[22],[23],[24]. |
|
What types of patients were included |
Three trials included patients diagnosed with idiopathic pulmonary fibrosis according to ATS / ERS 2011 criteria [6],[18],[19]. All of the trials included patients over 40 years of age. One trial included patients with PaO2 ≥55 mmHg at rest and room air, patients diagnosed with idiopathic pulmonary fibrosis within 5 years prior to enrollment and patients with high resolution computed tomography performed less than 1 year prior to enrollment [6]. Four trials included patients with functional vital capacity greater than or equal to 50% [6],[18],[19],[20]. Three trials included patients with a test of carbon monoxide diffusion capacity between 30% and 79% [6],[18],[19]. |
|
What types of interventions were included |
All of the trials used nintedanib as monotherapy at a dose of 300 mg daily. Three trials used an increasing dose of nintedanib until reaching the target dose of 300 mg daily [6],[20],[21]. All trials compared against placebo or standard treatment. |
|
What types of outcomes |
The different systematic reviews identified grouped the outcomes as follows:
|
The information on the effects of nintedanib is based on three randomized trials [6],[18],[19] which included 1231 patients. The other four trials were not used in this analysis because they did not report enough data to be incorporated into a meta-analysis. All trials measured mortality, acute exacerbations, and serious adverse events. The summary of findings is as follows:
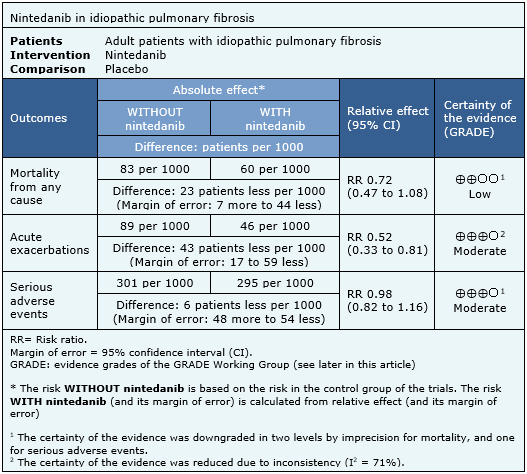

|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
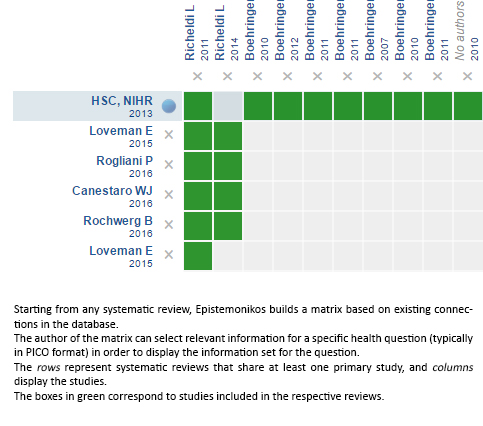
Follow the link to access the interactive version: Nintedanib for idiopathic pulmonary fibrosis
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Idiopathic pulmonary fibrosis has poor prognosis and effective therapies are scarce. In the search for treatments that can modify the course of the disease, nintedanib (BIBF 1120), a tyrosine kinase inhibitor, has emerged as an alternative. However, its role is still unclear. To answer this question, we searched in Epistemonikos database, which is maintained by screening multiple sources of information. We identified seven systematic reviews including seven randomized trials overall. We extracted data, conducted a meta-analysis and generated a summary of findings table using the GRADE approach. We concluded nintedanib probably decreases the risk of acute exacerbations, and might reduce mortality in idiopathic pulmonary fibrosis. On the other hand, it is probably not associated with serious adverse events.
 Autores:
Alejandro Jeldres[1,2], Gonzalo Labarca[2,3,4,5]
Autores:
Alejandro Jeldres[1,2], Gonzalo Labarca[2,3,4,5]

Citación: Jeldres A, Labarca G. Is nintedanib effective for idiopathic pulmonary fibrosis?. Medwave2017;17(Suppl2):e6918 doi: 10.5867/medwave.2017.6918
Fecha de publicación: 10/4/2017

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011 Mar 15;183(6):788-824 | CrossRef | PubMed |
Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011 Mar 15;183(6):788-824 | CrossRef | PubMed | Spagnolo P, Del Giovane C, Luppi F, Cerri S, Balduzzi S, Walters EH, et al. Non-steroid agents for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev. 2010 Sep 8;(9):CD003134 | CrossRef | PubMed |
Spagnolo P, Del Giovane C, Luppi F, Cerri S, Balduzzi S, Walters EH, et al. Non-steroid agents for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev. 2010 Sep 8;(9):CD003134 | CrossRef | PubMed | Vancheri C, Failla M, Crimi N, Raghu G. Idiopathic pulmonary fibrosis: a disease with similarities and links to cancer biology. Eur Respir J. 2010 Mar;35(3):496-504 | CrossRef | PubMed |
Vancheri C, Failla M, Crimi N, Raghu G. Idiopathic pulmonary fibrosis: a disease with similarities and links to cancer biology. Eur Respir J. 2010 Mar;35(3):496-504 | CrossRef | PubMed | Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006 Oct 1;174(7):810-6 | PubMed |
Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006 Oct 1;174(7):810-6 | PubMed | Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001 Jan 16;134(2):136-51 | PubMed |
Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001 Jan 16;134(2):136-51 | PubMed | Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011 Sep 22;365(12):1079-87 | CrossRef | PubMed |
Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011 Sep 22;365(12):1079-87 | CrossRef | PubMed | Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014 May 29;370(22):2071-82 | CrossRef | PubMed |
Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014 May 29;370(22):2071-82 | CrossRef | PubMed | Fda.gov [Online] Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205832Orig1s000Lbl.pdf. [Accessed 28 November 2016]. | Link |
Fda.gov [Online] Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205832Orig1s000Lbl.pdf. [Accessed 28 November 2016]. | Link | Atkins CP, Loke YK, Wilson AM. Outcomes in idiopathic pulmonary fibrosis: a meta-analysis from placebo controlled trials. Respir Med. 2014 Feb;108(2):376-87 | CrossRef | PubMed |
Atkins CP, Loke YK, Wilson AM. Outcomes in idiopathic pulmonary fibrosis: a meta-analysis from placebo controlled trials. Respir Med. 2014 Feb;108(2):376-87 | CrossRef | PubMed | Canestaro WJ, Forrester SH, Raghu G, Ho L, Devine BE. Drug Treatment of Idiopathic Pulmonary Fibrosis: Systematic Review and Network Meta-Analysis. Chest. 2016 Mar;149(3):756-66 | CrossRef | PubMed |
Canestaro WJ, Forrester SH, Raghu G, Ho L, Devine BE. Drug Treatment of Idiopathic Pulmonary Fibrosis: Systematic Review and Network Meta-Analysis. Chest. 2016 Mar;149(3):756-66 | CrossRef | PubMed | Loveman E, Copley VR, Colquitt JL, Scott DA, Clegg AJ, Jones J, et al. The effectiveness and cost-effectiveness of treatments for idiopathic pulmonary fibrosis: systematic review, network meta-analysis and health economic evaluation. BMC Pharmacol Toxicol. 2014 Nov 19;15:63 | CrossRef | PubMed |
Loveman E, Copley VR, Colquitt JL, Scott DA, Clegg AJ, Jones J, et al. The effectiveness and cost-effectiveness of treatments for idiopathic pulmonary fibrosis: systematic review, network meta-analysis and health economic evaluation. BMC Pharmacol Toxicol. 2014 Nov 19;15:63 | CrossRef | PubMed | Loveman E, Copley VR, Colquitt J, Scott DA, Clegg A, Jones J, et al. The clinical effectiveness and cost-effectiveness of treatments for idiopathic pulmonary fibrosis: a systematic review and economic evaluation. Health Technol Assess. 2015 Mar;19(20):i-xxiv, 1-336 | CrossRef | PubMed |
Loveman E, Copley VR, Colquitt J, Scott DA, Clegg A, Jones J, et al. The clinical effectiveness and cost-effectiveness of treatments for idiopathic pulmonary fibrosis: a systematic review and economic evaluation. Health Technol Assess. 2015 Mar;19(20):i-xxiv, 1-336 | CrossRef | PubMed | Loveman E, Copley VR, Scott DA, Colquitt JL, Clegg AJ, O'Reilly KM. Comparing new treatments for idiopathic pulmonary fibrosis--a network meta-analysis. BMC Pulm Med. 2015 Apr 18;15:37 | CrossRef | PubMed |
Loveman E, Copley VR, Scott DA, Colquitt JL, Clegg AJ, O'Reilly KM. Comparing new treatments for idiopathic pulmonary fibrosis--a network meta-analysis. BMC Pulm Med. 2015 Apr 18;15:37 | CrossRef | PubMed | Nintedanib for idiopathic pulmonary fibrosis – first line. Birmingham: NIHR Horizon Scanning Centre (NIHR HSC). Horizon Scanning Review. 2013 | Link |
Nintedanib for idiopathic pulmonary fibrosis – first line. Birmingham: NIHR Horizon Scanning Centre (NIHR HSC). Horizon Scanning Review. 2013 | Link | Rochwerg B, Neupane B, Zhang Y, Garcia CC, Raghu G, Richeldi L, et al. Treatment of idiopathic pulmonary fibrosis: a network meta-analysis. BMC Med. 2016 Feb 3;14:18 | CrossRef | PubMed |
Rochwerg B, Neupane B, Zhang Y, Garcia CC, Raghu G, Richeldi L, et al. Treatment of idiopathic pulmonary fibrosis: a network meta-analysis. BMC Med. 2016 Feb 3;14:18 | CrossRef | PubMed | Rogliani P, Calzetta L, Cavalli F, Matera MG, Cazzola M. Pirfenidone, nintedanib and N-acetylcysteine for the treatment of idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Pulm Pharmacol Ther. 2016 Oct;40:95-103 | CrossRef | PubMed |
Rogliani P, Calzetta L, Cavalli F, Matera MG, Cazzola M. Pirfenidone, nintedanib and N-acetylcysteine for the treatment of idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Pulm Pharmacol Ther. 2016 Oct;40:95-103 | CrossRef | PubMed | ClinicalTrials.gov. Safety and efficacy of BIBF 1120 in idiopathic pulmonary fibrosis. [Online] Available from: http://www.clinicaltrials.gov/ct2/show/NCT00514683. | Link |
ClinicalTrials.gov. Safety and efficacy of BIBF 1120 in idiopathic pulmonary fibrosis. [Online] Available from: http://www.clinicaltrials.gov/ct2/show/NCT00514683. | Link | ClinicalTrials.gov. Safety and efficacy of BIBF 1120 at high dose in idiopathic pulmonary fibrosis patients. [Online] Available from: http://www.clinicaltrials.gov/ct2/show/record/NCT01335464. | Link |
ClinicalTrials.gov. Safety and efficacy of BIBF 1120 at high dose in idiopathic pulmonary fibrosis patients. [Online] Available from: http://www.clinicaltrials.gov/ct2/show/record/NCT01335464. | Link | ClinicalTrials.gov. Safety and Efficacy of BIBF 1120 at high dose in idiopathic pulmonary fibrosis patients II. [Online] Available from: http://www.clinicaltrials.gov/ct2/show/NCT01335477. | Link |
ClinicalTrials.gov. Safety and Efficacy of BIBF 1120 at high dose in idiopathic pulmonary fibrosis patients II. [Online] Available from: http://www.clinicaltrials.gov/ct2/show/NCT01335477. | Link | ClinicalTrials.gov. Safety and PK study of BIBF 1120 in Japanese patients with IPF. [Online] Available from: http://www.clinicaltrials.gov/ct2/show/NCT01136174. | Link |
ClinicalTrials.gov. Safety and PK study of BIBF 1120 in Japanese patients with IPF. [Online] Available from: http://www.clinicaltrials.gov/ct2/show/NCT01136174. | Link | ClinicalTrials.gov. Roll over study from 1199.30 BIBF 1120 in idiopathic pulmonary fibrosis (IPF). [Online] Available from: http://www.clinicaltrials.gov/ct2/show/NCT01170065. | Link |
ClinicalTrials.gov. Roll over study from 1199.30 BIBF 1120 in idiopathic pulmonary fibrosis (IPF). [Online] Available from: http://www.clinicaltrials.gov/ct2/show/NCT01170065. | Link | ClinicalTrials.gov. Follow up Study From 1199.31(NCT01136174). [Online] Available from: http://www.clinicaltrials.gov/ct2/show/NCT01417156. | Link |
ClinicalTrials.gov. Follow up Study From 1199.31(NCT01136174). [Online] Available from: http://www.clinicaltrials.gov/ct2/show/NCT01417156. | Link | ClinicalTrials.gov. extension trial of the long term safety of BIBF 1120 in patients with idiopathic pulmonary fibrosis. [Online] Available from: http://www.clinicaltrials.gov/ct2/show/NCT01619085. | Link |
ClinicalTrials.gov. extension trial of the long term safety of BIBF 1120 in patients with idiopathic pulmonary fibrosis. [Online] Available from: http://www.clinicaltrials.gov/ct2/show/NCT01619085. | Link | EU Clinical Trials Register. A phase II open label, roll over study of the long term tolerability, safety and efficacy of oral BIBF 1120 in patients with Idiopathic Pulmonary Fibrosis.
https://www.clinicaltrialsregister.eu/ctr-search/trial/2009-013788-21/BE.
| Link |
EU Clinical Trials Register. A phase II open label, roll over study of the long term tolerability, safety and efficacy of oral BIBF 1120 in patients with Idiopathic Pulmonary Fibrosis.
https://www.clinicaltrialsregister.eu/ctr-search/trial/2009-013788-21/BE.
| Link |