 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Idiopathic pulmonary fibrosis is a progressive chronic respiratory disease that in final stages carries high mortality. Several treatment options have been proposed, including N-acetylcysteine, but its role is not clearly established. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified eight systematic reviews including 16 trials addressing the question of this article. We combined the evidence using meta-analysis and generated a summary of findings following the GRADE approach. We concluded N-acetylcysteine might increase the risk of hospitalizations and exacerbations. While it is unclear whether this leads to increased mortality because the certainty of the evidence is very low, in general there is consensus that it should not be used except in the context of a new clinical trial.
A substantial amount of evidence has appeared in the last years regarding different therapies potentially effective for idiopathic pulmonary fibrosis. This is not surprising since the only intervention that clearly increases survival is lung transplantation [1].
N-acetylcysteine, by its reducing character, exerts a cytoprotective activity in the human respiratory tract, acting against the harmful action of oxidative stress generated by free radicals of diverse etiology. Based on its structure derived from cysteine, N-acetylcysteine has a precursor role in the synthesis of the antioxidant molecule glutathione and normalizes its levels when they are reduced by a continuous oxidizing action on the respiratory system. This mechanism would explain a potential benefit in idiopathic pulmonary fibrosis [2].
In addition, N-acetylcysteine is widely available, and according to the ATS/ERS 2011 guideline, when used alone or in combination (with prednisolone and azathioprine) was a reasonable choice in a minority of patients with this disease [1]. However, an update of the same guideline in 2015 proposed banning its use due to an increased risk of hospitalization and death in a pivotal trial [3].
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
|
What is the evidence. |
We found eight systematic reviews published in nine references [4],[5],[6],[7],[8],[9],[10],[11],[12] including 16 randomized controlled trials published in 18 references [13],[14],[15],[16],[17],[18],[19],[20],[21],[22],[23],[24], [25],[26],[27],[28],[29],[30]. |
|
What types of patients were included |
All of the trials included patients over 18 years. Another trial also required markers of lung injury [14] and in one trial diagnosis was only based on images and biopsy [18]. |
|
What types of interventions were included |
Two trials used oral N-acetylcysteine monotherapy [15], [17], one trial used inhaled monotherapy [14], ten trials used a combination with prednisone 0,4 to 0,5 mg/kg/day [13],[20],[21],[23],[24],[25],[26],[27],[30], one trial used it associated with interferon [22], one study refers it associated N-acetylcysteine to antiinflammatory treatment but does not specifies which one [29], one trial administered it associated to prednisolone and azathioprine [18], and another trial that used N-acetylcysteine associated to prednisolone and azathioprine was interrupted early due to an increase in mortality risk [16]. |
|
What types of outcomes |
The systematic reviews identified pooled outcomes as follows:
|
The information on the effects of N-acetylcysteine is based on four randomized trials [14],[15],[16],[18] including 694 patients. The rest of the trials did not report the outcomes of interest, or did not present data in a format suitable for meta-analysis. Four trials [14],[15],[16],[18] measured the outcome mortality, and only one trial [16] reported hospitalizations and acute exacerbations. The summary of findings is as follows:
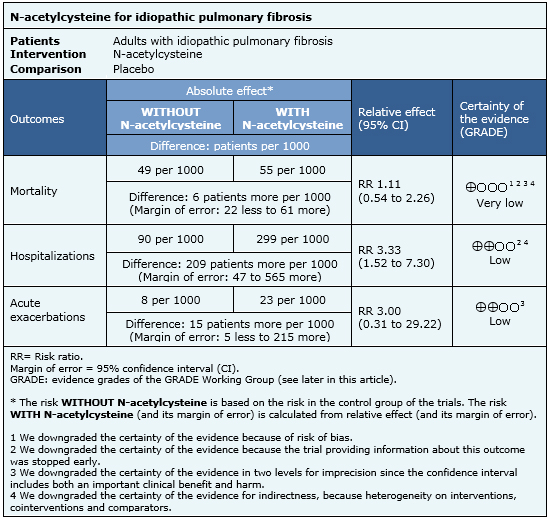

|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
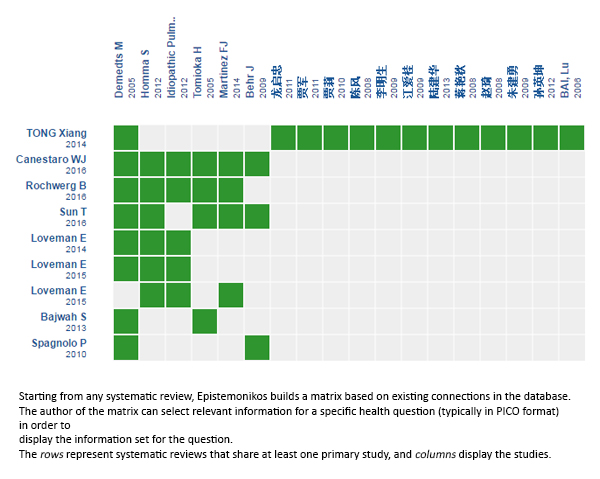
Follow the link to access the interactive version: N-acetylcysteine for idiopathic pulmonary fibrosis
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Idiopathic pulmonary fibrosis is a progressive chronic respiratory disease that in final stages carries high mortality. Several treatment options have been proposed, including N-acetylcysteine, but its role is not clearly established. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified eight systematic reviews including 16 trials addressing the question of this article. We combined the evidence using meta-analysis and generated a summary of findings following the GRADE approach. We concluded N-acetylcysteine might increase the risk of hospitalizations and exacerbations. While it is unclear whether this leads to increased mortality because the certainty of the evidence is very low, in general there is consensus that it should not be used except in the context of a new clinical trial.
 Autores:
Alejandro Jeldres Pulgar[1,2], Gonzalo Labarca[2,3,4]
Autores:
Alejandro Jeldres Pulgar[1,2], Gonzalo Labarca[2,3,4]

Citación: Jeldres Pulgar A, Labarca G. Is N-acetylcysteine effective in the treatment of pulmonary fibrosis?. Medwave 2016;16(Suppl 3):e6555 doi: 10.5867/medwave.2016.6555
Fecha de publicación: 29/9/2016

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011 Mar 15;183(6):788-824.
| CrossRef | PubMed |
Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011 Mar 15;183(6):788-824.
| CrossRef | PubMed | National Institute for Health and Care Excellence. Diagnosis and Management of Suspected Idiopathic Pulmonary Fibrosis. Clinical guideline CG163. London: NICE; 2013.
National Institute for Health and Care Excellence. Diagnosis and Management of Suspected Idiopathic Pulmonary Fibrosis. Clinical guideline CG163. London: NICE; 2013.  Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med. 2015 Jul 15;192(2):e3-19. | CrossRef | PubMed |
Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med. 2015 Jul 15;192(2):e3-19. | CrossRef | PubMed | Rochwerg B, Neupane B, Zhang Y, Garcia CC, Raghu G, Richeldi L, et alH. Treatment of idiopathic pulmonary fibrosis: a network meta-analysis. BMC Med. 2016 Feb 3;14:18. | CrossRef | PubMed |
Rochwerg B, Neupane B, Zhang Y, Garcia CC, Raghu G, Richeldi L, et alH. Treatment of idiopathic pulmonary fibrosis: a network meta-analysis. BMC Med. 2016 Feb 3;14:18. | CrossRef | PubMed | Loveman E, Copley VR, Colquitt JL, Scott DA, Clegg AJ, Jones J, et al. The effectiveness and cost-effectiveness of treatments for idiopathic pulmonary fibrosis: systematic review, network meta-analysis and health economic evaluation. BMC Pharmacol Toxicol. 2014 Nov 19;15:63. | CrossRef | PubMed |
Loveman E, Copley VR, Colquitt JL, Scott DA, Clegg AJ, Jones J, et al. The effectiveness and cost-effectiveness of treatments for idiopathic pulmonary fibrosis: systematic review, network meta-analysis and health economic evaluation. BMC Pharmacol Toxicol. 2014 Nov 19;15:63. | CrossRef | PubMed | Loveman E, Copley VR, Colquitt J, Scott DA, Clegg A, Jones J, et al. The clinical effectiveness and cost-effectiveness of treatments for idiopathic pulmonary fibrosis: a systematic review and economic evaluation. Health Technol Assess. 2015 Mar;19(20):i-xxiv, 1-336. | CrossRef | PubMed |
Loveman E, Copley VR, Colquitt J, Scott DA, Clegg A, Jones J, et al. The clinical effectiveness and cost-effectiveness of treatments for idiopathic pulmonary fibrosis: a systematic review and economic evaluation. Health Technol Assess. 2015 Mar;19(20):i-xxiv, 1-336. | CrossRef | PubMed | Bajwah S, Ross JR, Peacock JL, Higginson IJ, Wells AU, Patel AS, et al. Interventions to improve symptoms and quality of life of patients with fibrotic interstitial lung disease: a systematic review of the literature. Thorax. 2013 Sep;68(9):867-79. | CrossRef | PubMed |
Bajwah S, Ross JR, Peacock JL, Higginson IJ, Wells AU, Patel AS, et al. Interventions to improve symptoms and quality of life of patients with fibrotic interstitial lung disease: a systematic review of the literature. Thorax. 2013 Sep;68(9):867-79. | CrossRef | PubMed | Fu XW, Tong X, Liu ST, Fan H. Efficacy of N-acetylcysteine for patients with idiopathic pulmonary fibrosis: a meta-analysis. Chinese Journal of Evidence-Based Medicine 2014; 14(4): 449-455. | CrossRef |
Fu XW, Tong X, Liu ST, Fan H. Efficacy of N-acetylcysteine for patients with idiopathic pulmonary fibrosis: a meta-analysis. Chinese Journal of Evidence-Based Medicine 2014; 14(4): 449-455. | CrossRef | Spagnolo P, Del Giovane C, Luppi F, Cerri S, Balduzzi S, Walters EH, et al. Non-steroid agents for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev. 2010 Sep 8;(9):CD003134. | CrossRef | PubMed |
Spagnolo P, Del Giovane C, Luppi F, Cerri S, Balduzzi S, Walters EH, et al. Non-steroid agents for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev. 2010 Sep 8;(9):CD003134. | CrossRef | PubMed | Loveman E, Copley VR, Scott DA, Colquitt JL, Clegg AJ, O'Reilly KM. Comparing new treatments for idiopathic pulmonary fibrosis--a network meta-analysis. BMC Pulm Med. 2015 Apr 18;15:37.
| CrossRef | PubMed |
Loveman E, Copley VR, Scott DA, Colquitt JL, Clegg AJ, O'Reilly KM. Comparing new treatments for idiopathic pulmonary fibrosis--a network meta-analysis. BMC Pulm Med. 2015 Apr 18;15:37.
| CrossRef | PubMed | Canestaro WJ, Forrester SH, Raghu G, Ho L, Devine BE. Drug Treatment of Idiopathic Pulmonary Fibrosis: Systematic Review and Network Meta-Analysis. Chest. 2016 Mar;149(3):756-66.
| CrossRef | PubMed |
Canestaro WJ, Forrester SH, Raghu G, Ho L, Devine BE. Drug Treatment of Idiopathic Pulmonary Fibrosis: Systematic Review and Network Meta-Analysis. Chest. 2016 Mar;149(3):756-66.
| CrossRef | PubMed | Sun T, Liu J, Zhao de W. Efficacy of N-Acetylcysteine in Idiopathic Pulmonary Fibrosis: A Systematic Review and Meta-Analysis. Medicine (Baltimore). 2016 May;95(19):e3629. | CrossRef | PubMed |
Sun T, Liu J, Zhao de W. Efficacy of N-Acetylcysteine in Idiopathic Pulmonary Fibrosis: A Systematic Review and Meta-Analysis. Medicine (Baltimore). 2016 May;95(19):e3629. | CrossRef | PubMed | Chen Feng, Li Songkun, Liuzhang Bo. Shi Fu Lu large doses (N- acetylcysteine) efficacy in the treatment of idiopathic pulmonary fibrosis Analysis. Chinese Journal of Medical Device). 2008;21(7):33-34. | CrossRef |
Chen Feng, Li Songkun, Liuzhang Bo. Shi Fu Lu large doses (N- acetylcysteine) efficacy in the treatment of idiopathic pulmonary fibrosis Analysis. Chinese Journal of Medical Device). 2008;21(7):33-34. | CrossRef | Homma S, Azuma A, Taniguchi H, Ogura T, Mochiduki Y, Sugiyama Y, et al. Efficacy of inhaled N-acetylcysteine monotherapy in patients with early stage idiopathic pulmonary fibrosis. Respirology. 2012 Apr;17(3):467-77. | CrossRef | PubMed |
Homma S, Azuma A, Taniguchi H, Ogura T, Mochiduki Y, Sugiyama Y, et al. Efficacy of inhaled N-acetylcysteine monotherapy in patients with early stage idiopathic pulmonary fibrosis. Respirology. 2012 Apr;17(3):467-77. | CrossRef | PubMed | Tomioka H, Kuwata Y, Imanaka K, Hashimoto K, Ohnishi H, Tada K, et al. A pilot study of aerosolized N-acetylcysteine for idiopathic pulmonary fibrosis. Respirology. 2005 Sep;10(4):449-55. | PubMed |
Tomioka H, Kuwata Y, Imanaka K, Hashimoto K, Ohnishi H, Tada K, et al. A pilot study of aerosolized N-acetylcysteine for idiopathic pulmonary fibrosis. Respirology. 2005 Sep;10(4):449-55. | PubMed | Idiopathic Pulmonary Fibrosis Clinical Research Network, Raghu G, Anstrom KJ, King TE, Lasky JA, Martinez FJ. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012 May 24;366(21):1968-77. | CrossRef | PubMed |
Idiopathic Pulmonary Fibrosis Clinical Research Network, Raghu G, Anstrom KJ, King TE, Lasky JA, Martinez FJ. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012 May 24;366(21):1968-77. | CrossRef | PubMed | Idiopathic Pulmonary Fibrosis Clinical Research Network, Martinez FJ, de Andrade JA, Anstrom KJ, King TE Jr, Raghu G. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2014 May 29;370(22):2093-101. | CrossRef | PubMed |
Idiopathic Pulmonary Fibrosis Clinical Research Network, Martinez FJ, de Andrade JA, Anstrom KJ, King TE Jr, Raghu G. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2014 May 29;370(22):2093-101. | CrossRef | PubMed | Behr J, Demedts M, Buhl R, Costabel U, Dekhuijzen RP, Jansen HM, et al. Lung function in idiopathic pulmonary fibrosis--extended analyses of the IFIGENIA trial. Respir Res. 2009 Oct 27;10:101. | CrossRef | PubMed |
Behr J, Demedts M, Buhl R, Costabel U, Dekhuijzen RP, Jansen HM, et al. Lung function in idiopathic pulmonary fibrosis--extended analyses of the IFIGENIA trial. Respir Res. 2009 Oct 27;10:101. | CrossRef | PubMed | Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005 Nov 24;353(21):2229-42. | PubMed |
Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005 Nov 24;353(21):2229-42. | PubMed | Jia Jun, Chen Bo-Jiang, Pu Qing, Wang Bo. Effects of cyclophosphamide and high-dose N-acetylcysteine in the treatment of idiopathic pulmonary fibrosis. Journal of Clinical Pulmonary Medicine. 2011;16(6). | CrossRef |
Jia Jun, Chen Bo-Jiang, Pu Qing, Wang Bo. Effects of cyclophosphamide and high-dose N-acetylcysteine in the treatment of idiopathic pulmonary fibrosis. Journal of Clinical Pulmonary Medicine. 2011;16(6). | CrossRef | Jia Li, Meng Hongxia. Idiopathic pulmonary fibrosis Clinical Shi Fu Lu combined with prednisone therapy. Medical Information. 2010;5(7):1890-1891. | CrossRef |
Jia Li, Meng Hongxia. Idiopathic pulmonary fibrosis Clinical Shi Fu Lu combined with prednisone therapy. Medical Information. 2010;5(7):1890-1891. | CrossRef | Long Qizhong, Du Juan, Zhang Xianming, Ma Wen, Gui Kun. Curative Effect of Combination of Interferon-γ, N-acetylcysteine and Low Dose Prednisone on Idiopathic Pulmonary Interstitial Fibrosis. Journal of Guiyang Medical College. 2011;36(5):465-469. | CrossRef |
Long Qizhong, Du Juan, Zhang Xianming, Ma Wen, Gui Kun. Curative Effect of Combination of Interferon-γ, N-acetylcysteine and Low Dose Prednisone on Idiopathic Pulmonary Interstitial Fibrosis. Journal of Guiyang Medical College. 2011;36(5):465-469. | CrossRef | Li Mingsheng, Luming Usted, Zheng Xiaolu, Li Jifu, Shi Liya, Chen Yongde. Effects of N-acetylcysteine TGF-β1 and IL-13 on idiopathic pulmonary fibrosis. Chinese Medicine and Pharmacology. 2009;4(9):691-693. | CrossRef |
Li Mingsheng, Luming Usted, Zheng Xiaolu, Li Jifu, Shi Liya, Chen Yongde. Effects of N-acetylcysteine TGF-β1 and IL-13 on idiopathic pulmonary fibrosis. Chinese Medicine and Pharmacology. 2009;4(9):691-693. | CrossRef | Jiang Ai-Gui, Lu Hui-Yu, Duan De-Jun. N- acetylcysteine efficacy associated with idiopathic pulmonary fibrosis was observed with methylprednisolone therapy. Journal of Clinical Medicine in Practice. 2009;(8):69-70. | Link |
Jiang Ai-Gui, Lu Hui-Yu, Duan De-Jun. N- acetylcysteine efficacy associated with idiopathic pulmonary fibrosis was observed with methylprednisolone therapy. Journal of Clinical Medicine in Practice. 2009;(8):69-70. | Link | Lu Jianhua, Gao Jingzhen. Idiopathic pulmonary fibrosis nitric oxide levels in the United acetylcysteine glucocorticoid treatment. Chinese Journal of Laboratory Diagnosis. 2013;17(9):1692-1693. | CrossRef |
Lu Jianhua, Gao Jingzhen. Idiopathic pulmonary fibrosis nitric oxide levels in the United acetylcysteine glucocorticoid treatment. Chinese Journal of Laboratory Diagnosis. 2013;17(9):1692-1693. | CrossRef | Jiang Yanqiu, Jiang Hong. Clinical analysis of 20 cases of N- acetylcysteine treatment of idiopathic pulmonary fibrosis. Chinese Community Doctor. 2008;10(15):43-44. | Link |
Jiang Yanqiu, Jiang Hong. Clinical analysis of 20 cases of N- acetylcysteine treatment of idiopathic pulmonary fibrosis. Chinese Community Doctor. 2008;10(15):43-44. | Link | Zhao Qi, Lu Fuzhen, Mao Ziyuan. The N-acetyl Cysteine Treatment Idiopathic Pulmomary Fibrosis Curative Effect Observation. Heilongjiang Medical Journal. 2008;32(1):30-31. | CrossRef |
Zhao Qi, Lu Fuzhen, Mao Ziyuan. The N-acetyl Cysteine Treatment Idiopathic Pulmomary Fibrosis Curative Effect Observation. Heilongjiang Medical Journal. 2008;32(1):30-31. | CrossRef | Zhu Jianyong, Zeng Yuqin, Yuan Liangjun, Chen Gong, Wang Yonglan, Hu Ke. Study on the Treatment of Idiopathic Pulmonary Fibrosis with Prednisone, N-acetylcysteine Combined Captopril. Journal of Yunyang Medical College. 2009;28(6):582-584. | Link |
Zhu Jianyong, Zeng Yuqin, Yuan Liangjun, Chen Gong, Wang Yonglan, Hu Ke. Study on the Treatment of Idiopathic Pulmonary Fibrosis with Prednisone, N-acetylcysteine Combined Captopril. Journal of Yunyang Medical College. 2009;28(6):582-584. | Link | Sun Yingkun, Zhao Qi, Shao Yuxia. Clinical analysis of treatment of idiopathic pulmonary fibrosis with N-acetylcysteine. Journal of Harbin Medical University. 2012;46(5):465-467.
Sun Yingkun, Zhao Qi, Shao Yuxia. Clinical analysis of treatment of idiopathic pulmonary fibrosis with N-acetylcysteine. Journal of Harbin Medical University. 2012;46(5):465-467.  Bai, Lu, Wu, Xiao-Mei. Effect of High Dose Fluimucil on the Pulmonary Function of Patients with Idiopathic Pulmonary Fibrosis. Chinese Journal of Rehabilitation Theory and Practice. 2006;12(7):618-619. | Link |
Bai, Lu, Wu, Xiao-Mei. Effect of High Dose Fluimucil on the Pulmonary Function of Patients with Idiopathic Pulmonary Fibrosis. Chinese Journal of Rehabilitation Theory and Practice. 2006;12(7):618-619. | Link | Bradley B, Branley HM, Egan JJ, Greaves MS, Hansell DM, Harrison NK, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008 Sep;63 Suppl 5:v1-58. | CrossRef | PubMed |
Bradley B, Branley HM, Egan JJ, Greaves MS, Hansell DM, Harrison NK, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008 Sep;63 Suppl 5:v1-58. | CrossRef | PubMed | National Institute for Health and Clinical Excellence ) Idiopathic pulmonary fibrosis in adults: diagnosis and management. NICE guideline (CG163). 2013 [on line] | Link |
National Institute for Health and Clinical Excellence ) Idiopathic pulmonary fibrosis in adults: diagnosis and management. NICE guideline (CG163). 2013 [on line] | Link |