 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Cannabinoids have been proposed for the treatment of patients with cancer pain, especially if standard treatment does not control symptoms. Using Epistemonikos database, which is maintained by searching 30 databases, we identified nine systematic reviews including seven trials that answer the question of interest, of which six are randomized trials. We performed a meta-analysis and generated a summary of findings table using the GRADE approach. We concluded it is unclear whether cannabinoids decrease pain and improve quality of life in patients with refractory cancer pain because the certainty of the evidence is very low, and it probably increases adverse effects substantially.
Cannabis has been used for centuries both recreationally and therapeutically. However, its use has been restricted since the United Nations banned it in 1971. The interest in potential therapeutic uses led to the discovery of endocannabinoid receptors CB1 and CB2, which through a G-protein associated mechanism, would lead to reduced pain. Considering many cancer patients persist with pain despite maximal analgesic therapy, plant extracts with the active agent delta-9-tetrahydrocannabinol or its analogues have been proposed. However, it is unclear what their real clinical role is.
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
|
What is the evidence. |
We identified nine systematic reviews [1],[2],[3],[4],[5], [6],[7],[8],[9], including seven primary studies [10],[11], [12],[13],[14],[15],[16] of which six are randomized controlled trials [10],[11],[12],[13],[14],[15]. This table and the summary in general are based on the latter. |
|
What types of patients were included |
All of the trials included adults with active cancer and pain of diverse etiology (cancer, bone or neuropathic) [10],[11],[12],[13],[14],[15]. Four trials included inpatients [10],[11],[12],[13], and two outpatients [14],[15]. Only two trials [14],[15] included patients that were refractory to treatment, defined as moderate or intense pain despite treatment with 80 mg or more morphine equivalents. |
|
What types of interventions were included |
Three trials evaluated delta-9-tetrahydrocannabinol extracts [10],[11],[14], two trials [14],[15] addressed nabiximol, a preparation of tetrahydrocannabinol and cannabidiol, one trial tested a nitrogen analogue of Cannabis (NIB) [12] and one trial evaluated benzopyranoperidine [13]. All of the studies compared against placebo or treatment with codeine (in less than standard dose). One trial had several arms, including comparison against other cannabinoids [14]. |
|
What types of outcomes |
The main outcome pooled by the systematic reviews identified was pain reduction evaluated with Houde’s scale, visual-analogue scale or numerical-rating scale. Other outcomes pooled were quality of life, and adverse effects such as gastrointestinal (nausea, vomiting), central nervous system (ataxia, memory impairment, disorientation, confusion), psychiatric (euphoria, depression, anxiety, psychosis), and death, among others. |
The information on the effects of cannabinoids is based on two randomized trials (290 participants) that evaluated patients with refractory pain [14],[15]. Both trials measured reduction of pain and one reported quality of life [14]. The information on adverse effects is based on a systematic review [7] evaluating adverse effects of cannabinoids in different populations, and includes 29 studies reporting this outcome.
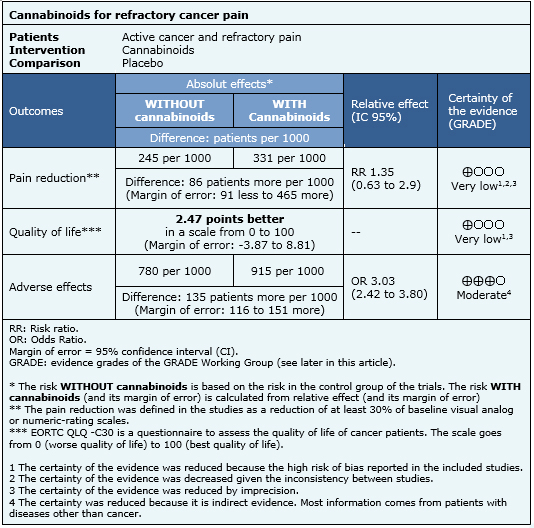

|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
| Feasibility and acceptability |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
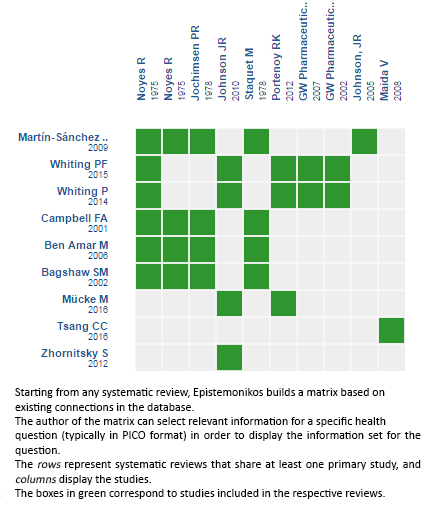
Follow the link to access the interactive version: Cannabinoids for treatment of pain in patients with active cancer
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Cannabinoids have been proposed for the treatment of patients with cancer pain, especially if standard treatment does not control symptoms. Using Epistemonikos database, which is maintained by searching 30 databases, we identified nine systematic reviews including seven trials that answer the question of interest, of which six are randomized trials. We performed a meta-analysis and generated a summary of findings table using the GRADE approach. We concluded it is unclear whether cannabinoids decrease pain and improve quality of life in patients with refractory cancer pain because the certainty of the evidence is very low, and it probably increases adverse effects substantially.
 Autores:
Diego Lobos Urbina[1,2], José Peña Durán [2,3,4]
Autores:
Diego Lobos Urbina[1,2], José Peña Durán [2,3,4]

Citación: Lobos Urbina D, Peña Durán J. Are cannabinoids effective for treatment of pain in patients with active cancer?. Medwave 2016;16(Suppl3):e6539 doi: 10.5867/medwave.2016.6539
Fecha de publicación: 14/9/2016

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Campbell FA, Tramèr MR, Carroll D, Reynolds DJ, Moore RA, McQuay HJ. Are cannabinoids an effective and safe treatment option in the management of pain? A qualitative systematic review. BMJ. 2001 Jul 7;323(7303):13-6. | PubMed |
Campbell FA, Tramèr MR, Carroll D, Reynolds DJ, Moore RA, McQuay HJ. Are cannabinoids an effective and safe treatment option in the management of pain? A qualitative systematic review. BMJ. 2001 Jul 7;323(7303):13-6. | PubMed | Bagshaw SM, Hagen NA. Medical efficacy of cannabinoids and marijuana: a comprehensive review of the literature. J Palliat Care. 2002 Summer;18(2):111-22. | PubMed |
Bagshaw SM, Hagen NA. Medical efficacy of cannabinoids and marijuana: a comprehensive review of the literature. J Palliat Care. 2002 Summer;18(2):111-22. | PubMed | Ben Amar M. Cannabinoids in medicine: A review of their therapeutic potential.J Ethnopharmacol. 2006 Apr 21;105(1-2):1-25. | PubMed |
Ben Amar M. Cannabinoids in medicine: A review of their therapeutic potential.J Ethnopharmacol. 2006 Apr 21;105(1-2):1-25. | PubMed | Martín-Sánchez E, Furukawa TA, Taylor J, Martin JL. Systematic review and meta-analysis of cannabis treatment for chronic pain. Pain Med. 2009 Nov;10 (8):1353-68. | CrossRef | PubMed |
Martín-Sánchez E, Furukawa TA, Taylor J, Martin JL. Systematic review and meta-analysis of cannabis treatment for chronic pain. Pain Med. 2009 Nov;10 (8):1353-68. | CrossRef | PubMed | Zhornitsky S, Potvin S. Cannabidiol in humans-the quest for therapeutic targets. Pharmaceuticals (Basel). 2012 May 21;5(5):529-52. | CrossRef | PubMed |
Zhornitsky S, Potvin S. Cannabidiol in humans-the quest for therapeutic targets. Pharmaceuticals (Basel). 2012 May 21;5(5):529-52. | CrossRef | PubMed | Whiting P, Wolff R, Westwood M, Duffy S, Misso K, Keurentjes C, et al. Systematic Review of Cannabis for Medical Use. N.p., 2014. [on line] | Link |
Whiting P, Wolff R, Westwood M, Duffy S, Misso K, Keurentjes C, et al. Systematic Review of Cannabis for Medical Use. N.p., 2014. [on line] | Link | Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015 Jun 23-30;313(24):2456-73. | CrossRef | PubMed |
Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015 Jun 23-30;313(24):2456-73. | CrossRef | PubMed | Tsang CC, Giudice MG. Nabilone for the Management of Pain. Pharmacotherapy. 2016 Mar;36(3):273-86. | CrossRef | PubMed |
Tsang CC, Giudice MG. Nabilone for the Management of Pain. Pharmacotherapy. 2016 Mar;36(3):273-86. | CrossRef | PubMed | Tsang CC, Giudice MG. Nabilone for the Management of Pain. Pharmacotherapy. 2016 Mar;36(3):273-86. | CrossRef | PubMed |
Tsang CC, Giudice MG. Nabilone for the Management of Pain. Pharmacotherapy. 2016 Mar;36(3):273-86. | CrossRef | PubMed | Noyes R Jr, Brunk SF, Baram DA, Canter A. Analgesic effect of delta-9-tetrahydrocannabinol. J Clin Pharmacol. 1975 Feb-Mar;15(2-3):139-43. | PubMed |
Noyes R Jr, Brunk SF, Baram DA, Canter A. Analgesic effect of delta-9-tetrahydrocannabinol. J Clin Pharmacol. 1975 Feb-Mar;15(2-3):139-43. | PubMed | Noyes R Jr, Brunk SF, Avery DA, Canter AC. The analgesic properties of delta-9-tetrahydrocannabinol and codeine. Clin Pharmacol Ther. 1975 Jul;18(1):84-9. | PubMed |
Noyes R Jr, Brunk SF, Avery DA, Canter AC. The analgesic properties of delta-9-tetrahydrocannabinol and codeine. Clin Pharmacol Ther. 1975 Jul;18(1):84-9. | PubMed | Staquet M, Gantt C, Machin D. Effect of a nitrogen analog of tetrahydrocannabinol on cancer pain. Clin Pharmacol Ther. 1978 Apr;23(4):397-401. | PubMed |
Staquet M, Gantt C, Machin D. Effect of a nitrogen analog of tetrahydrocannabinol on cancer pain. Clin Pharmacol Ther. 1978 Apr;23(4):397-401. | PubMed | Jochimsen PR, Lawton RL, VerSteeg K, Noyes R Jr. Effect of benzopyranoperidine, a delta-9-THC congener, on pain. Clin Pharmacol Ther. 1978 Aug;24(2):223-7. | PubMed |
Jochimsen PR, Lawton RL, VerSteeg K, Noyes R Jr. Effect of benzopyranoperidine, a delta-9-THC congener, on pain. Clin Pharmacol Ther. 1978 Aug;24(2):223-7. | PubMed | Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage. 2010 Feb;39(2):167-79. | CrossRef | PubMed |
Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage. 2010 Feb;39(2):167-79. | CrossRef | PubMed | Portenoy RK, Ganae-Motan ED, Allende S, Yanagihara R, Shaiova L, Weinstein S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain. 2012 May;13(5):438-49. | CrossRef | PubMed |
Portenoy RK, Ganae-Motan ED, Allende S, Yanagihara R, Shaiova L, Weinstein S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain. 2012 May;13(5):438-49. | CrossRef | PubMed | Maida V, Ennis M, Irani S, Corbo M, Dolzhykov M. Adjunctive nabilone in cancer pain and symptom management: a prospective observational study using propensity scoring. J Support Oncol. 2008 Mar;6(3):119-24. | PubMed |
Maida V, Ennis M, Irani S, Corbo M, Dolzhykov M. Adjunctive nabilone in cancer pain and symptom management: a prospective observational study using propensity scoring. J Support Oncol. 2008 Mar;6(3):119-24. | PubMed | National Institute for Health and Clinical Excellence. End of Life Care for Adults. nice.org.uk. [on line] | Link |
National Institute for Health and Clinical Excellence. End of Life Care for Adults. nice.org.uk. [on line] | Link | Ripamonti CI, Santini D, Maranzano E, Berti M, Roila F; ESMO Guidelines Working Group. Management of cancer pain: ESMO Clinical Practice Guidelines. Ann Oncol. 2012 Oct;23 Suppl 7:vii139-54. | PubMed |
Ripamonti CI, Santini D, Maranzano E, Berti M, Roila F; ESMO Guidelines Working Group. Management of cancer pain: ESMO Clinical Practice Guidelines. Ann Oncol. 2012 Oct;23 Suppl 7:vii139-54. | PubMed | Paice JA, Portenoy R, Lacchetti C, Campbell T, Cheville A, Citron M, et al. Management of Chronic Pain in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016 Jul 25. pii: JCO685206. | PubMed |
Paice JA, Portenoy R, Lacchetti C, Campbell T, Cheville A, Citron M, et al. Management of Chronic Pain in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016 Jul 25. pii: JCO685206. | PubMed | Martinez D; New York State Psychiatric Institute. Investigation of Cannabis for Pain and Inflammation in Lung Cancer. Bethesda (MD): National Library of Medicine; 2000. ClinicalTrials.gov [on line]. | Link |
Martinez D; New York State Psychiatric Institute. Investigation of Cannabis for Pain and Inflammation in Lung Cancer. Bethesda (MD): National Library of Medicine; 2000. ClinicalTrials.gov [on line]. | Link | Martinez D, New York State Psychiatric Institute. Investigation of Cannabis for Chronic Pain and Palliative Care. Bethesda (MD): National Library of Medicine; 2000. ClinicalTrials.gov [on line]. | Link |
Martinez D, New York State Psychiatric Institute. Investigation of Cannabis for Chronic Pain and Palliative Care. Bethesda (MD): National Library of Medicine; 2000. ClinicalTrials.gov [on line]. | Link |