 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
During an episode of sepsis, the systemic inflammatory response phenomenon triggers a deficit in the action and/or secretion of cortisol. It has been suggested that the use of corticosteroids may have a role in the management of sepsis, but there is no consensus. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified 16 systematic reviews including 66 randomized controlled trials addressing the question of this article. We combined the evidence using meta-analysis and generated a summary of findings following the GRADE approach. We concluded the use of corticosteroids during a sepsis episode probably favors reversal of shock, briefly shortens the stay in intensive care unit and might reduce mortality, with few clinically relevant adverse effects.
Sepsis remains the leading cause of morbidity and mortality in intensive care units worldwide. Its incidence has been increasing, with more complications and more resistant infectious agents. While there has been a tendency to a decrease in mortality due to some interventions, effective therapeutic tools remain limited.
During an episode of sepsis, systemic inflammatory response phenomenon triggers a deficit in action and/or cortisol secretion secondary to proinflammatory cytokines. For this reason, it has been postulated the use of steroids may play a role in the management of sepsis. However, evidence has been mixed regarding its actual effect, and there is still no consensus on the role played by this treatment in sepsis.
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
|
What is the evidence. |
We found sixteen systematic reviews [1],[2],[3],[4],[5], [6],[7],[8],[9],[10],[11],[12],[13],[14],[15],[16], including 66 primary studies (64 randomized controlled trials). Two systematic reviews were excluded from the analysis. One was exclusively focused on pediatric population (whose protocols and evolution differs regarding adult population) [9] and the other was restricted to patients with dengue [6]. The remaining reviews include fifty-three randomized controlled trials [17],[18],[19],[20],[21],[22],[23],[24],[25],[26],[27],[28],[29],[30],[31],[32],[33],[34],[36],[37], [38],[39],[40],[41],[42],[43],[44],[45],[46],[47],[48],[49], [50],[51],[52],[53],[54],[55],[56],[57],[58],[59],[60],[61], [62],[63],[64],[65],[66],[67],[68],[69]. |
|
What types of patients were included |
Nineteen studies included were conducted prior to 1991, when the first Consensus Definitions for Sepsis and Septic Shock [70] was released. So, the definition and classification of the severity of sepsis was heterogeneous among studies, added to the fact the third Consensus Definitions for Sepsis and Septic Shock has been published recently [71]. |
|
What types of interventions were included |
The most commonly used steroid was hydrocortisone (53.8%), then dexamethasone (21.1%), methylprednisolone (17.3%) and others (e.g. betamethasone, prednisolone; 7.8%). The route of administration was intravenous in all of the studies. Regarding the dose (equivalent to hydrocortisone), doses ranged from 30 mg to 4200 mg per day. The different systematic reviews used different definitions to consider low-dose corticosteroids. Some used a dose equivalent <300 mg hydrocortisone per day, another 400 mg and even 500 mg. 50% of the studies used less than or equal to 300 mg daily dose. Only two studies added mineralocorticoids [18],[28]. All of the studies compared against placebo or standard treatment. |
|
What types of outcomes |
The different systematic reviews pooled the following outcomes: - Mortality at 28 days |
Information on the effects of using steroids in sepsis is based on 44 randomized trials that provided information usable for meta-analysis involving 5618 patients. Forty-four trials reported mortality [17],[18],[19],[20],[21],[22],[23],[24],[25],[26],[27],[29],[30],[32],[33],[34],[39],[40],[41],[42],[43],[44],[45],[46],[47],[48],[51],[52],[53],[55],[56],[57],[58],[59],[60],[61],[62],[63],[64],[65],[66],[67],[68],[69], 12 trials reported reversal of shock [18],[19],[22],[23],[24],[25],[32],[34],[55],[57],[60],[61], 12 trials reported stay in intensive care [18],[19],[22],[24],[25],[29],[32],[34],[44],[59],[61],[66], 20 trials reported gastrointestinal bleeding [18],[19],[20],[22],[24],[25],[27],[29],[32],[42],[44],[46],[55],[57],[59],[61],[63],[67],[68],[69], 21 trials reported superinfection [17],[18],[19],[20],[22],[23],[24],[25],[27],[29],[32],[39],[42],[46],[57],[59],[60],[61],[63],[68],[69], 13 trials reported hyperglycemia [18],[19],[22],[42],[44],[46],[57],[59],[60],[61],[63], [68],[69] and three trials reported myoneuropathy of critically ill patients [18],[19],[61]. The summary of findings is the following:
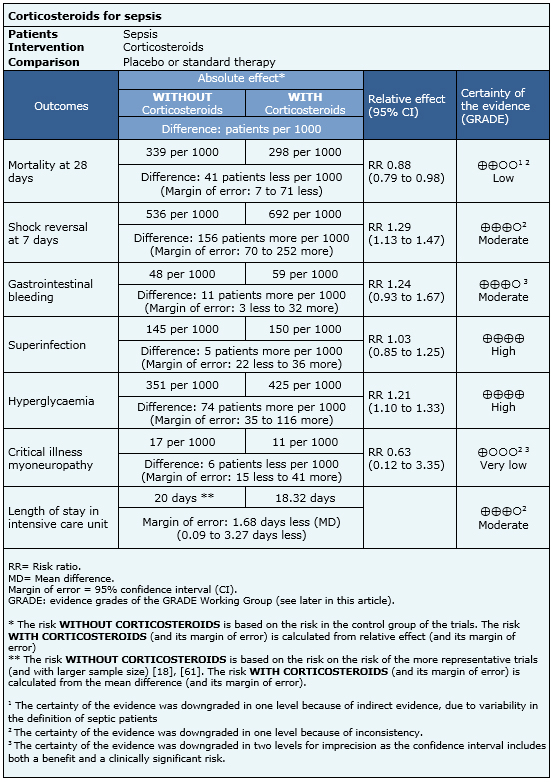

|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
Follow the link to access the interactive version:Corticosteroids for sepsis
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

During an episode of sepsis, the systemic inflammatory response phenomenon triggers a deficit in the action and/or secretion of cortisol. It has been suggested that the use of corticosteroids may have a role in the management of sepsis, but there is no consensus. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified 16 systematic reviews including 66 randomized controlled trials addressing the question of this article. We combined the evidence using meta-analysis and generated a summary of findings following the GRADE approach. We concluded the use of corticosteroids during a sepsis episode probably favors reversal of shock, briefly shortens the stay in intensive care unit and might reduce mortality, with few clinically relevant adverse effects.
 Autores:
Joaquín Jerez[1,2], Ricardo Castro[2,3]
Autores:
Joaquín Jerez[1,2], Ricardo Castro[2,3]

Citación: Jerez J, Castro R. What is the role of corticosteroids in the management of sepsis?. Medwave 2016;16(Suppl3):e6522 doi: 10.5867/medwave.2016.6522
Fecha de publicación: 30/8/2016

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y. Corticosteroids for treating sepsis. The Cochrane database of systematic reviews. 2015;12:CD002243. | Link |
Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y. Corticosteroids for treating sepsis. The Cochrane database of systematic reviews. 2015;12:CD002243. | Link | Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y. Corticosteroids for severe sepsis and septic shock: a systematic review and meta-analysis. BMJ. 2004 Aug 28;329(7464):480. | CrossRef | PubMed |
Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y. Corticosteroids for severe sepsis and septic shock: a systematic review and meta-analysis. BMJ. 2004 Aug 28;329(7464):480. | CrossRef | PubMed | Annane D, Bellissant E, Bollaert PE, Briegel J, Confalonieri M, De Gaudio R,et al. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA. 2009 Jun 10;301(22):2362-75.
| CrossRef | PubMed |
Annane D, Bellissant E, Bollaert PE, Briegel J, Confalonieri M, De Gaudio R,et al. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA. 2009 Jun 10;301(22):2362-75.
| CrossRef | PubMed | Burry LD, Wax RS. Role of corticosteroids in septic shock. The Annals of pharmacotherapy. 2004;38(3):464-72. | Link |
Burry LD, Wax RS. Role of corticosteroids in septic shock. The Annals of pharmacotherapy. 2004;38(3):464-72. | Link | Cronin L, Cook DJ, Carlet J, Heyland DK, King D, Lansang MA, .Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med. 1995 Aug;23(8):1430-9. | PubMed |
Cronin L, Cook DJ, Carlet J, Heyland DK, King D, Lansang MA, .Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med. 1995 Aug;23(8):1430-9. | PubMed | Zhang F, Kramer CV.orticosteroids for dengue infection. Cochrane Database Syst Rev. 2014 Jul 1;(7):CD003488. | CrossRef | PubMed |
Zhang F, Kramer CV.orticosteroids for dengue infection. Cochrane Database Syst Rev. 2014 Jul 1;(7):CD003488. | CrossRef | PubMed | Ho KM, Tan JA. Use of L'Abbé and pooled calibration plots to assess the relationship between severity of illness and effectiveness in studies ofcorticosteroids for severe sepsis. Br J Anaesth. 2011 Apr;106(4):528-36. | CrossRef | PubMed |
Ho KM, Tan JA. Use of L'Abbé and pooled calibration plots to assess the relationship between severity of illness and effectiveness in studies ofcorticosteroids for severe sepsis. Br J Anaesth. 2011 Apr;106(4):528-36. | CrossRef | PubMed | Lefering R, Neugebauer EA. Steroid controversy in sepsis and septic shock: a meta-analysis. Crit Care Med. 1995 Jul;23(7):1294-303. | PubMed |
Lefering R, Neugebauer EA. Steroid controversy in sepsis and septic shock: a meta-analysis. Crit Care Med. 1995 Jul;23(7):1294-303. | PubMed | Menon K, McNally D, Choong K, Sampson M. A systematic review and meta-analysis on the effect of steroids in pediatric shock. Pediatr Crit Care Med. 2013 Jun;14(5):474-80.
| CrossRef | PubMed |
Menon K, McNally D, Choong K, Sampson M. A systematic review and meta-analysis on the effect of steroids in pediatric shock. Pediatr Crit Care Med. 2013 Jun;14(5):474-80.
| CrossRef | PubMed | Minneci PC, Deans KJ, Banks SM, Eichacker PQ, Natanson C. Meta-analysis: the effect of steroids on survival and shock during sepsis depends on the dose. Ann Intern Med. 2004 Jul 6;141(1):47-56. | PubMed |
Minneci PC, Deans KJ, Banks SM, Eichacker PQ, Natanson C. Meta-analysis: the effect of steroids on survival and shock during sepsis depends on the dose. Ann Intern Med. 2004 Jul 6;141(1):47-56. | PubMed | Minneci PC, Deans KJ, Eichacker PQ, Natanson C. The effects of steroids during sepsis depend on dose and severity of illness: an updated meta-analysis. Clin Microbiol Infect. 2009 Apr;15(4):308-18. | CrossRef | PubMed |
Minneci PC, Deans KJ, Eichacker PQ, Natanson C. The effects of steroids during sepsis depend on dose and severity of illness: an updated meta-analysis. Clin Microbiol Infect. 2009 Apr;15(4):308-18. | CrossRef | PubMed | Moran JL, Graham PL, Rockliff S, Bersten AD. Updating the evidence for the role of corticosteroids in severe sepsis and septic shock: a Bayesian meta-analytic perspective. Crit Care. 2010;14(4):R134. | CrossRef | PubMed |
Moran JL, Graham PL, Rockliff S, Bersten AD. Updating the evidence for the role of corticosteroids in severe sepsis and septic shock: a Bayesian meta-analytic perspective. Crit Care. 2010;14(4):R134. | CrossRef | PubMed | Patel GP, Balk RA. Systemic steroids in severe sepsis and septic shock. Am JRespir Crit Care Med. 2012 Jan 15;185(2):133-9. | CrossRef | PubMed |
Patel GP, Balk RA. Systemic steroids in severe sepsis and septic shock. Am JRespir Crit Care Med. 2012 Jan 15;185(2):133-9. | CrossRef | PubMed | Sherwin RL, Garcia AJ, Bilkovski R. Do low-dose corticosteroids improvemortality or shock reversal in patients with septic shock? A systematic reviewand position statement prepared for the American Academy of Emergency Medicine. J Emerg Med. 2012 Jul;43(1):7-12. | CrossRef | PubMed |
Sherwin RL, Garcia AJ, Bilkovski R. Do low-dose corticosteroids improvemortality or shock reversal in patients with septic shock? A systematic reviewand position statement prepared for the American Academy of Emergency Medicine. J Emerg Med. 2012 Jul;43(1):7-12. | CrossRef | PubMed | Sligl WI, Milner DA Jr, Sundar S, Mphatswe W, Majumdar SR. Safety andefficacy of corticosteroids for the treatment of septic shock: A systematic review and meta-analysis. Clin Infect Dis. 2009 Jul 1;49(1):93-101.
| CrossRef | PubMed |
Sligl WI, Milner DA Jr, Sundar S, Mphatswe W, Majumdar SR. Safety andefficacy of corticosteroids for the treatment of septic shock: A systematic review and meta-analysis. Clin Infect Dis. 2009 Jul 1;49(1):93-101.
| CrossRef | PubMed | Volbeda M, Wetterslev J, Gluud C, Zijlstra JG, van der Horst IC, Keus F.Glucocorticosteroids for sepsis: systematic review with meta-analysis and trialsequential analysis. Intensive Care Med. 2015 Jul;41(7):1220-34. | CrossRef | PubMed |
Volbeda M, Wetterslev J, Gluud C, Zijlstra JG, van der Horst IC, Keus F.Glucocorticosteroids for sepsis: systematic review with meta-analysis and trialsequential analysis. Intensive Care Med. 2015 Jul;41(7):1220-34. | CrossRef | PubMed | Abdelsalam Rezk N, Mohamed Ibrahim A. Effects of methyl prednisolone in early ARDS. Egyptian Journal of Chest Diseases and Tuberculosis. 2013;62(1):167-172. | CrossRef |
Abdelsalam Rezk N, Mohamed Ibrahim A. Effects of methyl prednisolone in early ARDS. Egyptian Journal of Chest Diseases and Tuberculosis. 2013;62(1):167-172. | CrossRef | Annane D, Sébille V, Charpentier C, Bollaert PE, François B, Korach JM, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone onmortality in patients with septic shock. JAMA. 2002 Aug 21;288(7):862-71.
| PubMed |
Annane D, Sébille V, Charpentier C, Bollaert PE, François B, Korach JM, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone onmortality in patients with septic shock. JAMA. 2002 Aug 21;288(7):862-71.
| PubMed | Arabi YM, Aljumah A, Dabbagh O, Tamim HM, Rishu AH, Al-Abdulkareem A, et al. Low-dose hydrocortisone in patients with cirrhosis and septic shock: a randomized controlled trial. CMAJ. 2010 Dec14;182(18):1971-7. | CrossRef | PubMed |
Arabi YM, Aljumah A, Dabbagh O, Tamim HM, Rishu AH, Al-Abdulkareem A, et al. Low-dose hydrocortisone in patients with cirrhosis and septic shock: a randomized controlled trial. CMAJ. 2010 Dec14;182(18):1971-7. | CrossRef | PubMed | Bennett IL Jr, Finland M, Hamburger M, Kass EH, Lepper M, Waisbren BA. Adouble-blind study of the effectiveness of cortisol in the management of severe infections. Trans Assoc Am Physicians. 1962;75:198-207. | PubMed |
Bennett IL Jr, Finland M, Hamburger M, Kass EH, Lepper M, Waisbren BA. Adouble-blind study of the effectiveness of cortisol in the management of severe infections. Trans Assoc Am Physicians. 1962;75:198-207. | PubMed | Bernard GR, Luce JM, Sprung CL, Rinaldo JE, Tate RM, Sibbald WJ, et al. High-dose corticosteroids in patients withthe adult respiratory distress syndrome. N Engl J Med. 1987 Dec 17;317(25):1565-70. | PubMed |
Bernard GR, Luce JM, Sprung CL, Rinaldo JE, Tate RM, Sibbald WJ, et al. High-dose corticosteroids in patients withthe adult respiratory distress syndrome. N Engl J Med. 1987 Dec 17;317(25):1565-70. | PubMed | Bollaert PE, Charpentier C, Levy B, Debouverie M, Audibert G, Larcan A. Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Crit Care Med. 1998 Apr;26(4):645-50. | PubMed |
Bollaert PE, Charpentier C, Levy B, Debouverie M, Audibert G, Larcan A. Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Crit Care Med. 1998 Apr;26(4):645-50. | PubMed | Bone RC, Fisher CJ Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA. A controlledclinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987 Sep 10;317(11):653-8. | PubMed |
Bone RC, Fisher CJ Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA. A controlledclinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987 Sep 10;317(11):653-8. | PubMed | Briegel J, Forst H, Haller M, Schelling G, Kilger E, Kuprat G, et al. Stress doses of hydrocortisonereverse hyperdynamic septic shock: a prospective, randomized, double-blind, single-center study. Crit Care Med. 1999 Apr;27(4):723-32. | PubMed |
Briegel J, Forst H, Haller M, Schelling G, Kilger E, Kuprat G, et al. Stress doses of hydrocortisonereverse hyperdynamic septic shock: a prospective, randomized, double-blind, single-center study. Crit Care Med. 1999 Apr;27(4):723-32. | PubMed | Chawla, Kabu, Kupfer, Yizhak, Goldman, Isa, Tessler, Sidney. Hydrocortisone reverses refractory septic shock. Critical Care Medicine. 1999;27(1). | Link |
Chawla, Kabu, Kupfer, Yizhak, Goldman, Isa, Tessler, Sidney. Hydrocortisone reverses refractory septic shock. Critical Care Medicine. 1999;27(1). | Link | Cicarelli DD, Vieira JE, Benseñor FE. Early dexamethasone treatment for septic shock patients: a prospective randomized clinical trial. Sao Paulo Med J. 2007 Jul 5;125(4):237-41. | PubMed |
Cicarelli DD, Vieira JE, Benseñor FE. Early dexamethasone treatment for septic shock patients: a prospective randomized clinical trial. Sao Paulo Med J. 2007 Jul 5;125(4):237-41. | PubMed | Cicarelli DD, Benseñor FE, Vieira JE. Effects of single dose of dexamethasoneon patients with systemic inflammatory response. Sao Paulo Med J. 2006 Mar 2;124(2):90-5. | PubMed |
Cicarelli DD, Benseñor FE, Vieira JE. Effects of single dose of dexamethasoneon patients with systemic inflammatory response. Sao Paulo Med J. 2006 Mar 2;124(2):90-5. | PubMed | COIITSS Study Investigators, Annane D, Cariou A, Maxime V, Azoulay E, D'honneur G, et al. Corticosteroid treatment and intensive insulin therapy for septic shock in adults: a randomized controlled trial. JAMA. 2010 Jan 27;303(4):341-8. | CrossRef | PubMed |
COIITSS Study Investigators, Annane D, Cariou A, Maxime V, Azoulay E, D'honneur G, et al. Corticosteroid treatment and intensive insulin therapy for septic shock in adults: a randomized controlled trial. JAMA. 2010 Jan 27;303(4):341-8. | CrossRef | PubMed | Confalonieri M, Urbino R, Potena A, Piattella M, Parigi P, Puccio G, et al. Hydrocortisone infusion forsevere community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med. 2005 Feb 1;171(3):242-8. | PubMed |
Confalonieri M, Urbino R, Potena A, Piattella M, Parigi P, Puccio G, et al. Hydrocortisone infusion forsevere community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med. 2005 Feb 1;171(3):242-8. | PubMed | de Gans J, van de Beek D. Dexamethasone in adults with bacterial meningitis. N Engl J Med. 2002 Nov 14;347(20):1549-56. | CrossRef | PubMed |
de Gans J, van de Beek D. Dexamethasone in adults with bacterial meningitis. N Engl J Med. 2002 Nov 14;347(20):1549-56. | CrossRef | PubMed | Gordon AC, Mason AJ, Perkins GD, Stotz M, Terblanche M, Ashby D, et al. The interaction of vasopressin and corticosteroids in septic shock: a ilotrandomized controlled trial. Crit Care Med. 2014 Jun;42(6):1325-33. | CrossRef | PubMed |
Gordon AC, Mason AJ, Perkins GD, Stotz M, Terblanche M, Ashby D, et al. The interaction of vasopressin and corticosteroids in septic shock: a ilotrandomized controlled trial. Crit Care Med. 2014 Jun;42(6):1325-33. | CrossRef | PubMed | Hoffman SL, Woodward TE, Hornick RB, Punjabi NH, Greisman SE. Effectivetreatment and prevention of typhoid fever: updated. Trans Am Clin Climatol Assoc. 1984;95:52-65. | PubMed |
Hoffman SL, Woodward TE, Hornick RB, Punjabi NH, Greisman SE. Effectivetreatment and prevention of typhoid fever: updated. Trans Am Clin Climatol Assoc. 1984;95:52-65. | PubMed | Hu B, Li JG, Liang H, Zhou Q, Yu Z, Li L, et al. [The effect oflow-dose hydrocortisone on requirement of norepinephrine and lactate clearance in patients with refractory septic shock]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2009 Sep;21(9):529-31. | PubMed |
Hu B, Li JG, Liang H, Zhou Q, Yu Z, Li L, et al. [The effect oflow-dose hydrocortisone on requirement of norepinephrine and lactate clearance in patients with refractory septic shock]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2009 Sep;21(9):529-31. | PubMed | Hughes GS Jr. Naloxone and methylprednisolone sodium succinate enhancesympathomedullary discharge in patients with septic shock. Life Sci. 1984 Dec 3;35(23):2319-26. | PubMed |
Hughes GS Jr. Naloxone and methylprednisolone sodium succinate enhancesympathomedullary discharge in patients with septic shock. Life Sci. 1984 Dec 3;35(23):2319-26. | PubMed | Huh, Jin Won, Lim, Chae-Man, Koh, Younsuck, Hong, Sang-Bum. Effect of low doses of hydrocortisone in patient with septic shock and relative adrenal insufficiency: 3 days versus 7 days treatment.: 369. Crit Care Med. 2006;34(12):A101-A101. | Link |
Huh, Jin Won, Lim, Chae-Man, Koh, Younsuck, Hong, Sang-Bum. Effect of low doses of hydrocortisone in patient with septic shock and relative adrenal insufficiency: 3 days versus 7 days treatment.: 369. Crit Care Med. 2006;34(12):A101-A101. | Link | Kaufmann I, Briegel J, Schliephake F, Hoelzl A, Chouker A, Hummel T, et al. Stress doses of hydrocortisone in septic shock: beneficial effects on opsonization-dependent neutrophil functions. Intensive Care Med. 2008 Feb;34(2):344-9. | PubMed |
Kaufmann I, Briegel J, Schliephake F, Hoelzl A, Chouker A, Hummel T, et al. Stress doses of hydrocortisone in septic shock: beneficial effects on opsonization-dependent neutrophil functions. Intensive Care Med. 2008 Feb;34(2):344-9. | PubMed | Keh D, Boehnke T, Weber-Cartens S, Schulz C, Ahlers O, Bercker S, et al. Immunologic and hemodynamic effects of "low-dose" hydrocortisone in septic shock: a double-blind, randomized, placebo-controlled, crossover study. Am J Respir Crit Care Med. 2003 Feb 15;167(4):512-20. | CrossRef | PubMed |
Keh D, Boehnke T, Weber-Cartens S, Schulz C, Ahlers O, Bercker S, et al. Immunologic and hemodynamic effects of "low-dose" hydrocortisone in septic shock: a double-blind, randomized, placebo-controlled, crossover study. Am J Respir Crit Care Med. 2003 Feb 15;167(4):512-20. | CrossRef | PubMed | Keh D, Boehnke T, Weber-Cartens S, Schulz C, Ahlers O, Bercker S, et al. Immunologic and hemodynamic effects of "low-dose" hydrocortisone in septic shock: a double-blind, randomized, placebo-controlled, crossover study. Am J Respir Crit Care Med. 2003 Feb 15;167(4):512-20. | PubMed |
Keh D, Boehnke T, Weber-Cartens S, Schulz C, Ahlers O, Bercker S, et al. Immunologic and hemodynamic effects of "low-dose" hydrocortisone in septic shock: a double-blind, randomized, placebo-controlled, crossover study. Am J Respir Crit Care Med. 2003 Feb 15;167(4):512-20. | PubMed | Klastersky J, Cappel R, Debusscher L. Effectiveness of betamethasone in management of severe infections. A double-blind study. N Engl J Med. 1971 Jun 3;284(22):1248-50. | PubMed |
Klastersky J, Cappel R, Debusscher L. Effectiveness of betamethasone in management of severe infections. A double-blind study. N Engl J Med. 1971 Jun 3;284(22):1248-50. | PubMed | Liu L, Li J, Huang YZ, Liu SQ, Yang CS, Guo FM, Qiu HB, Yang Y. [The effect of stress dose glucocorticoid on patients with acute respiratory distress syndrome combined with critical illness-related corticosteroid insufficiency]. Zhonghua Nei Ke Za Zhi. 2012 Aug;51(8):599-603. | PubMed |
Liu L, Li J, Huang YZ, Liu SQ, Yang CS, Guo FM, Qiu HB, Yang Y. [The effect of stress dose glucocorticoid on patients with acute respiratory distress syndrome combined with critical illness-related corticosteroid insufficiency]. Zhonghua Nei Ke Za Zhi. 2012 Aug;51(8):599-603. | PubMed | Lucas CE, Ledgerwood AM. The cardiopulmonary response to massive doses of steroids in patients with septic shock. Arch Surg. 1984 May;119(5):537-41. | PubMed |
Lucas CE, Ledgerwood AM. The cardiopulmonary response to massive doses of steroids in patients with septic shock. Arch Surg. 1984 May;119(5):537-41. | PubMed | Luce JM, Montgomery AB, Marks JD, Turner J, Metz CA, Murray JF. Ineffectiveness of high-dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am Rev Respir Dis. 1988 Jul;138(1):62-8. | PubMed |
Luce JM, Montgomery AB, Marks JD, Turner J, Metz CA, Murray JF. Ineffectiveness of high-dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am Rev Respir Dis. 1988 Jul;138(1):62-8. | PubMed | Marik P, Kraus P, Sribante J, Havlik I, Lipman J, Johnson DW. Hydrocortisone and tumor necrosis factor in severe community-acquired pneumonia. A randomized controlled study. Chest. 1993 Aug;104(2):389-92. | PubMed |
Marik P, Kraus P, Sribante J, Havlik I, Lipman J, Johnson DW. Hydrocortisone and tumor necrosis factor in severe community-acquired pneumonia. A randomized controlled study. Chest. 1993 Aug;104(2):389-92. | PubMed | Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007 Apr;131(4):954-63. | PubMed |
Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007 Apr;131(4):954-63. | PubMed | Meduri, Gianfranco U, Golden, Emmel, Umberger, Reba. PRospective double-blind randomized clinical trial on the effects of low-dose hydrocortisone infusion in patients with severe sepsis. Chest. 2009;136(4_MeetingAbstracts):45S-h-45S. | Link |
Meduri, Gianfranco U, Golden, Emmel, Umberger, Reba. PRospective double-blind randomized clinical trial on the effects of low-dose hydrocortisone infusion in patients with severe sepsis. Chest. 2009;136(4_MeetingAbstracts):45S-h-45S. | Link | Meijvis SC, Hardeman H, Remmelts HH, Heijligenberg R, Rijkers GT, van Velzen-Blad H, et al. Dexamethasone and length of hospital stay in patients with community-acquired pneumonia: a randomised, double-blind, placebo-controlled trial. Lancet. 2011 Jun 11;377(9782):2023-30. | CrossRef | PubMed |
Meijvis SC, Hardeman H, Remmelts HH, Heijligenberg R, Rijkers GT, van Velzen-Blad H, et al. Dexamethasone and length of hospital stay in patients with community-acquired pneumonia: a randomised, double-blind, placebo-controlled trial. Lancet. 2011 Jun 11;377(9782):2023-30. | CrossRef | PubMed | Mikami K, Suzuki M, Kitagawa H, Kawakami M, Hirota N, Yamaguchi H, et al. Efficacy of corticosteroids in the treatment of community-acquired pneumonia requiring hospitalization. Lung. 2007 Sep-Oct;185(5):249-55. | PubMed |
Mikami K, Suzuki M, Kitagawa H, Kawakami M, Hirota N, Yamaguchi H, et al. Efficacy of corticosteroids in the treatment of community-acquired pneumonia requiring hospitalization. Lung. 2007 Sep-Oct;185(5):249-55. | PubMed | Mirea, L, Ungureanu, R, Pavelescu, D, Grintescu, IC, Dumitrache, C, Grintescu, I, Mirea, D. Continuous administration of corticosteroids in septic shock can reduce risk of hypernatremia. Critical Care. 2014;18(Suppl 1):P239-P239. | CrossRef |
Mirea, L, Ungureanu, R, Pavelescu, D, Grintescu, IC, Dumitrache, C, Grintescu, I, Mirea, D. Continuous administration of corticosteroids in septic shock can reduce risk of hypernatremia. Critical Care. 2014;18(Suppl 1):P239-P239. | CrossRef | Mussack T, Briegel J, Schelling G, Biberthaler P, Jochum M. Effect of stress doses of hydrocortisone on S-100B vs. interleukin-8 and polymorphonuclear elastase levels in human septic shock. Clin Chem Lab Med. 2005;43(3):259-68. | PubMed |
Mussack T, Briegel J, Schelling G, Biberthaler P, Jochum M. Effect of stress doses of hydrocortisone on S-100B vs. interleukin-8 and polymorphonuclear elastase levels in human septic shock. Clin Chem Lab Med. 2005;43(3):259-68. | PubMed | Mussack T, Briegel J, Schelling G, Jochum M. Hemofiltrastion does not influence early S-100B serum levels in septic shock patients receiving stress doses of hydrocortisone or placebo. European journal of medical research. 2005;10(1):11-7. | Link |
Mussack T, Briegel J, Schelling G, Jochum M. Hemofiltrastion does not influence early S-100B serum levels in septic shock patients receiving stress doses of hydrocortisone or placebo. European journal of medical research. 2005;10(1):11-7. | Link | Nafae, Ramadan M., Ragab, Mostafa I., Amany, Fawzy M., Rashed, Shimaa B.. Adjuvant role of corticosteroids in the treatment of community-acquired pneumonia. Egyptian Journal of Chest Diseases and Tuberculosis. 2013;62(3):439-445. | Link |
Nafae, Ramadan M., Ragab, Mostafa I., Amany, Fawzy M., Rashed, Shimaa B.. Adjuvant role of corticosteroids in the treatment of community-acquired pneumonia. Egyptian Journal of Chest Diseases and Tuberculosis. 2013;62(3):439-445. | Link | Oppert M, Schindler R, Husung C, Offermann K, Gräf KJ, Boenisch O, et al. Low-dose hydrocortisone improves shock reversal and reduces cytokine levels in early hyperdynamic septic shock. Crit Care Med. 2005 Nov;33(11):2457-64. | PubMed |
Oppert M, Schindler R, Husung C, Offermann K, Gräf KJ, Boenisch O, et al. Low-dose hydrocortisone improves shock reversal and reduces cytokine levels in early hyperdynamic septic shock. Crit Care Med. 2005 Nov;33(11):2457-64. | PubMed | Rinaldi S, Adembri C, Grechi S, De Gaudio AR. Low-dose hydrocortisone during severe sepsis: effects on microalbuminuria. Crit Care Med. 2006 Sep;34(9):2334-9. | PubMed |
Rinaldi S, Adembri C, Grechi S, De Gaudio AR. Low-dose hydrocortisone during severe sepsis: effects on microalbuminuria. Crit Care Med. 2006 Sep;34(9):2334-9. | PubMed | Sabry, Nirmeen A., Omar, Emad El-Din. Corticosteroids and ICU Course of Community Acquired Pneumonia in Egyptian Settings. Pharmacology & Pharmacy. 2011;2:73-81. | CrossRef |
Sabry, Nirmeen A., Omar, Emad El-Din. Corticosteroids and ICU Course of Community Acquired Pneumonia in Egyptian Settings. Pharmacology & Pharmacy. 2011;2:73-81. | CrossRef | Scarborough M, Gordon SB, Whitty CJ, French N, Njalale Y, Chitani A, et al. Corticosteroids for bacterial meningitis in adults in sub-Saharan Africa. N Engl J Med. 2007 Dec 13;357(24):2441-50. | PubMed |
Scarborough M, Gordon SB, Whitty CJ, French N, Njalale Y, Chitani A, et al. Corticosteroids for bacterial meningitis in adults in sub-Saharan Africa. N Engl J Med. 2007 Dec 13;357(24):2441-50. | PubMed | Schumer W. Steroids in the treatment of clinical septic shock. Ann Surg. 1976 Sep;184(3):333-41. | PubMed |
Schumer W. Steroids in the treatment of clinical septic shock. Ann Surg. 1976 Sep;184(3):333-41. | PubMed | Slusher T, Gbadero D, Howard C, Lewison L, Giroir B, Toro L, et al. Randomized, placebo-controlled, double blinded trial of dexamethasone in African children with sepsis. Pediatr Infect Dis J. 1996 Jul;15(7):579-83. | PubMed |
Slusher T, Gbadero D, Howard C, Lewison L, Giroir B, Toro L, et al. Randomized, placebo-controlled, double blinded trial of dexamethasone in African children with sepsis. Pediatr Infect Dis J. 1996 Jul;15(7):579-83. | PubMed | Snijders D, Daniels JM, de Graaff CS, van der Werf TS, Boersma WG. Efficacy of corticosteroids in community-acquired pneumonia: a randomized double-blinded clinical trial. Am J Respir Crit Care Med. 2010 May 1;181(9):975-82. | CrossRef | PubMed |
Snijders D, Daniels JM, de Graaff CS, van der Werf TS, Boersma WG. Efficacy of corticosteroids in community-acquired pneumonia: a randomized double-blinded clinical trial. Am J Respir Crit Care Med. 2010 May 1;181(9):975-82. | CrossRef | PubMed | Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008 Jan 10;358(2):111-24. | CrossRef | PubMed |
Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008 Jan 10;358(2):111-24. | CrossRef | PubMed | Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008 Jan 10;358(2):111-24. | CrossRef | PubMed |
Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008 Jan 10;358(2):111-24. | CrossRef | PubMed | Tandan, SM, Guleria, R, Gupta, N. Low dose steroids and adrenocortical insufficiency in septic shock: a double-blind randomised controlled trial from India. Am J Respir Crit Care Med. 2005;171:A43. | Link |
Tandan, SM, Guleria, R, Gupta, N. Low dose steroids and adrenocortical insufficiency in septic shock: a double-blind randomised controlled trial from India. Am J Respir Crit Care Med. 2005;171:A43. | Link | Veterans Administration Systemic Sepsis Cooperative Study Group. Effect of high-dose glucocorticoid therapy on mortality in patients with clinical signs of systemic sepsis. N Engl J Med. 1987 Sep 10;317(11):659-65. | PubMed |
Veterans Administration Systemic Sepsis Cooperative Study Group. Effect of high-dose glucocorticoid therapy on mortality in patients with clinical signs of systemic sepsis. N Engl J Med. 1987 Sep 10;317(11):659-65. | PubMed | Thompson, WL, Gurley, HT, Lutz, BA, Jackson, DL, Kvols, LK, Morris, IA. Inefficacy of glucocorticoids in shock (double-blind-study). CLINICAL RESEARCH. 1976;24(3):A258-A258. | Link |
Thompson, WL, Gurley, HT, Lutz, BA, Jackson, DL, Kvols, LK, Morris, IA. Inefficacy of glucocorticoids in shock (double-blind-study). CLINICAL RESEARCH. 1976;24(3):A258-A258. | Link | Thwaites GE, Nguyen DB, Nguyen HD, Hoang TQ, Do TT, Nguyen TC, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004 Oct 21;351(17):1741-51. | PubMed |
Thwaites GE, Nguyen DB, Nguyen HD, Hoang TQ, Do TT, Nguyen TC, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004 Oct 21;351(17):1741-51. | PubMed | Torres A, Sibila O, Ferrer M, Polverino E, Menendez R, Mensa J, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015 Feb 17;313(7):677-86. | CrossRef | PubMed |
Torres A, Sibila O, Ferrer M, Polverino E, Menendez R, Mensa J, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015 Feb 17;313(7):677-86. | CrossRef | PubMed | Wagner HN Jr, Bennett IL Jr, Lasagna L, Cluff LE, Rosenthal MB, Mirick GS. The effect of hydrocortisone upon the course of pneumococcal pneumonia treated with penicillin. Bull Johns Hopkins Hosp. 1956 Mar;98(3):197-215. | PubMed |
Wagner HN Jr, Bennett IL Jr, Lasagna L, Cluff LE, Rosenthal MB, Mirick GS. The effect of hydrocortisone upon the course of pneumococcal pneumonia treated with penicillin. Bull Johns Hopkins Hosp. 1956 Mar;98(3):197-215. | PubMed | Yildiz O, Doganay M, Aygen B, Güven M, Keleştimur F, Tutuû A. Physiological-dose steroid therapy in sepsis [ISRCTN36253388]. Crit Care. 2002 Jun;6(3):251-9. | PubMed |
Yildiz O, Doganay M, Aygen B, Güven M, Keleştimur F, Tutuû A. Physiological-dose steroid therapy in sepsis [ISRCTN36253388]. Crit Care. 2002 Jun;6(3):251-9. | PubMed | Yildiz O, Tanriverdi F, Simsek S, Aygen B, Kelestimur F. The effects of moderate-dose steroid therapy in sepsis: A placebo-controlled, randomized study. J Res Med Sci. 2011 Nov;16(11):1410-21. | PubMed |
Yildiz O, Tanriverdi F, Simsek S, Aygen B, Kelestimur F. The effects of moderate-dose steroid therapy in sepsis: A placebo-controlled, randomized study. J Res Med Sci. 2011 Nov;16(11):1410-21. | PubMed | Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest. 1992 Jun;101(6):1481-3. | PubMed |
Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest. 1992 Jun;101(6):1481-3. | PubMed | Singer, Mervyn, et al. "The third international consensus definitions for sepsis and septic shock (sepsis-3). Jama (2016): 801-810. | Link |
Singer, Mervyn, et al. "The third international consensus definitions for sepsis and septic shock (sepsis-3). Jama (2016): 801-810. | Link | Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Crit care med. 2013;41(2):580-637. | Link |
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Crit care med. 2013;41(2):580-637. | Link |