 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Patients with prolonged febrile neutropenia are at high risk of invasive fungal infection, so it has been standard practice to initiate empirical antifungal therapy in these cases. However, this strategy is associated with important toxicity, so diagnostic test-guided preemptive antifungal therapy has been proposed as an alternative. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified three systematic reviews including twelve studies overall. Four randomized controlled trials addressed the question of this article. We combined the evidence using meta-analysis and generated a summary of findings following the GRADE approach. We concluded it is not clear whether preemptive strategy affects mortality because the certainty of the evidence is very low, but it might slightly decrease the use of antifungal agents in patients with prolonged febrile neutropenia.
During chemotherapy-induced neutropenia fever occurs frequently but clinical infection is documented in only 20-30% of febrile episodes [1]. Invasive fungal infections usually appear after the first week of prolonged neutropenia and empirical antibiotic therapy, and constitute an important cause of morbidity and mortality [2]. It has been standard practice to initiate empirical therapy for fungal infections in patients with prolonged febrile neutropenia, and this approach has been incorporated into several guidelines. Approximately 40–50% of patients are treated with antifungals using this empirical approach, whereas the estimated actual incidence of invasive fungal infection is around 10–15% [3]. As a result, many patients are potentially exposed to unnecessary toxic treatment and considerable financial burden. Diagnostic test-guided preemptive antifungal therapy, that is antifungal treatment instituted if indicators of possible invasive fungal are identified, has been proposed as an alternative treatment strategy in these patients.
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
|
What is the evidence. |
We identified three systematic reviews [4],[5],[6] including twelve primary studies [7],[8],[9],[10],[11],[12],[13],[14],[15],[16],[17],[18] from which five correspond to randomized controlled trials [9],[10],[13],[15],[18]. One of the trials was excluded from this summary since it does not compare against empirical strategy [9]. This table and the summary in general are based on the four pertinent randomized controlled trials [10],[13],[15],[18]. |
|
What types of patients were included |
Two studies included only adults [10],[15], one study included both adults and children [18] and one study did not report the age of the patients [13]. All studies included only patients with hematological malignancies. One study included only patients who received allogenic stem cell transplant [13], and the other three included patients undergoing chemotherapy or allogenic stem cell transplant [10],[15],[18]. |
|
What types of interventions were included |
All studies used different criteria for starting antifungal therapy in the preemptive strategy, and different tests for early diagnosis of invasive fungal disease. Three studies used galactomannan test twice a week [10],[15],[18]; one study used nested PCR for Aspergillus [15] and one study non-nested PCR for Aspergillus and Candida [13]; only one study performed blood cultures [13]. All studies used imaging as diagnostic test; two studies performed chest computed tomography if serological tests were positive [15],[18], one study performed chest and abdomen computed tomography to all patients [13], and one study performed chest radiography followed by chest computed tomography [10]. |
|
What types of outcomes |
Different systematic reviews reported meta-analysis for the following outcomes:
|
Information on the effects of preemptive strategy is based on four randomized controlled studies involving 988 patients [10],[13],[15],[18]. All of them provided information on the use of antifungal therapy, and three studies provided information on all-cause mortality [10],[13],[15]. The summary of findings is the following:
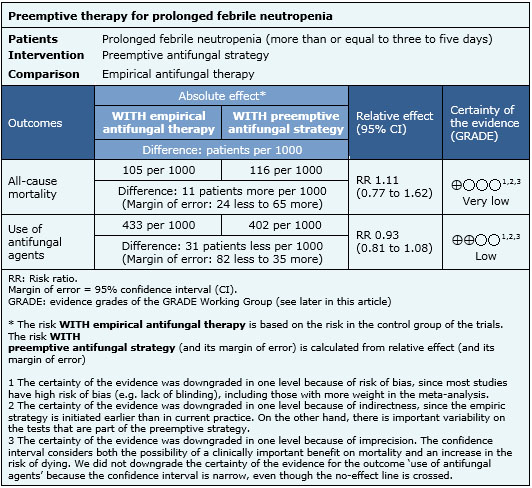

|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
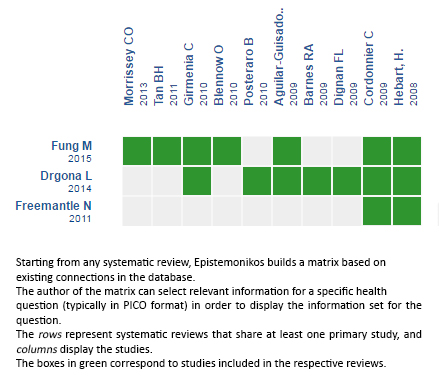
Follow the link to access the interactive version: Preemptive antifungal strategies in febrile neutropenia
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Patients with prolonged febrile neutropenia are at high risk of invasive fungal infection, so it has been standard practice to initiate empirical antifungal therapy in these cases. However, this strategy is associated with important toxicity, so diagnostic test-guided preemptive antifungal therapy has been proposed as an alternative. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified three systematic reviews including twelve studies overall. Four randomized controlled trials addressed the question of this article. We combined the evidence using meta-analysis and generated a summary of findings following the GRADE approach. We concluded it is not clear whether preemptive strategy affects mortality because the certainty of the evidence is very low, but it might slightly decrease the use of antifungal agents in patients with prolonged febrile neutropenia.
 Autores:
Erica Koch[1,2], Gabriel Rada[1,2,3,4,5]
Autores:
Erica Koch[1,2], Gabriel Rada[1,2,3,4,5]

Citación: Koch E, Rada G. Is preemptive antifungal therapy a good alternative to empirical treatment in prolonged febrile neutropenia?. Medwave 2016;16(Suppl 2):36463 doi: 10.5867/medwave.2016.6463
Fecha de publicación: 9/6/2016

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011 Feb 15;52(4):e56-93.
| CrossRef | PubMed |
Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011 Feb 15;52(4):e56-93.
| CrossRef | PubMed | Dignan FL, Evans SO, Ethell ME, Shaw BE, Davies FE, Dearden CE, et al. An early CT-diagnosis-based treatment strategy for invasive fungal infection in allogeneic transplant recipients using caspofungin first line: an effective strategy with low mortality. Bone Marrow Transplant. 2009 Jul;44(1):51-6. | CrossRef | PubMed |
Dignan FL, Evans SO, Ethell ME, Shaw BE, Davies FE, Dearden CE, et al. An early CT-diagnosis-based treatment strategy for invasive fungal infection in allogeneic transplant recipients using caspofungin first line: an effective strategy with low mortality. Bone Marrow Transplant. 2009 Jul;44(1):51-6. | CrossRef | PubMed | Mahfouz T, Anaissie E.Prevention of fungal infections in the immunocompromised host. Curr Opin Investig Drugs. 2003 Aug;4(8):974-90. | PubMed |
Mahfouz T, Anaissie E.Prevention of fungal infections in the immunocompromised host. Curr Opin Investig Drugs. 2003 Aug;4(8):974-90. | PubMed | Freemantle N, Tharmanathan P, Herbrecht R. Systematic review and mixed treatment comparison of randomized evidence for empirical, pre-emptive and directed treatment strategies for invasive mould disease. J Antimicrob Chemother. 2011 Jan;66 Suppl 1:i25-35. | CrossRef | PubMed |
Freemantle N, Tharmanathan P, Herbrecht R. Systematic review and mixed treatment comparison of randomized evidence for empirical, pre-emptive and directed treatment strategies for invasive mould disease. J Antimicrob Chemother. 2011 Jan;66 Suppl 1:i25-35. | CrossRef | PubMed | Drgona L, Khachatryan A, Stephens J, Charbonneau C, Kantecki M, Haider S, et al. Clinical and economic burden of invasive fungal diseases in Europe: focus on pre-emptive and empirical treatment of Aspergillus and Candida species. Eur J Clin Microbiol Infect Dis. 2014 Jan;33(1):7-21. | CrossRef | PubMed |
Drgona L, Khachatryan A, Stephens J, Charbonneau C, Kantecki M, Haider S, et al. Clinical and economic burden of invasive fungal diseases in Europe: focus on pre-emptive and empirical treatment of Aspergillus and Candida species. Eur J Clin Microbiol Infect Dis. 2014 Jan;33(1):7-21. | CrossRef | PubMed | Fung M, Kim J, Marty FM, Schwarzinger M, Koo S. Meta-Analysis and Cost Comparison of Empirical versus Pre-Emptive Antifungal Strategies in Hematologic Malignancy Patients with High-Risk Febrile Neutropenia. PLoS One. 2015 Nov 10;10(11):e0140930. | CrossRef | PubMed |
Fung M, Kim J, Marty FM, Schwarzinger M, Koo S. Meta-Analysis and Cost Comparison of Empirical versus Pre-Emptive Antifungal Strategies in Hematologic Malignancy Patients with High-Risk Febrile Neutropenia. PLoS One. 2015 Nov 10;10(11):e0140930. | CrossRef | PubMed | Aguilar-Guisado M, Espigado I, Cordero E, Noguer M, Parody R, Pachón J, et al. Empirical antifungal therapy in selected patients with persistent febrile neutropenia. Bone Marrow Transplant. 2010 Jan;45(1):159-64.
| CrossRef | PubMed |
Aguilar-Guisado M, Espigado I, Cordero E, Noguer M, Parody R, Pachón J, et al. Empirical antifungal therapy in selected patients with persistent febrile neutropenia. Bone Marrow Transplant. 2010 Jan;45(1):159-64.
| CrossRef | PubMed | Barnes RA, White PL, Bygrave C, Evans N, Healy B, Kell J. Clinical impact of enhanced diagnosis of invasive fungal disease in high-risk haematology and stem cell transplant patients. J Clin Pathol. 2009 Jan;62(1):64-9. | CrossRef | PubMed |
Barnes RA, White PL, Bygrave C, Evans N, Healy B, Kell J. Clinical impact of enhanced diagnosis of invasive fungal disease in high-risk haematology and stem cell transplant patients. J Clin Pathol. 2009 Jan;62(1):64-9. | CrossRef | PubMed | Blennow O, Remberger M, Klingspor L, Omazic B, Fransson K, Ljungman P, et al. Randomized PCR-based therapy and risk factors for invasive fungal infection following reduced-intensity conditioning and hematopoietic SCT. Bone Marrow Transplant. 2010 Dec;45(12):1710-8. | CrossRef | PubMed |
Blennow O, Remberger M, Klingspor L, Omazic B, Fransson K, Ljungman P, et al. Randomized PCR-based therapy and risk factors for invasive fungal infection following reduced-intensity conditioning and hematopoietic SCT. Bone Marrow Transplant. 2010 Dec;45(12):1710-8. | CrossRef | PubMed | Cordonnier C, Pautas C, Maury S, Vekhoff A, Farhat H, Suarez F, et al. Empirical versus preemptive antifungal therapy for high-risk, febrile, neutropenic patients: a randomized, controlled trial. Clin Infect Dis. 2009 Apr 15;48(8):1042-51. | CrossRef | PubMed |
Cordonnier C, Pautas C, Maury S, Vekhoff A, Farhat H, Suarez F, et al. Empirical versus preemptive antifungal therapy for high-risk, febrile, neutropenic patients: a randomized, controlled trial. Clin Infect Dis. 2009 Apr 15;48(8):1042-51. | CrossRef | PubMed | Dignan FL, Evans SO, Ethell ME, Shaw BE, Davies FE, Dearden CE, et al. An early CT-diagnosis-based treatment strategy for invasive fungal infection in allogeneic transplant recipients using caspofungin first line: an effective strategy with low mortality. Bone Marrow Transplant. 2009 Jul;44(1):51-6. | CrossRef | PubMed |
Dignan FL, Evans SO, Ethell ME, Shaw BE, Davies FE, Dearden CE, et al. An early CT-diagnosis-based treatment strategy for invasive fungal infection in allogeneic transplant recipients using caspofungin first line: an effective strategy with low mortality. Bone Marrow Transplant. 2009 Jul;44(1):51-6. | CrossRef | PubMed | Girmenia C, Micozzi A, Gentile G, Santilli S, Arleo E, Cardarelli L, et al. Clinically driven diagnostic antifungal approach in neutropenic patients: a prospective feasibility study. J Clin Oncol. 2010 Feb 1;28(4):667-74. | CrossRef | PubMed |
Girmenia C, Micozzi A, Gentile G, Santilli S, Arleo E, Cardarelli L, et al. Clinically driven diagnostic antifungal approach in neutropenic patients: a prospective feasibility study. J Clin Oncol. 2010 Feb 1;28(4):667-74. | CrossRef | PubMed | Hebart H, Klingspor L, Klingebiel T, Loeffler J, Tollemar J, Ljungman P, et al. A prospective randomized controlled trial comparing PCR-based and empirical treatment with liposomal amphotericin B in patients after allo-SCT. Bone Marrow Transplant. 2009 Apr;43(7):553-61. | CrossRef | PubMed |
Hebart H, Klingspor L, Klingebiel T, Loeffler J, Tollemar J, Ljungman P, et al. A prospective randomized controlled trial comparing PCR-based and empirical treatment with liposomal amphotericin B in patients after allo-SCT. Bone Marrow Transplant. 2009 Apr;43(7):553-61. | CrossRef | PubMed | Maertens J, Theunissen K, Verhoef G, Verschakelen J, Lagrou K, Verbeken E, et al. Galactomannan and computed tomography-based preemptive antifungal therapy in neutropenic patients at high risk for invasive fungal infection: a prospective feasibility study. Clin Infect Dis. 2005 Nov 1;41(9):1242-50. | PubMed |
Maertens J, Theunissen K, Verhoef G, Verschakelen J, Lagrou K, Verbeken E, et al. Galactomannan and computed tomography-based preemptive antifungal therapy in neutropenic patients at high risk for invasive fungal infection: a prospective feasibility study. Clin Infect Dis. 2005 Nov 1;41(9):1242-50. | PubMed | Morrissey CO, Chen SC, Sorrell TC, Milliken S, Bardy PG, Bradstock KF, et al. Galactomannan and PCR versus culture and histology for directing use of antifungal treatment for invasive aspergillosis in high-risk haematology patients: a randomised controlled trial. Lancet Infect Dis. 2013 Jun;13(6):519-28. | CrossRef | PubMed |
Morrissey CO, Chen SC, Sorrell TC, Milliken S, Bardy PG, Bradstock KF, et al. Galactomannan and PCR versus culture and histology for directing use of antifungal treatment for invasive aspergillosis in high-risk haematology patients: a randomised controlled trial. Lancet Infect Dis. 2013 Jun;13(6):519-28. | CrossRef | PubMed | Oshima K, Kanda Y, Asano-Mori Y, Nishimoto N, Arai S, Nagai S, et al. Presumptive treatment strategy for aspergillosis in allogeneic haematopoietic stem cell transplant recipients. J Antimicrob Chemother. 2007 Aug;60(2):350-5. | PubMed |
Oshima K, Kanda Y, Asano-Mori Y, Nishimoto N, Arai S, Nagai S, et al. Presumptive treatment strategy for aspergillosis in allogeneic haematopoietic stem cell transplant recipients. J Antimicrob Chemother. 2007 Aug;60(2):350-5. | PubMed | Posteraro B, Sanguinetti M, Boccia S, De Feo E, La Sorda M, Tana M, et al. Early mannan detection in bronchoalveolar lavage fluid with preemptive treatment reduces the incidence of invasive Candida infections in preterm infants. Pediatr Infect Dis J. 2010 Sep;29(9):844-8. | CrossRef | PubMed |
Posteraro B, Sanguinetti M, Boccia S, De Feo E, La Sorda M, Tana M, et al. Early mannan detection in bronchoalveolar lavage fluid with preemptive treatment reduces the incidence of invasive Candida infections in preterm infants. Pediatr Infect Dis J. 2010 Sep;29(9):844-8. | CrossRef | PubMed |