 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Intrahepatic cholestasis of pregnancy is a condition associated with fetal morbidity and mortality. Ursodeoxycholic acid has been proposed as a treatment alternative, but its use remains controversial. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified three systematic reviews including eight randomized trials. We combined the evidence using meta-analysis and generated a summary of findings table following the GRADE approach. We concluded ursodeoxycholic acid reduces prematurity risk and need for admission in neonatal intensive care units. It might also reduce maternal pruritus.
Intrahepatic obstetric cholestasis is an exclusive condition of pregnancy. The highest prevalence is in South America and Scandinavia, where it is about 6-29% [1]. It is characterized by the appearance of severe palmoplantar pruritus predominantly nocturnal, during the third trimester of pregnancy, which may be associated with elevated liver enzymes and bile acids. The etiology of this condition is not completely understood, but genetic, hormonal and environmental factors would be involved [2]]. It has been associated to an increase in premature delivery, neonatal respiratory distress syndrome and spontaneous fetal death, among others.
Ursodeoxycholic acid could reduce symptoms and adverse outcomes decreasing hepatocellular damage and placental transfer of bile acids by replacing toxic endogenous bile acids without altering the biliary function [3],[4]. However, there is controversy about the real usefulness of this intervention.
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
|
What is the evidence. |
We found three systematic reviews [5],[6],[7], including 14 studies reported in 21 references [8],[9],[10],[11], |
|
What types of patients were included |
All of the studies included pregnant women with clinical diagnosis of obstetric cholestasis. Six studies reported when pruritus started; in four studies started at week 29 [11],[12],[22],[23] and in two at week 24 [10],[17]. Five studies also used laboratory test results (bile acids > 10 and/or aminotransferase increase) as inclusion criteria [10],[11],[12],[17],[23]. Two studies did not describe inclusion criteria [14],[16]. Age and race were not considered as inclusion criteria in any study. |
|
What types of interventions were included |
All of the studies used oral ursodeoxycholic acid. Daily dose was different across studies: 600 mg [11],[22], 900 mg [16],[17], 1000 mg [12],[23], 500-2000 mg [10] and it was not specified in one study [14]. The intervention was administered during 14 days in two studies [16],[17], 20 or more days in three studies [11],[12],[22] and it was not specified in three studies [10],[14],[23]. All of the studies compared against placebo. |
|
What types of outcomes |
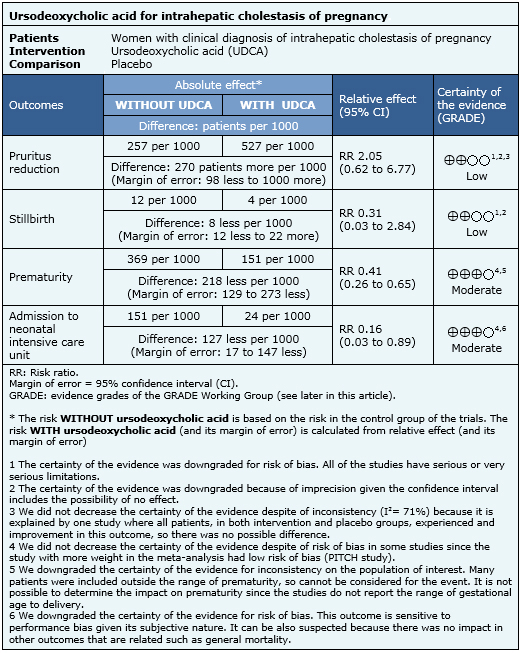
Several outcomes were measured in the studies. The outcomes were divided in maternal outcomes (reduction of pruritus, aminotransferase and bile acid levels, cesarean section and adverse effects) and neonatal outcomes (prematurity, stillbirth, admission to neonatal intensive care unit, meconium staining of amniotic fluid, birth weight and fetal distress). |
The information on the effects of ursodeoxycholic acid in intrahepatic cholestasis of pregnancy is based on eight randomized controlled trials that included 363 patients. Four studies reported pruritus improvement [11],[12],[22],[23], six studies stillbirth [10],[11],[12],[14],[17],[23], six studies prematurity [10],[11],[12],[17],[22],[23] and four studies reported admission to neonatal intensive care unit [10],[11],[22],[23].

|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
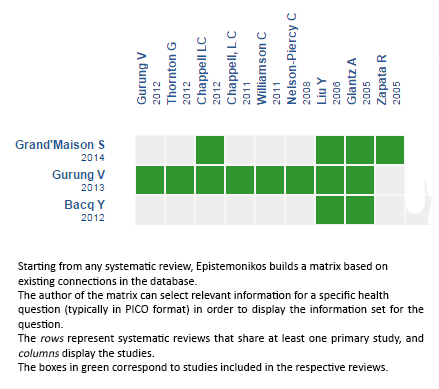
Follow the link to access the interactive version: Ursodeoxycholic acid versus placebo for intrahepatic cholestasis of pregnancy
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Intrahepatic cholestasis of pregnancy is a condition associated with fetal morbidity and mortality. Ursodeoxycholic acid has been proposed as a treatment alternative, but its use remains controversial. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified three systematic reviews including eight randomized trials. We combined the evidence using meta-analysis and generated a summary of findings table following the GRADE approach. We concluded ursodeoxycholic acid reduces prematurity risk and need for admission in neonatal intensive care units. It might also reduce maternal pruritus.
 Autores:
Sebastián Sepúlveda Marín[1,2], Valeria Contreras Maragaño[1,2], Claudio Vera[2,3,4]
Autores:
Sebastián Sepúlveda Marín[1,2], Valeria Contreras Maragaño[1,2], Claudio Vera[2,3,4]

Citación: Sepúlveda Marín S, Contreras Maragaño V, Vera C. Is ursodeoxycholic acid effective for intrahepatic cholestasis of pregnancy?. Medwave 2016;16(Suppl 1):e6361 doi: 10.5867/medwave.2015.6361
Fecha de publicación: 8/1/2016

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Gorelik J, Shevchuk AI, Diakonov I, de Swiet M, Lab M, Korchev Y, et al. Dexamethasone and ursodeoxycholic acid protect against the arrhythmogenic effect of taurocholate in an in vitro study of rat cardiomyocytes. BJOG. 2003 May;110(5):467-74. | PubMed |
Gorelik J, Shevchuk AI, Diakonov I, de Swiet M, Lab M, Korchev Y, et al. Dexamethasone and ursodeoxycholic acid protect against the arrhythmogenic effect of taurocholate in an in vitro study of rat cardiomyocytes. BJOG. 2003 May;110(5):467-74. | PubMed | Serrano MA, Brites D, Larena MG, Monte MJ, Bravo MP, Oliveira N, Marin JJ. Beneficial effect of ursodeoxycholic acid on alterations induced by cholestasis of pregnancy in bile acid transport across the human placenta. J Hepatol. 1998 May;28(5):829-39. | PubMed |
Serrano MA, Brites D, Larena MG, Monte MJ, Bravo MP, Oliveira N, Marin JJ. Beneficial effect of ursodeoxycholic acid on alterations induced by cholestasis of pregnancy in bile acid transport across the human placenta. J Hepatol. 1998 May;28(5):829-39. | PubMed | Stiehl A, Benz C, Sauer P. Mechanism of hepatoprotective action of bile salts in liver disease. Gastroenterol Clin North Am. 1999 Mar;28(1):195-209, viii. | PubMed |
Stiehl A, Benz C, Sauer P. Mechanism of hepatoprotective action of bile salts in liver disease. Gastroenterol Clin North Am. 1999 Mar;28(1):195-209, viii. | PubMed | Bacq Y, Sentilhes L, Reyes HB, Glantz A, Kondrackiene J, Binder T, et al. Efficacy of ursodeoxycholic acid in treating intrahepatic cholestasis of pregnancy: a meta-analysis. Gastroenterology. 2012 Dec;143(6):1492-501. | CrossRef | PubMed |
Bacq Y, Sentilhes L, Reyes HB, Glantz A, Kondrackiene J, Binder T, et al. Efficacy of ursodeoxycholic acid in treating intrahepatic cholestasis of pregnancy: a meta-analysis. Gastroenterology. 2012 Dec;143(6):1492-501. | CrossRef | PubMed | Grand'Maison S, Durand M, Mahone M. The effects of ursodeoxycholic acid treatment for intrahepatic cholestasis of pregnancy on maternal and fetal outcomes: a meta-analysis including non-randomized studies. J Obstet Gynaecol Can. 2014 Jul;36(7):632-41.
| PubMed |
Grand'Maison S, Durand M, Mahone M. The effects of ursodeoxycholic acid treatment for intrahepatic cholestasis of pregnancy on maternal and fetal outcomes: a meta-analysis including non-randomized studies. J Obstet Gynaecol Can. 2014 Jul;36(7):632-41.
| PubMed | Gurung V, Middleton P, Milan SJ, Hague W, Thornton JG. Interventions for treating cholestasis in pregnancy. Cochrane Database Syst Rev. 2013 Jun 24;6:CD000493. | CrossRef | PubMed |
Gurung V, Middleton P, Milan SJ, Hague W, Thornton JG. Interventions for treating cholestasis in pregnancy. Cochrane Database Syst Rev. 2013 Jun 24;6:CD000493. | CrossRef | PubMed | Brites D, Rodrigues CM. Elevated levels of bile acids in colostrum of patients with cholestasis of pregnancy are decreased following ursodeoxycholic acid therapy [see comemnts]. J Hepatol. 1998 Nov;29(5):743-51. | PubMed |
Brites D, Rodrigues CM. Elevated levels of bile acids in colostrum of patients with cholestasis of pregnancy are decreased following ursodeoxycholic acid therapy [see comemnts]. J Hepatol. 1998 Nov;29(5):743-51. | PubMed | Chappell LC, Gurung V, Chambers J, Seed PT, Williamson C, Thornton JG. Pitch: Two randomised controlled trials in obstetric cholestasis: ursodeoxycholic acid versus placebo and early delivery versus expectant management. Arch Dis Child Fetal Neonatal Ed. 2011;96:Fa110-Fa111 | CrossRef |
Chappell LC, Gurung V, Chambers J, Seed PT, Williamson C, Thornton JG. Pitch: Two randomised controlled trials in obstetric cholestasis: ursodeoxycholic acid versus placebo and early delivery versus expectant management. Arch Dis Child Fetal Neonatal Ed. 2011;96:Fa110-Fa111 | CrossRef | Chappell LC, Gurung V, Seed PT, Chambers J, Williamson C, Thornton JG. Ursodeoxycholic acid versus placebo, and early term delivery versus expectant management, in women with intrahepatic cholestasis of pregnancy: semifactorial randomised clinical trial. BMJ. 2012 Jun 13;344:e3799. | CrossRef | PubMed |
Chappell LC, Gurung V, Seed PT, Chambers J, Williamson C, Thornton JG. Ursodeoxycholic acid versus placebo, and early term delivery versus expectant management, in women with intrahepatic cholestasis of pregnancy: semifactorial randomised clinical trial. BMJ. 2012 Jun 13;344:e3799. | CrossRef | PubMed | Diaferia A, Nicastri PL, Tartagni M, Loizzi P, Iacovizzi C, Di Leo A. Ursodeoxycholic acid therapy in pregnant women with holestasis. Int J Gynaecol Obstet. 1996 Feb;52(2):133-40. | PubMed |
Diaferia A, Nicastri PL, Tartagni M, Loizzi P, Iacovizzi C, Di Leo A. Ursodeoxycholic acid therapy in pregnant women with holestasis. Int J Gynaecol Obstet. 1996 Feb;52(2):133-40. | PubMed | Glantz A, Marschall HU, Lammert F, Mattsson LA. Intrahepatic cholestasis of pregnancy: a randomized controlled trial comparing dexamethasone and ursodeoxycholic acid. Hepatology. 2005 Dec;42(6):1399-405. | PubMed |
Glantz A, Marschall HU, Lammert F, Mattsson LA. Intrahepatic cholestasis of pregnancy: a randomized controlled trial comparing dexamethasone and ursodeoxycholic acid. Hepatology. 2005 Dec;42(6):1399-405. | PubMed | Gurung V, Chappell L, Seed P, Chambers J, Williamson C, Thornton J. Pitch: Ursodeoxycholic acid versus placebo, and early delivery versus expectant management in the management of intrahepatic cholestasis of pregnancy: Two randomised controlled trials. International journal of gynaecology and obstetrics. 2012;119:S363-S4. | CrossRef |
Gurung V, Chappell L, Seed P, Chambers J, Williamson C, Thornton J. Pitch: Ursodeoxycholic acid versus placebo, and early delivery versus expectant management in the management of intrahepatic cholestasis of pregnancy: Two randomised controlled trials. International journal of gynaecology and obstetrics. 2012;119:S363-S4. | CrossRef | Isla CR, Cappelletti CA, Tielli G, Ameigeiras B, Caiafa HM, Dunaiewsky A, et al. Value of ursodeoxicholic acid in the treatment of intrahepatic cholestasis of pregnancy. American Association for the Study of Liver Diseases (published in: Gastroenterology); 1996: A1219. | Link |
Isla CR, Cappelletti CA, Tielli G, Ameigeiras B, Caiafa HM, Dunaiewsky A, et al. Value of ursodeoxicholic acid in the treatment of intrahepatic cholestasis of pregnancy. American Association for the Study of Liver Diseases (published in: Gastroenterology); 1996: A1219. | Link | Laifer SA, Stiller RJ, Siddiqui DS, Dunston-Boone G, Whetham JC. Ursodeoxycholic acid for the treatment of intrahepatic cholestasis of pregnancy. J Matern Fetal Med. 2001 Apr;10(2):131-5. | PubMed |
Laifer SA, Stiller RJ, Siddiqui DS, Dunston-Boone G, Whetham JC. Ursodeoxycholic acid for the treatment of intrahepatic cholestasis of pregnancy. J Matern Fetal Med. 2001 Apr;10(2):131-5. | PubMed | Leino R, Ekblad U, Irjala K, Erkkola R. Ursodeoxycholic acid in the treatment of intrahepatic cholestasis of pregnancy. Prenatal and neonatal medicine. 1998;3 Suppl 1:67.
| Link |
Leino R, Ekblad U, Irjala K, Erkkola R. Ursodeoxycholic acid in the treatment of intrahepatic cholestasis of pregnancy. Prenatal and neonatal medicine. 1998;3 Suppl 1:67.
| Link | Liu Y, Qiao F, Liu H, Liu D. Ursodeoxycholic acid in the treatment of intraheptic cholestasis of pregnancy. J Huazhong Univ Sci Technolog Med Sci. 2006;26(3):350-2. | PubMed |
Liu Y, Qiao F, Liu H, Liu D. Ursodeoxycholic acid in the treatment of intraheptic cholestasis of pregnancy. J Huazhong Univ Sci Technolog Med Sci. 2006;26(3):350-2. | PubMed | Mazzella G, Rizzo N, Azzaroli F, Simoni P, Bovicelli L, Miracolo A,et al. Ursodeoxycholic acid administration in patients with cholestasis of pregnancy: effects on primary bile acids in babies and mothers. Hepatology. 2001 Mar;33(3):504-8. | PubMed |
Mazzella G, Rizzo N, Azzaroli F, Simoni P, Bovicelli L, Miracolo A,et al. Ursodeoxycholic acid administration in patients with cholestasis of pregnancy: effects on primary bile acids in babies and mothers. Hepatology. 2001 Mar;33(3):504-8. | PubMed | Meng LJ, Reyes H, Axelson M, Palma J, Hernandez I, Ribalta J, Sjövall J. Progesterone metabolites and bile acids in serum of patients with intrahepatic cholestasis of pregnancy: effect of ursodeoxycholic acid therapy. Hepatology. 1997 Dec;26(6):1573-9. | PubMed |
Meng LJ, Reyes H, Axelson M, Palma J, Hernandez I, Ribalta J, Sjövall J. Progesterone metabolites and bile acids in serum of patients with intrahepatic cholestasis of pregnancy: effect of ursodeoxycholic acid therapy. Hepatology. 1997 Dec;26(6):1573-9. | PubMed | Milkiewicz P, Gallagher R, Chambers J, Eggington E, Weaver J, Elias E.Obstetric cholestasis with elevated gamma glutamyl transpeptidase: incidence, presentation and treatment. J Gastroenterol Hepatol. 2003 Nov;18(11):1283-6. | PubMed |
Milkiewicz P, Gallagher R, Chambers J, Eggington E, Weaver J, Elias E.Obstetric cholestasis with elevated gamma glutamyl transpeptidase: incidence, presentation and treatment. J Gastroenterol Hepatol. 2003 Nov;18(11):1283-6. | PubMed | Nicastri PL, Diaferia A, Tartagni M, Loizzi P, Fanelli M. A randomised placebo-controlled trial of ursodeoxycholic acid and S adenosylmethionine in the treatment of intrahepatic cholestasis of pregnancy. Br J Obstet Gynaecol. 1998 Nov;105(11):1205-7. | PubMed |
Nicastri PL, Diaferia A, Tartagni M, Loizzi P, Fanelli M. A randomised placebo-controlled trial of ursodeoxycholic acid and S adenosylmethionine in the treatment of intrahepatic cholestasis of pregnancy. Br J Obstet Gynaecol. 1998 Nov;105(11):1205-7. | PubMed | Palma J, Reyes H, Ribalta J, Hernández I, Sandoval L, Almuna R, et al. Ursodeoxycholic acid in the treatment of cholestasis of pregnancy: a randomized, double-blind study controlled with placebo. J Hepatol. 1997 Dec;27(6):1022-8. | PubMed |
Palma J, Reyes H, Ribalta J, Hernández I, Sandoval L, Almuna R, et al. Ursodeoxycholic acid in the treatment of cholestasis of pregnancy: a randomized, double-blind study controlled with placebo. J Hepatol. 1997 Dec;27(6):1022-8. | PubMed | Palma J, Reyes H, Ribalta J, Hernandez I, Sandoval L, Silva O, et al. Ursodeoxycholic acid in cholestasis of pregnancy: final report of a randomized, double-blind, placebo controlled study [AASLD abstract]. Hepatology (Baltimore, Md). 1996;24(4 (Pt 2) ):373A. | Link |
Palma J, Reyes H, Ribalta J, Hernandez I, Sandoval L, Silva O, et al. Ursodeoxycholic acid in cholestasis of pregnancy: final report of a randomized, double-blind, placebo controlled study [AASLD abstract]. Hepatology (Baltimore, Md). 1996;24(4 (Pt 2) ):373A. | Link | Rodrigues CM, Marín JJ, Brites D. Bile acid patterns in meconium are influenced by cholestasis of pregnancy and not altered by ursodeoxycholic acid treatment. Gut. 1999 Sep;45(3):446-52. | PubMed |
Rodrigues CM, Marín JJ, Brites D. Bile acid patterns in meconium are influenced by cholestasis of pregnancy and not altered by ursodeoxycholic acid treatment. Gut. 1999 Sep;45(3):446-52. | PubMed | Thornton G, Gurung V, Chappel C, Williamson C, Chambers J. Ursodeoxycholic acid versus placebo, and early delivery versus expectant management, in women with obstetric cholestasis: Two semi-factorial randomised clinical trials. Journal of maternal-fetal & neonatal medicine. 2012;25:100-1.
Thornton G, Gurung V, Chappel C, Williamson C, Chambers J. Ursodeoxycholic acid versus placebo, and early delivery versus expectant management, in women with obstetric cholestasis: Two semi-factorial randomised clinical trials. Journal of maternal-fetal & neonatal medicine. 2012;25:100-1.  Williamson C, Chappell LC, Gurung V, Chambers J, Seed PT, Thornton JG. Pitch: two randomised controlled trials in intrahepatic cholestasis of pregnancy: ursodeoxycholic acid vs. placebo and early delivery vs. expectant management. Hepatology (Baltimore, Md). 2011;54(4 Suppl):925A. | Link |
Williamson C, Chappell LC, Gurung V, Chambers J, Seed PT, Thornton JG. Pitch: two randomised controlled trials in intrahepatic cholestasis of pregnancy: ursodeoxycholic acid vs. placebo and early delivery vs. expectant management. Hepatology (Baltimore, Md). 2011;54(4 Suppl):925A. | Link | Zapata R, Sandoval L, Palma J, Hernández I, Ribalta J, Reyes H, et al. Ursodeoxycholic acid in the treatment of intrahepatic cholestasis of pregnancy. A 12-year experience. Liver Int. 2005 Jun;25(3):548-54. | PubMed |
Zapata R, Sandoval L, Palma J, Hernández I, Ribalta J, Reyes H, et al. Ursodeoxycholic acid in the treatment of intrahepatic cholestasis of pregnancy. A 12-year experience. Liver Int. 2005 Jun;25(3):548-54. | PubMed | Gynecologists. RCoOa. Obstetric Cholestasis. Guideline N° 43. 2011.
Gynecologists. RCoOa. Obstetric Cholestasis. Guideline N° 43. 2011.