 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Chronic spontaneous urticaria is a disorder mediated by mast cells, characterized by the development of wheals, angioedema or both, lasting six weeks or more, with or without a known trigger agent. First and second line treatment are antihistamines, but some refractory cases require other alternatives, such as omalizumab. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified four systematic reviews including five pertinent randomized controlled trials overall. We combined the evidence using meta-analysis and generated a summary of findings following the GRADE approach. We concluded omalizumab reduces symptoms and improves quality of life in patients with chronic spontaneous urticaria.
Chronic spontaneous urticaria is a disorder mediated by mast cells, characterized by wheals, angioedema or both, lasting six weeks or more. Usual treatment is based on H1 antihistamines, but some patients do not achieve an optimal clinical response even with maximal doses. Different alternatives have emerged for second and third line treatment of this condition, such as omalizumab, a monoclonal antibody that selectively binds to IgE. It has been postulated that omalizumab would improve symptoms and quality of life. The main potential adverse effects are headache, abdominal pain and injection site reaction.
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
|
What is the evidence. |
We found four systematic reviews [1],[2],[3],[4] that include five primary studies [5],[6],[7],[8],[9], all of which correspond to randomized controlled trials. |
|
What types of patients were included |
Five studies included adults with chronic spontaneous urticaria defined by increased itching or hives lasting for 6-8 weeks despite treatment with antihistamines. Three studies included patients with UAS7 (Urticaria Activity Score) > 16 points [5],[6],[7], one study with UAS > 4 points [8] and one study with UAS7 > 10 points [9]. |
|
What types of interventions were included |
The intervention was omalizumab versus placebo in all the studies, maintaining the antihistamine baseline treatment. One study used omalizumab 300 mg/day [5], one study used 75-375 mg/day [9], two studies used doses of 75, 150 and 300 mg/day [6],[7], and one study used doses of 75, 300 and 600 mg/day [8]. The treatment period with omalizumab lasted 24 weeks in three studies [5],[6],[9], 12 weeks in one study [7] and 4 weeks in one study [8]. |
|
What types of outcomes |
The outcomes measured were the change in the index of disease and quality of life. Index of disease was measured with UAS7, which evaluates itching and hives for seven days with a scale of 0-6 points per day, with a minimum of 0 points and a maximum of 42 points. Quality of life was evaluated with CU-Q2oL (Chronic Urticaria Quality of Life Questionnaire) which evaluates 23 factors ranging from 0 to 5 points, with a minimum of 0 points and a maximum of 115. |
The information on the effects of omalizumab is based on five randomized studies including 1117 patients. All studies measured improvement with UAS7 scale and four studies measured improvement with Q2oL [5],[6],[7],[8],[9].
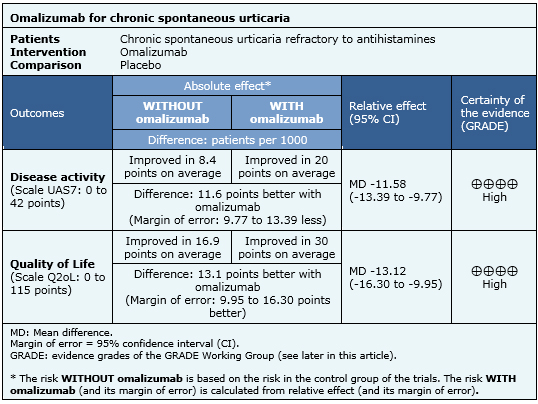
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
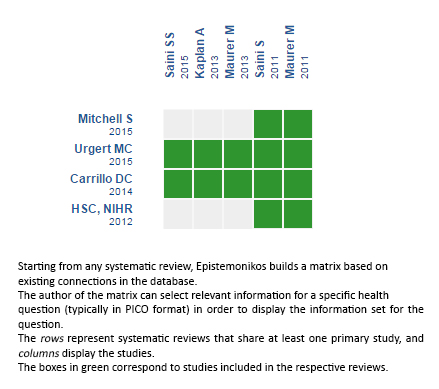
Follow the link to access the interactive version: Omalizumab versus placebo for chronic spontaneous urticaria
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Chronic spontaneous urticaria is a disorder mediated by mast cells, characterized by the development of wheals, angioedema or both, lasting six weeks or more, with or without a known trigger agent. First and second line treatment are antihistamines, but some refractory cases require other alternatives, such as omalizumab. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified four systematic reviews including five pertinent randomized controlled trials overall. We combined the evidence using meta-analysis and generated a summary of findings following the GRADE approach. We concluded omalizumab reduces symptoms and improves quality of life in patients with chronic spontaneous urticaria.
 Autor:
Mario López[1,3], Lucas Navajas-Galimany[1,2,3]
Autor:
Mario López[1,3], Lucas Navajas-Galimany[1,2,3]

Citación: López M, Navajas-Galimany L. What are the effects of omalizumab in refractory chronic spontaneous urticaria?. Medwave 2015;15(Suppl 3):e6346 doi: 10.5867/medwave.2015.6346
Fecha de publicación: 24/12/2015

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Mitchell S, Balp MM, Samuel M, McBride D, Maurer M. Systematic review of treatments for chronic spontaneous urticaria with inadequate response to licensed first-line treatments. Int J Dermatol. 2015 Sep;54(9):1088-104. | CrossRef | PubMed |
Mitchell S, Balp MM, Samuel M, McBride D, Maurer M. Systematic review of treatments for chronic spontaneous urticaria with inadequate response to licensed first-line treatments. Int J Dermatol. 2015 Sep;54(9):1088-104. | CrossRef | PubMed | Carrillo DC, Borges MS, García E, Egea E, Serrano CD. Omalizumab vs. placebo in the management of chronic idiopathic urticaria: a systematic review. World Allergy Organ J. 2014 Dec 31;7(1):72.
| CrossRef | PubMed |
Carrillo DC, Borges MS, García E, Egea E, Serrano CD. Omalizumab vs. placebo in the management of chronic idiopathic urticaria: a systematic review. World Allergy Organ J. 2014 Dec 31;7(1):72.
| CrossRef | PubMed | Urgert MC, van den Elzen MT, Knulst AC, Fedorowicz Z, van Zuuren EJ. Omalizumab in patients with chronic spontaneous urticaria: a systematic review and GRADE assessment. Br J Dermatol. 2015 Aug;173(2):404-15. | CrossRef | PubMed |
Urgert MC, van den Elzen MT, Knulst AC, Fedorowicz Z, van Zuuren EJ. Omalizumab in patients with chronic spontaneous urticaria: a systematic review and GRADE assessment. Br J Dermatol. 2015 Aug;173(2):404-15. | CrossRef | PubMed | HSC, NIHR. Omalizumab for chronic spontaneous urticaria- second line. Birmingham NIHR Horizon Scanning Centre (NIHR HSC) 2012. | Link |
HSC, NIHR. Omalizumab for chronic spontaneous urticaria- second line. Birmingham NIHR Horizon Scanning Centre (NIHR HSC) 2012. | Link | Kaplan A, Ledford D, Ashby M, Canvin J, Zazzali JL, Conner E, et al. Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticaria despite standard combination therapy. J Allergy Clin Immunol. 2013 Jul;132(1):101-9. | CrossRef | PubMed |
Kaplan A, Ledford D, Ashby M, Canvin J, Zazzali JL, Conner E, et al. Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticaria despite standard combination therapy. J Allergy Clin Immunol. 2013 Jul;132(1):101-9. | CrossRef | PubMed | Saini SS, Bindslev-Jensen C, Maurer M, Grob JJ, Bülbül Baskan E, Bradley MS, et al. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on H1 antihistamines: a randomized, placebo-controlled study. J Invest Dermatol. 2015 Jan;135(1):67-75. | CrossRef | PubMed |
Saini SS, Bindslev-Jensen C, Maurer M, Grob JJ, Bülbül Baskan E, Bradley MS, et al. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on H1 antihistamines: a randomized, placebo-controlled study. J Invest Dermatol. 2015 Jan;135(1):67-75. | CrossRef | PubMed | Maurer M, Rosén K, Hsieh HJ, Saini S, Grattan C, Gimenéz-Arnau A, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013 Mar 7;368(10):924-35. | CrossRef | PubMed |
Maurer M, Rosén K, Hsieh HJ, Saini S, Grattan C, Gimenéz-Arnau A, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013 Mar 7;368(10):924-35. | CrossRef | PubMed | Saini S, Rosen KE, Hsieh HJ, Wong DA, Conner E, Kaplan A, et al. A randomized, placebo-controlled, dose-ranging study of single-dose omalizumab in patients with H1-antihistamine-refractory chronic idiopathic urticaria. J Allergy Clin Immunol. 2011 Sep;128(3):567-73.e1. | CrossRef | PubMed |
Saini S, Rosen KE, Hsieh HJ, Wong DA, Conner E, Kaplan A, et al. A randomized, placebo-controlled, dose-ranging study of single-dose omalizumab in patients with H1-antihistamine-refractory chronic idiopathic urticaria. J Allergy Clin Immunol. 2011 Sep;128(3):567-73.e1. | CrossRef | PubMed | Maurer M, Altrichter S, Bieber T, Biedermann T, Bräutigam M, Seyfried S, et al. Efficacy and safety of omalizumab in patients with chronic urticaria who exhibit IgE against thyroperoxidase. J Allergy Clin Immunol. 2011 Jul;128(1):202-209.e5. | CrossRef | PubMed |
Maurer M, Altrichter S, Bieber T, Biedermann T, Bräutigam M, Seyfried S, et al. Efficacy and safety of omalizumab in patients with chronic urticaria who exhibit IgE against thyroperoxidase. J Allergy Clin Immunol. 2011 Jul;128(1):202-209.e5. | CrossRef | PubMed | Har D, Patel S, Khan DA. Outcomes of using omalizumab for more than 1 year in refractory chronic urticaria. Ann Allergy Asthma Immunol. 2015 Aug;115(2):126-9. | CrossRef | PubMed |
Har D, Patel S, Khan DA. Outcomes of using omalizumab for more than 1 year in refractory chronic urticaria. Ann Allergy Asthma Immunol. 2015 Aug;115(2):126-9. | CrossRef | PubMed | British Medical Association and Royal Pharmaceutical Company of Great Britain. British National Formulary. BNF 63. London: BMJ Group and RPS Publishing; 2012.
British Medical Association and Royal Pharmaceutical Company of Great Britain. British National Formulary. BNF 63. London: BMJ Group and RPS Publishing; 2012.