 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
HIV infection is a worldwide epidemic. Antiretroviral therapy has dramatically changed the outcome of the disease but there is still controversy about the best time to initiate it, especially in patients with CD4 counts over 350 cells/µL. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified two systematic reviews including four pertinent randomized controlled trials overall. We concluded early initiation of antiretroviral therapy probably reduces mortality, risk of opportunistic infections and tuberculosis, but increases the risk of important adverse effects.
Human immunodeficiency virus (HIV) infection is a worldwide epidemic. The use of antiretroviral therapy has changed the outcome of the disease, clearly and consistently reducing mortality. There is wide agreement about the benefits of starting antiretroviral therapy in patients at B or C stage, CD4 count below 350, during pregnancy or lactation, in patients co-infected with hepatitis C virus, hepatitis B virus or tuberculosis, HCV, and in patients over 50 years of age. However, there is no consensus about the effects of early initiation of antiretroviral therapy in patients with CD4 counts over 350.
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
|
What is the evidence. |
We found two systematic reviews [1],[2], that consider 23 primary studies reported in 25 references [3],[4],[5],[6], [7],[8],[9],[10],[11],[12],[13],[14],[15],[16],[17],[18], [19],[20],[21],[22],[23],[24],[25],[26],[27], including four randomized controlled trials reported in six references [6],[11],[12],[13],[16],[20]. This table and the summary in general are based on the latter. |
|
What types of patients were included |
Two studies were conducted in multiple countries [6],[11], one in Haiti [13] and one in the USA [16]. All of the studies included patients over 18 years of age, except one study that included younger patients (over 13 years) [11]. All studies included HIV infected adults, naïve to treatment and willing to be treated. One study included patients with CD4 counts equal or greater than 350 [11], one with CD4 counts between 200 and 350 [13], one with CD4 counts between 350 and 500 [6] and other with CD4 counts equal or greater than 350 [16]. Average of CD4 counts across studies was 337. All studies excluded pregnant or nursing women, patients in advanced stage of disease (AIDS) or with previous use of antiretroviral therapy. Two studies included normal laboratory parameters among their inclusion criteria [6],[16]. |
|
What types of interventions were included |
The interventions were:
|
|
What types of outcomes |
The following outcomes were measured:
|
The information on the effects of early initiation of antiretroviral therapy in HIV infected patients with CD4 counts over 350 is based on four randomized controlled trials [6],[11],[13],[16], that include 4,686 patients. Two studies [6],[11], measured the outcome opportunistic infections, three [6],[11],[13] the outcome tuberculosis, three [6],[11],[13] measured mortality and two studies [6],[13] measured grade 3-4 adverse events.
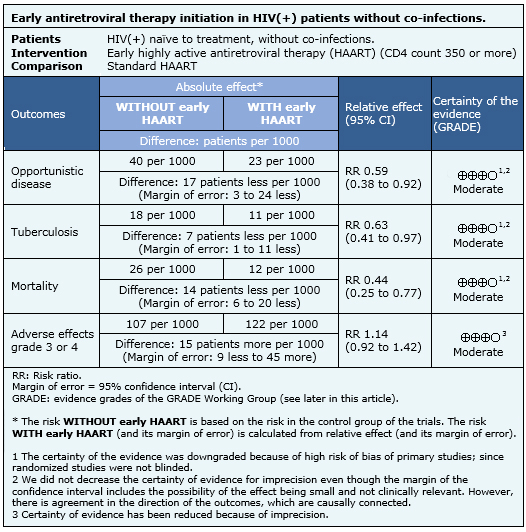

|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
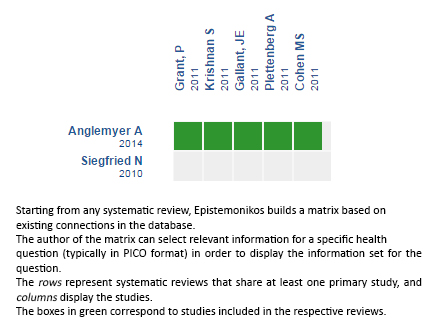
Follow the link to access the interactive version: Early initiation of antiretroviral therapy in HIV-infected adults without coinfection
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

HIV infection is a worldwide epidemic. Antiretroviral therapy has dramatically changed the outcome of the disease but there is still controversy about the best time to initiate it, especially in patients with CD4 counts over 350 cells/µL. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified two systematic reviews including four pertinent randomized controlled trials overall. We concluded early initiation of antiretroviral therapy probably reduces mortality, risk of opportunistic infections and tuberculosis, but increases the risk of important adverse effects.
 Autores:
Verónica Chauriye[1,2], Ximena Monsalve[1,2,3]
Autores:
Verónica Chauriye[1,2], Ximena Monsalve[1,2,3]

Citación: Chauriye V, Monsalve X. Is early antiretroviral therapy initiation useful in HIV(+) adults without co-infections?. Medwave 2015;15(Suppl 3):e6326 doi: 10.5867/medwave.2015.6326
Fecha de publicación: 2/12/2015

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Siegfried N, Uthman OA, Rutherford GW. Optimal time for initiation of antiretroviral therapy in asymptomatic, HIV-infected, treatment-naive adults. Cochrane Database Syst Rev. 2010 Mar 17;(3):CD008272. | CrossRef | PubMed |
Siegfried N, Uthman OA, Rutherford GW. Optimal time for initiation of antiretroviral therapy in asymptomatic, HIV-infected, treatment-naive adults. Cochrane Database Syst Rev. 2010 Mar 17;(3):CD008272. | CrossRef | PubMed | Anglemyer A, Rutherford GW, Easterbrook PJ, Horvath T, Vitória M, Jan M, et al. Early initiation of antiretroviral therapy in HIV-infected adults and adolescents: a systematic review. AIDS. 2014 Mar;28 Suppl 2:S105-18. | CrossRef | PubMed |
Anglemyer A, Rutherford GW, Easterbrook PJ, Horvath T, Vitória M, Jan M, et al. Early initiation of antiretroviral therapy in HIV-infected adults and adolescents: a systematic review. AIDS. 2014 Mar;28 Suppl 2:S105-18. | CrossRef | PubMed | Ahdieh-Grant L, Yamashita TE, Phair JP, Detels R, Wolinsky SM, Margolick JB, et al. When to initiate highly active antiretroviral therapy: a cohort approach. Am J Epidemiol. 2003 Apr 15;157(8):738-46. | PubMed |
Ahdieh-Grant L, Yamashita TE, Phair JP, Detels R, Wolinsky SM, Margolick JB, et al. When to initiate highly active antiretroviral therapy: a cohort approach. Am J Epidemiol. 2003 Apr 15;157(8):738-46. | PubMed | Babiker A, Darbyshire J, Pezzotti P, Porter K, Prins M, Sabin C, et al. Short-term CD4 cell response after highly active antiretroviral therapy initiated at different times from seroconversion in 1,500 seroconverters. J Acquir Immune Defic Syndr. 2003 Mar 1;32(3):303-10. | PubMed |
Babiker A, Darbyshire J, Pezzotti P, Porter K, Prins M, Sabin C, et al. Short-term CD4 cell response after highly active antiretroviral therapy initiated at different times from seroconversion in 1,500 seroconverters. J Acquir Immune Defic Syndr. 2003 Mar 1;32(3):303-10. | PubMed | Chêne G, Sterne JA, May M, Costagliola D, Ledergerber B, Phillips AN, et al. Prognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy: analysis of prospective studies. Lancet. 2003 Aug 30;362(9385):679-86. | PubMed |
Chêne G, Sterne JA, May M, Costagliola D, Ledergerber B, Phillips AN, et al. Prognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy: analysis of prospective studies. Lancet. 2003 Aug 30;362(9385):679-86. | PubMed | Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011 Aug 11;365(6):493-505. | CrossRef | PubMed |
Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011 Aug 11;365(6):493-505. | CrossRef | PubMed | HIV-CAUSAL Collaboration, Cain LE, Logan R, Robins JM, Sterne JA, et al. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries: an observational study. Ann Intern Med. 2011 Apr 19;154(8):509-15. | CrossRef | PubMed |
HIV-CAUSAL Collaboration, Cain LE, Logan R, Robins JM, Sterne JA, et al. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries: an observational study. Ann Intern Med. 2011 Apr 19;154(8):509-15. | CrossRef | PubMed | HIV-CAUSAL Collaboration, Ray M, Logan R, Sterne JA, Hernández-Díaz S, Robins JM, et al. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS. 2010 Jan 2;24(1):123-37. | CrossRef | PubMed |
HIV-CAUSAL Collaboration, Ray M, Logan R, Sterne JA, Hernández-Díaz S, Robins JM, et al. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS. 2010 Jan 2;24(1):123-37. | CrossRef | PubMed | Cozzi Lepri A, Phillips AN, d'Arminio Monforte A, Castelli F, Antinori A, de Luca A, et al. When to start highly active antiretroviral therapy in chronically HIV-infected patients: evidence from the ICONA study. AIDS. 2001 May 25;15(8):983-90. | PubMed |
Cozzi Lepri A, Phillips AN, d'Arminio Monforte A, Castelli F, Antinori A, de Luca A, et al. When to start highly active antiretroviral therapy in chronically HIV-infected patients: evidence from the ICONA study. AIDS. 2001 May 25;15(8):983-90. | PubMed | Egger M, May M, Chêne G, Phillips AN, Ledergerber B, Dabis F, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002 Jul 13;36(9327):119-29. | PubMed |
Egger M, May M, Chêne G, Phillips AN, Ledergerber B, Dabis F, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002 Jul 13;36(9327):119-29. | PubMed | Strategies for Management of Antiretroviral Therapy (SMART) Study Group, El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006 Nov 30;355(22):2283-96. | PubMed |
Strategies for Management of Antiretroviral Therapy (SMART) Study Group, El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006 Nov 30;355(22):2283-96. | PubMed | Strategies for Management of Antiretroviral Therapy (SMART) Study Group, Emery S, Neuhaus JA, Phillips AN, Babiker A, Cohen CJ, Get al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008 Apr 15;197(8):1133-44.
| CrossRef | PubMed |
Strategies for Management of Antiretroviral Therapy (SMART) Study Group, Emery S, Neuhaus JA, Phillips AN, Babiker A, Cohen CJ, Get al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008 Apr 15;197(8):1133-44.
| CrossRef | PubMed | Fitzgerald D. A randomized clinical trial of early versus strandard antiretroviral therapy for HIV-infected patients with a CD4 T cell count of 200 - 350 cells/ml (CIPRA HT 001). International AIDS Society Conference, Cape Town; 2009.
Fitzgerald D. A randomized clinical trial of early versus strandard antiretroviral therapy for HIV-infected patients with a CD4 T cell count of 200 - 350 cells/ml (CIPRA HT 001). International AIDS Society Conference, Cape Town; 2009.  Gallant JE, Hulbert E, Harley C. Health outcomes associated with the timing of antiretroviral therapy initiation. 6th IAS Conference on HIV Pathogenesis and Treatment Rome, Italy; 2011: Abstract no. CDB320.
Gallant JE, Hulbert E, Harley C. Health outcomes associated with the timing of antiretroviral therapy initiation. 6th IAS Conference on HIV Pathogenesis and Treatment Rome, Italy; 2011: Abstract no. CDB320.  García F, de Lazzari E, Plana M, Castro P, Mestre G, Nomdedeu M, et al. Long-term CD4+ T-cell response to highly active antiretroviral therapy according to baseline CD4+ T-cell count. J Acquir Immune Defic Syndr. 2004 Jun 1;36(2):702-13. | PubMed |
García F, de Lazzari E, Plana M, Castro P, Mestre G, Nomdedeu M, et al. Long-term CD4+ T-cell response to highly active antiretroviral therapy according to baseline CD4+ T-cell count. J Acquir Immune Defic Syndr. 2004 Jun 1;36(2):702-13. | PubMed | Grant P, Tierney C, Katzenstein D. Association of baseline viral load, CD4 count, and Week 4 virologic response (VR) with virologic failure (VF) in ACTG study A5202. 18th Conference on Retroviruses and Opportunistic Infections, Boston, Massachusetts; 2011: Abstract 535.
Grant P, Tierney C, Katzenstein D. Association of baseline viral load, CD4 count, and Week 4 virologic response (VR) with virologic failure (VF) in ACTG study A5202. 18th Conference on Retroviruses and Opportunistic Infections, Boston, Massachusetts; 2011: Abstract 535.  Gras L, Kesselring AM, Griffin JT, van Sighem AI, Fraser C, Ghani AC, et al. CD4 cell counts of 800 cells/mm3 or greater after 7 years of highly active antiretroviral therapy are feasible in most patients starting with 350 cells/mm3 or greater. J Acquir Immune Defic Syndr. 2007 Jun 1;45(2):183-92. | PubMed |
Gras L, Kesselring AM, Griffin JT, van Sighem AI, Fraser C, Ghani AC, et al. CD4 cell counts of 800 cells/mm3 or greater after 7 years of highly active antiretroviral therapy are feasible in most patients starting with 350 cells/mm3 or greater. J Acquir Immune Defic Syndr. 2007 Jun 1;45(2):183-92. | PubMed | Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009 Apr 30;360(18):1815-26. | CrossRef | PubMed |
Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009 Apr 30;360(18):1815-26. | CrossRef | PubMed | Krishnan S, Schouten JT, Jacobson DL, Benson CA, Collier AC, Koletar SL, et al. Incidence of non-AIDS-defining cancer in antiretroviral treatment-naïve subjects after antiretroviral treatment initiation: an ACTG longitudinal linked randomized trials analysis. Oncology. 2011;80(1-2):42-9. | CrossRef | PubMed |
Krishnan S, Schouten JT, Jacobson DL, Benson CA, Collier AC, Koletar SL, et al. Incidence of non-AIDS-defining cancer in antiretroviral treatment-naïve subjects after antiretroviral treatment initiation: an ACTG longitudinal linked randomized trials analysis. Oncology. 2011;80(1-2):42-9. | CrossRef | PubMed | Strategies for Management of Antiretroviral Therapy (SMART) Study Group, Lundgren JD, Babiker A, El-Sadr W, Emery S, Grund B, et al. Inferior clinical outcome of the CD4+ cell count-guided antiretroviral treatment interruption strategy in the SMART study: role of CD4+ Cell counts and HIV RNA levels during follow-up. J Infect Dis. 2008 Apr 15;197(8):1145-55. | CrossRef | PubMed |
Strategies for Management of Antiretroviral Therapy (SMART) Study Group, Lundgren JD, Babiker A, El-Sadr W, Emery S, Grund B, et al. Inferior clinical outcome of the CD4+ cell count-guided antiretroviral treatment interruption strategy in the SMART study: role of CD4+ Cell counts and HIV RNA levels during follow-up. J Infect Dis. 2008 Apr 15;197(8):1145-55. | CrossRef | PubMed | Merito M, Pezzotti P; ICONA Study Group. Comparing costs and effectiveness of different starting points for highly active antiretroviral therapy in HIV-positive patients. Evidence from the ICONA cohort. Eur J Health Econ. 2006 Mar;7(1):30-6. | PubMed |
Merito M, Pezzotti P; ICONA Study Group. Comparing costs and effectiveness of different starting points for highly active antiretroviral therapy in HIV-positive patients. Evidence from the ICONA cohort. Eur J Health Econ. 2006 Mar;7(1):30-6. | PubMed | Moore DM, Harris R, Lima V, Hogg B, May M, Yip B, et al. Effect of baseline CD4 cell counts on the clinical significance of short-term immunologic response to antiretroviral therapy in individuals with virologic suppression. J Acquir Immune Defic Syndr. 2009 Nov 1;52(3):357-63. | CrossRef | PubMed |
Moore DM, Harris R, Lima V, Hogg B, May M, Yip B, et al. Effect of baseline CD4 cell counts on the clinical significance of short-term immunologic response to antiretroviral therapy in individuals with virologic suppression. J Acquir Immune Defic Syndr. 2009 Nov 1;52(3):357-63. | CrossRef | PubMed | Opravil M, Ledergerber B, Furrer H, Hirschel B, Imhof A, Gallant S, et al. Clinical efficacy of early initiation of HAART in patients with asymptomatic HIV infection and CD4 cell count > 350 x 10(6) /l. AIDS. 2002 Jul 5;16(10):1371-81. | PubMed |
Opravil M, Ledergerber B, Furrer H, Hirschel B, Imhof A, Gallant S, et al. Clinical efficacy of early initiation of HAART in patients with asymptomatic HIV infection and CD4 cell count > 350 x 10(6) /l. AIDS. 2002 Jul 5;16(10):1371-81. | PubMed | Palella FJ Jr, Deloria-Knoll M, Chmiel JS, Moorman AC, Wood KC, Greenberg AE, et al. Survival benefit of initiating
antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Ann Intern Med. 2003 Apr 15;138(8):620-6.
| PubMed |
Palella FJ Jr, Deloria-Knoll M, Chmiel JS, Moorman AC, Wood KC, Greenberg AE, et al. Survival benefit of initiating
antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Ann Intern Med. 2003 Apr 15;138(8):620-6.
| PubMed | Phillips AN, Staszewski S, Weber R, Kirk O, Francioli P, Miller V, et al. HIV viral load response to antiretroviral therapy according to the baseline CD4 cell count and viral load. JAMA. 2001 Nov 28;286(20):2560-7. | PubMed |
Phillips AN, Staszewski S, Weber R, Kirk O, Francioli P, Miller V, et al. HIV viral load response to antiretroviral therapy according to the baseline CD4 cell count and viral load. JAMA. 2001 Nov 28;286(20):2560-7. | PubMed | Plettenberg A, Brockmeyer NH, Haastert B, Michalik C, Dupke S, Schewe K, et al. Impact of earlier HAART initiation on the immune status and clinical course of treated patients on the basis of cohort data of the German Competence Network for HIV/AIDS. Infection. 2011 Feb;39(1):3-12. | CrossRef | PubMed |
Plettenberg A, Brockmeyer NH, Haastert B, Michalik C, Dupke S, Schewe K, et al. Impact of earlier HAART initiation on the immune status and clinical course of treated patients on the basis of cohort data of the German Competence Network for HIV/AIDS. Infection. 2011 Feb;39(1):3-12. | CrossRef | PubMed | Writing Committee for the CASCADE Collaboration. Timing of HAART initiation and clinical outcomes in human immunodeficiency virus type 1 seroconverters. Arch Intern Med. 2011 Sep 26;171(17):1560-9. | CrossRef | PubMed |
Writing Committee for the CASCADE Collaboration. Timing of HAART initiation and clinical outcomes in human immunodeficiency virus type 1 seroconverters. Arch Intern Med. 2011 Sep 26;171(17):1560-9. | CrossRef | PubMed | When To Start Consortium, Sterne JA, May M, Costagliola D, de Wolf F, Phillips AN, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009 Apr 18;373(9672):1352-63. | CrossRef | PubMed |
When To Start Consortium, Sterne JA, May M, Costagliola D, de Wolf F, Phillips AN, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009 Apr 18;373(9672):1352-63. | CrossRef | PubMed | Adolescents PoAGfAa. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Bethesda (MD): Department of Health and Human Services (DHHS); 2014. | Link |
Adolescents PoAGfAa. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Bethesda (MD): Department of Health and Human Services (DHHS); 2014. | Link |