 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Antiparasitic treatment of patients with Chagas’ disease in chronic stage could prevent the complications related to the disease. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified five systematic reviews including eight randomized trials and 11 observational studies. We combined the evidence using meta-analysis and generated a summary of findings table following the GRADE approach. We concluded it is not clear whether antiparasitic treatment improves survival or reduces complications related to chronic Chagas’ disease because the certainty of the evidence is very low.
Chagas' disease represents the third largest parasitic disease burden globally and the first in Latin America [1]. Chronic Chagas' cardiomyopathy is the most common form of non-ischemic cardiomyopathy worldwide, and one of the leading causes of morbidity and death in Latin America [2]. Chagas' disease has two clinical phases, acute infection that may be manifested by a self-limited febrile illness that lasts 4 to 8 weeks, and chronic phase characterized by an indeterminate stage in which patients are asymptomatic and free of complications that can last their whole life [3]. Ten to thirty years after the acute infection, about one third of the patients in chronic phase of Chagas’ disease develop cardiac or digestive complications. At the present time, the mechanism responsible for the mentioned complications is believed to be related to the presence of chronic parasitemia and its corresponding inflammatory reaction [4]. In this context, antiparasitic treatment for patients with Chagas’ disease in the chronic phase is proposed as a measure to prevent the disease’s visceral complications and their consequences.
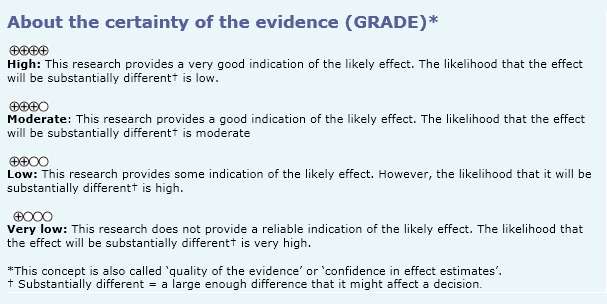
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
|
What is the evidence. |
We found five systematic reviews [5],[6],[7],[8],[9] considering 19 primary studies [10],[11],[12],[13], |
|
What types of patients were included |
Patients with Chagas’ disease in chronic phase indeterminate stage or chronic phase with visceral compromise. |
|
What types of interventions were included |
Benznidazole, nifurtimox, allopurinol or itraconazole for 30 to 60 days. |
|
What types of outcomes |
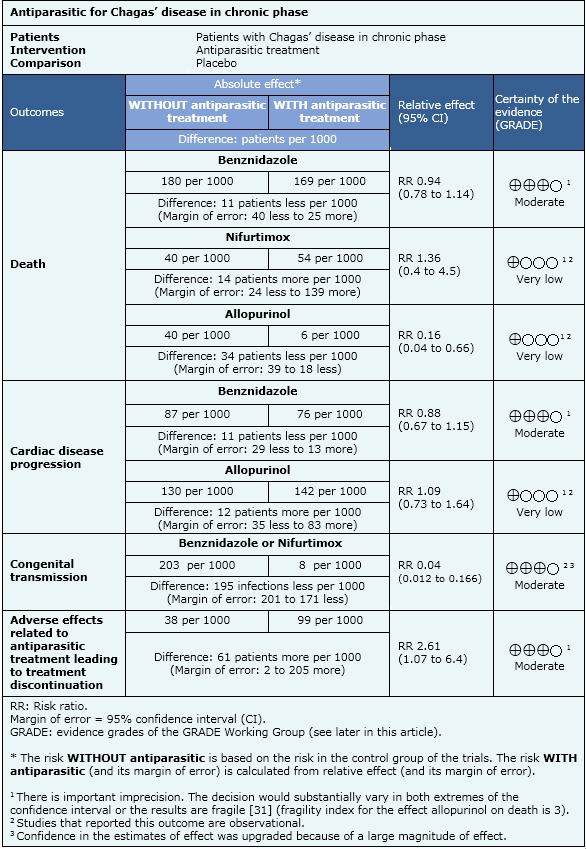
Death from any cause; cardiac disease progression; congenital transmission; electrocardiographic abnormalities; serological tests negativization; xenodiagnosis negativization; polymerase chain reaction negativization; adverse effects leading to treatment discontinuation |
The information about the antiparasitic effects is based on eight randomized controlled trials and eleven observational studies that included 7772 patients. Eight studies (one randomized and seven observational studies) reported death from any cause, six studies (one randomized and five observational studies) reported cardiac disease progression, one observational study reported congenital transmission, eleven studies (six randomized and five observational studies) reported adverse effects that lead to treatment discontinuation, four studies (three randomized and one observational study) reported electrocardiographic abnormalities, ten studies (three randomized and seven observational studies) reported serological tests negativization, four studies (two randomized and two observational studies) reported polymerase chain reaction negativization and seven studies (four randomized and three observational studies) reported xenodiagnosis negativization.
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
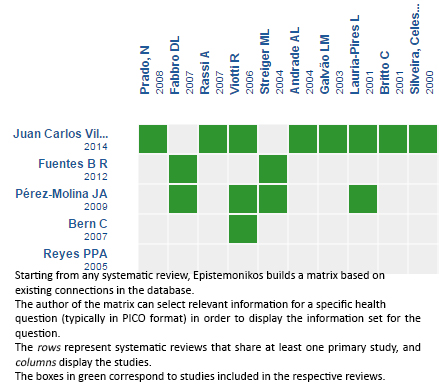
Follow the link to access the interactive version: Trypanocidal therapy to prevent chagasic cardiomyopathy
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Antiparasitic treatment of patients with Chagas’ disease in chronic stage could prevent the complications related to the disease. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified five systematic reviews including eight randomized trials and 11 observational studies. We combined the evidence using meta-analysis and generated a summary of findings table following the GRADE approach. We concluded it is not clear whether antiparasitic treatment improves survival or reduces complications related to chronic Chagas’ disease because the certainty of the evidence is very low.
 Autores:
Federico Popoff[1,2,3], Ariel Izcovich[1,2,3]
Autores:
Federico Popoff[1,2,3], Ariel Izcovich[1,2,3]

Citación: Popoff F, Izcovich A. Should trypanocidal therapy be used to treat patients in the chronic phase of Chagas disease?. Medwave 2015;15(Suppl 2):e6291 doi: 10.5867/medwave.2015.6291
Fecha de publicación: 26/10/2015

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Bank W. World Development Report 1993: Investing in health. Oxford University Press; 1993: xii + 329.
Bank W. World Development Report 1993: Investing in health. Oxford University Press; 1993: xii + 329.  Rassi A Jr, Rassi A, Little WC. Chagas' heart disease. Clin Cardiol. 2000 Dec;23(12):883-9. | PubMed |
Rassi A Jr, Rassi A, Little WC. Chagas' heart disease. Clin Cardiol. 2000 Dec;23(12):883-9. | PubMed | Dias JC. The indeterminate form of human chronic Chagas' disease A clinical epidemiological review. Rev Soc Bras Med Trop. 1989 Jul-Sep;22 (3):147-56.
| PubMed |
Dias JC. The indeterminate form of human chronic Chagas' disease A clinical epidemiological review. Rev Soc Bras Med Trop. 1989 Jul-Sep;22 (3):147-56.
| PubMed | Marin-Neto JA, Cunha-Neto E, Maciel BC, Simões MV. Pathogenesis of chronic Chagas heart disease. Circulation. 2007 Mar 6;115(9):1109-23. | PubMed |
Marin-Neto JA, Cunha-Neto E, Maciel BC, Simões MV. Pathogenesis of chronic Chagas heart disease. Circulation. 2007 Mar 6;115(9):1109-23. | PubMed | Reyes PPA, Vallejo M. Trypanocidal drugs for late stage, symptomatic Chagas disease (Trypanosoma cruzi infection). Cochrane Database of Systematic Reviews. 2005 2008/07/16(4):CD004102
Reyes PPA, Vallejo M. Trypanocidal drugs for late stage, symptomatic Chagas disease (Trypanosoma cruzi infection). Cochrane Database of Systematic Reviews. 2005 2008/07/16(4):CD004102  Pérez-Molina JA, Pérez-Ayala A, Moreno S, Fernández-González MC, Zamora J, López-Velez R. Use of benznidazole to treat chronic Chagas' disease: a systematic review with a meta-analysis. J Antimicrob Chemother. 2009 Dec;64(6):1139-47. | CrossRef | PubMed |
Pérez-Molina JA, Pérez-Ayala A, Moreno S, Fernández-González MC, Zamora J, López-Velez R. Use of benznidazole to treat chronic Chagas' disease: a systematic review with a meta-analysis. J Antimicrob Chemother. 2009 Dec;64(6):1139-47. | CrossRef | PubMed | Fuentes B R, Maturana A M, de la Cruz M R. [Efficacy of nifurtimox for the treatment of chronic Chagas disease]. Rev Chilena Infectol. 2012 Feb;29(1):82-6. | CrossRef | PubMed |
Fuentes B R, Maturana A M, de la Cruz M R. [Efficacy of nifurtimox for the treatment of chronic Chagas disease]. Rev Chilena Infectol. 2012 Feb;29(1):82-6. | CrossRef | PubMed | Villar JC, Perez JG, Cortes OL, Riarte A, Pepper M, Marin-Neto JA, et al. Trypanocidal drugs for chronic asymptomatic Trypanosoma cruzi infection. Cochrane Database Syst Rev. 2014 May 27;5:CD003463. | CrossRef | PubMed |
Villar JC, Perez JG, Cortes OL, Riarte A, Pepper M, Marin-Neto JA, et al. Trypanocidal drugs for chronic asymptomatic Trypanosoma cruzi infection. Cochrane Database Syst Rev. 2014 May 27;5:CD003463. | CrossRef | PubMed | Bern C, Montgomery SP, Herwaldt BL, Rassi A Jr, Marin-Neto JA, Dantas RO, et al. Evaluation and treatment of chagas disease in the United States: a systematic review. JAMA. 2007 Nov 14;298(18):2171-81. | PubMed |
Bern C, Montgomery SP, Herwaldt BL, Rassi A Jr, Marin-Neto JA, Dantas RO, et al. Evaluation and treatment of chagas disease in the United States: a systematic review. JAMA. 2007 Nov 14;298(18):2171-81. | PubMed | Coura JR, de Abreu LL, Willcox HP, Petana W. [Comparative controlled study on the use of benznidazole, nifurtimox and placebo, in the chronic form of Chagas' disease, in a field area with interrupted transmission. I. Preliminary evaluation]. Rev Soc Bras Med Trop. 1997 Mar-Apr;30(2):139-44. | PubMed |
Coura JR, de Abreu LL, Willcox HP, Petana W. [Comparative controlled study on the use of benznidazole, nifurtimox and placebo, in the chronic form of Chagas' disease, in a field area with interrupted transmission. I. Preliminary evaluation]. Rev Soc Bras Med Trop. 1997 Mar-Apr;30(2):139-44. | PubMed | Galvão LM, Chiari E, Macedo AM, Luquetti AO, Silva SA, Andrade AL. PCR assay for monitoring Trypanosoma cruzi parasitemia in childhood after specific chemotherapy. J Clin Microbiol. 2003 Nov;41(11):5066-70. | PubMed |
Galvão LM, Chiari E, Macedo AM, Luquetti AO, Silva SA, Andrade AL. PCR assay for monitoring Trypanosoma cruzi parasitemia in childhood after specific chemotherapy. J Clin Microbiol. 2003 Nov;41(11):5066-70. | PubMed | Andrade AL, Martelli CM, Oliveira RM, Silva SA, Aires AI, Soussumi LM, et al. Short report: benznidazole efficacy among Trypanosoma cruzi-infected adolescents after a six-year follow-up. Am J Trop Med Hyg. 2004 Nov;71(5):594-7. | PubMed |
Andrade AL, Martelli CM, Oliveira RM, Silva SA, Aires AI, Soussumi LM, et al. Short report: benznidazole efficacy among Trypanosoma cruzi-infected adolescents after a six-year follow-up. Am J Trop Med Hyg. 2004 Nov;71(5):594-7. | PubMed | Sosa Estani S, Segura EL, Ruiz AM, Velazquez E, Porcel BM, Yampotis C. Efficacy of chemotherapy with benznidazole in children in the indeterminate phase of Chagas' disease. Am J Trop Med Hyg. 1998 Oct;59(4):526-9. | PubMed |
Sosa Estani S, Segura EL, Ruiz AM, Velazquez E, Porcel BM, Yampotis C. Efficacy of chemotherapy with benznidazole in children in the indeterminate phase of Chagas' disease. Am J Trop Med Hyg. 1998 Oct;59(4):526-9. | PubMed | Gallerano RR, Sosa RR. [Interventional study in the natural evolution of Chagas disease. Evaluation of specific antiparasitic treatment. Retrospective-prospective study of antiparasitic therapy]. Rev Fac Cien Med Univ Nac Cordoba. 2000;57(2):135-62. | PubMed |
Gallerano RR, Sosa RR. [Interventional study in the natural evolution of Chagas disease. Evaluation of specific antiparasitic treatment. Retrospective-prospective study of antiparasitic therapy]. Rev Fac Cien Med Univ Nac Cordoba. 2000;57(2):135-62. | PubMed | de Andrade AL, Zicker F, de Oliveira RM, Almeida Silva S, Luquetti A, Travassos LR, Almeida IC, de Andrade SS, de Andrade JG, Martelli CM. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet. 1996 Nov 23;348(9039):1407-13. | PubMed |
de Andrade AL, Zicker F, de Oliveira RM, Almeida Silva S, Luquetti A, Travassos LR, Almeida IC, de Andrade SS, de Andrade JG, Martelli CM. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet. 1996 Nov 23;348(9039):1407-13. | PubMed | Apt W, Aguilera X, Arribada A, Pérez C, Miranda C, Sánchez G, Zulantay I, Cortés P, Rodriguez J, Juri D. Treatment of chronic Chagas' disease with itraconazole and allopurinol. Am J Trop Med Hyg. 1998 Jul;59(1):133-8. | PubMed |
Apt W, Aguilera X, Arribada A, Pérez C, Miranda C, Sánchez G, Zulantay I, Cortés P, Rodriguez J, Juri D. Treatment of chronic Chagas' disease with itraconazole and allopurinol. Am J Trop Med Hyg. 1998 Jul;59(1):133-8. | PubMed | Viotti R, Vigliano C, Lococo B, Bertocchi G, Petti M, Alvarez MG, et al. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: a nonrandomized trial. Ann Intern Med. 2006 May 16;144(10):724-34.
| PubMed |
Viotti R, Vigliano C, Lococo B, Bertocchi G, Petti M, Alvarez MG, et al. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: a nonrandomized trial. Ann Intern Med. 2006 May 16;144(10):724-34.
| PubMed | Ginella A, Holzman A, Iihoshi N, Barja Z, Peredo C. Efficacy of the Allopurinol in chronic Chagas disease. Boletín científico del CENETROP. 1997;16(1):25-9. | Link |
Ginella A, Holzman A, Iihoshi N, Barja Z, Peredo C. Efficacy of the Allopurinol in chronic Chagas disease. Boletín científico del CENETROP. 1997;16(1):25-9. | Link | Britto C, Silveira C, Cardoso MA, Marques P, Luquetti A, Macêdo V, et al. Parasite persistence in treated chagasic patients revealed by xenodiagnosis and polymerase chain reaction. Mem Inst Oswaldo Cruz. 2001 Aug;96(6):823-6. | PubMed |
Britto C, Silveira C, Cardoso MA, Marques P, Luquetti A, Macêdo V, et al. Parasite persistence in treated chagasic patients revealed by xenodiagnosis and polymerase chain reaction. Mem Inst Oswaldo Cruz. 2001 Aug;96(6):823-6. | PubMed | Lauria-Pires L, Braga MS, Vexenat AC, Nitz N, Simões-Barbosa A, Tinoco DL, et al. Progressive chronic Chagas heart disease ten years after treatment with anti-Trypanosoma cruzi nitroderivatives. Am J Trop Med Hyg. 2000 Sep-Oct;63(3-4):111-8. | PubMed |
Lauria-Pires L, Braga MS, Vexenat AC, Nitz N, Simões-Barbosa A, Tinoco DL, et al. Progressive chronic Chagas heart disease ten years after treatment with anti-Trypanosoma cruzi nitroderivatives. Am J Trop Med Hyg. 2000 Sep-Oct;63(3-4):111-8. | PubMed | Rassi A, Luquetti AO, Rassi A Jr, Rassi GG, Rassi SG, DA Silva IG,et al. Specific treatment for Trypanosoma cruzi: lack of efficacy of allopurinol in the human chronic phase of Chagas disease. Am J Trop Med Hyg. 2007 Jan;76(1):58-61. | PubMed |
Rassi A, Luquetti AO, Rassi A Jr, Rassi GG, Rassi SG, DA Silva IG,et al. Specific treatment for Trypanosoma cruzi: lack of efficacy of allopurinol in the human chronic phase of Chagas disease. Am J Trop Med Hyg. 2007 Jan;76(1):58-61. | PubMed | Fabbro DL, Streiger ML, Arias ED, Bizai ML, del Barco M, Amicone NA. Trypanocide treatment among adults with chronic Chagas disease living in Santa Fe city (Argentina), over a mean follow-up of 21 years: parasitological, serological and clinical evolution. Rev Soc Bras Med Trop. 2007 Jan-Feb;40(1):1-10. | PubMed |
Fabbro DL, Streiger ML, Arias ED, Bizai ML, del Barco M, Amicone NA. Trypanocide treatment among adults with chronic Chagas disease living in Santa Fe city (Argentina), over a mean follow-up of 21 years: parasitological, serological and clinical evolution. Rev Soc Bras Med Trop. 2007 Jan-Feb;40(1):1-10. | PubMed | Streiger ML, del Barco ML, Fabbro DL, Arias ED, Amicone NA. [Longitudinal study and specific chemotherapy in children with chronic Chagas' disease, residing in a low endemicity area of Argentina]. Rev Soc Bras Med Trop. 2004 Sep-Oct;37(5):365-75. | PubMed |
Streiger ML, del Barco ML, Fabbro DL, Arias ED, Amicone NA. [Longitudinal study and specific chemotherapy in children with chronic Chagas' disease, residing in a low endemicity area of Argentina]. Rev Soc Bras Med Trop. 2004 Sep-Oct;37(5):365-75. | PubMed | Fabbro De Suasnábar D, Arias E, Streiger M, Piacenza M, Ingaramo M, Del Barco M, Amicone N. Evolutive behavior towards cardiomyopathy of treated (nifurtimox or benznidazole) and untreated chronic chagasic patients. Rev Inst Med Trop Sao Paulo. 2000 Mar-Apr;42(2):99-109. | PubMed |
Fabbro De Suasnábar D, Arias E, Streiger M, Piacenza M, Ingaramo M, Del Barco M, Amicone N. Evolutive behavior towards cardiomyopathy of treated (nifurtimox or benznidazole) and untreated chronic chagasic patients. Rev Inst Med Trop Sao Paulo. 2000 Mar-Apr;42(2):99-109. | PubMed | Cerisola JA, Neves da Silva N, Prata A, Schenone H, Rohwedder R. [Evaluation of the efficacy of nifurtimox in chronic human chagasic infection by using xenodiagnosis (author's transl)]. Bol Chil Parasitol. 1977 Jul-Dec;32(3-4):51-62. | PubMed |
Cerisola JA, Neves da Silva N, Prata A, Schenone H, Rohwedder R. [Evaluation of the efficacy of nifurtimox in chronic human chagasic infection by using xenodiagnosis (author's transl)]. Bol Chil Parasitol. 1977 Jul-Dec;32(3-4):51-62. | PubMed | Prado N, Hernández Y, De Rissio AM, Esteve M, Riarte A. Security in a randomized control trial (RCT) - TRAENA Study - in adult patients with Chagas disease. Ministerio de Salud, Argentina; 2008.
Prado N, Hernández Y, De Rissio AM, Esteve M, Riarte A. Security in a randomized control trial (RCT) - TRAENA Study - in adult patients with Chagas disease. Ministerio de Salud, Argentina; 2008.  Silveira CAN. Evaluation in the long run of the specific treatment of the illness of Chagas. Faculdade de Medicina, Universidade de Brasília; 2000. | Link |
Silveira CAN. Evaluation in the long run of the specific treatment of the illness of Chagas. Faculdade de Medicina, Universidade de Brasília; 2000. | Link | Catalioti F, Acquatella H. Mortality comparison of 5 years follow up in subjects with chronic Chagas disease with and without benznidazol treatment. Rev BiolTropical. 1998 1998;27(Suppl):29-31.
| Link |
Catalioti F, Acquatella H. Mortality comparison of 5 years follow up in subjects with chronic Chagas disease with and without benznidazol treatment. Rev BiolTropical. 1998 1998;27(Suppl):29-31.
| Link | Morillo CA, Marin-Neto JA, Avezum A, Sosa-Estani S, Rassi A Jr, Rosas F, et al. Randomized Trial of Benznidazole for Chronic Chagas' Cardiomyopathy. N Engl J Med. 2015 Oct;373(14):1295-306.
| CrossRef | PubMed |
Morillo CA, Marin-Neto JA, Avezum A, Sosa-Estani S, Rassi A Jr, Rosas F, et al. Randomized Trial of Benznidazole for Chronic Chagas' Cardiomyopathy. N Engl J Med. 2015 Oct;373(14):1295-306.
| CrossRef | PubMed | Fabbro DL, Danesi E, Olivera V, Codebó MO, Denner S, Heredia C, et al. Trypanocide treatment of women infected with Trypanosoma cruzi and its effect on preventing congenital Chagas. PLoS Negl Trop Dis. 2014 Nov 20;8(11):e3312. | CrossRef | PubMed |
Fabbro DL, Danesi E, Olivera V, Codebó MO, Denner S, Heredia C, et al. Trypanocide treatment of women infected with Trypanosoma cruzi and its effect on preventing congenital Chagas. PLoS Negl Trop Dis. 2014 Nov 20;8(11):e3312. | CrossRef | PubMed | Walsh M, Srinathan SK, McAuley DF, Mrkobrada M, Levine O, Ribic C, et al. The statistical significance of randomized controlled trial results is frequently fragile: a case for a Fragility Index. J Clin Epidemiol. 2014 Jun;67(6):622-8. | CrossRef | PubMed |
Walsh M, Srinathan SK, McAuley DF, Mrkobrada M, Levine O, Ribic C, et al. The statistical significance of randomized controlled trial results is frequently fragile: a case for a Fragility Index. J Clin Epidemiol. 2014 Jun;67(6):622-8. | CrossRef | PubMed | Argentina, Ministerio de Salud de la Nación. Guías para la atención al paciente infectado con Trypanosoma cruzi (Enfermedad de Chagas). Buenos Aires, Argentina: MSAL; 2012. | Link |
Argentina, Ministerio de Salud de la Nación. Guías para la atención al paciente infectado con Trypanosoma cruzi (Enfermedad de Chagas). Buenos Aires, Argentina: MSAL; 2012. | Link | Gobierno de Chile, Ministerio de Salud. Guías de diagnóstico, tratamiento y prevención de la enfermedad de Chagas". Santiago, Chile: MINSAL;2011. | Link |
Gobierno de Chile, Ministerio de Salud. Guías de diagnóstico, tratamiento y prevención de la enfermedad de Chagas". Santiago, Chile: MINSAL;2011. | Link | Andrade JP, Marin-Neto JA, Paola AAV, Vilas-Boas F, Oliveira GM, Bacal F, et al. I Directriz Latinoamericana para el diagnóstico y el tratamiento de la cardiopatía de la enfermedad de chagas. Arq Bras Cardiol 2011; 97(2 supl.3): 1-47. | CrossRef |
Andrade JP, Marin-Neto JA, Paola AAV, Vilas-Boas F, Oliveira GM, Bacal F, et al. I Directriz Latinoamericana para el diagnóstico y el tratamiento de la cardiopatía de la enfermedad de chagas. Arq Bras Cardiol 2011; 97(2 supl.3): 1-47. | CrossRef |