 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Patients with systemic sclerosis frequently have Raynaud's phenomenon and digital ischemic ulcers. Iloprost, a synthetic prostacyclin analogue, may be effective in these cases. Searching in Epistemonikos database, which is maintained by screening 20 databases, we identified three systematic reviews including seven randomized trials. We combined the evidence using meta-analysis and generated a summary of findings table following the GRADE approach. We concluded iloprost may lead to little or no difference in the frequency or severity of secondary Raynaud, and it is associated to adverse effects and important costs.
Systemic sclerosis is a connective tissue disease characterized by fibrosis and vascular phenomena that frequently affect the skin and other organs. About 90% of patients with systemic sclerosis suffer from secondary Raynaud’s phenomenon, which is usually more severe and it is accompanied by digital ischemic ulcers that carry the risk of infection, gangrene, osteomyelitis and amputation.
Iloprost, a synthetic prostacyclin analogue with vasodilatory effect, has been proposed as a treatment for Raynaud’s phenomenon associated to systemic sclerosis.
We used Epistemonikos database, which is maintained by screening more than 20 databases, to identify systematic reviews and their included primary studies.
With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.

|
What is the evidence. See evidence matrix in Epistemonikos later |
We found three systematic reviews [1],[2],[3] including seven randomized controlled studies comparing iloprost vs placebo [4],[5],[6],[7],[8],[9],[10]. One of the studies did not report usable data for our analysis, so it is not considered in this table or in the summary of findings [5]. |
| What types of patients were included. |
All studies included patients with systemic sclerosis exclusively, except one that also included patients with severe primary Raynaud’s [6], but presented data in a way that allowed to separate the information of the systemic sclerosis group. Patients included in the studies presented a mean of 18 to 28 attacks per week [4],[7],[9],[10]. Two studies included patients with more than 12 episodes per week [6], but did not report average baseline frequency, and one study did not report any data on this regard [8]. |
|
What types of interventions were included. |
Two studies evaluated oral iloprost [4],[7], and the rest administered it intravenously. The duration of treatment was variable, from three days to six weeks in the different studies. |
|
What types of outcomes were measured. |
Frequency, severity and duration of attacks; global evaluation of the impact of Raynaud’s phenomenon made by patients or physicians; digital ulcer healing; Raynaud’s score, etc. |
This information is based on six of seven randomized studies identified, including 595 patients. Five studies reported frequency [4],[6],[7],[9],[10], and four severity of attacks [4],[6],[8],[9]. Three studies measured digital ulcer healing [6],[8],[9], but only two reported data adequately [8],[9]. Adverse effects were reported in four studies [4],[7],[8],[9].
• Iloprost may lead to little or no difference in the frequency or severity of secondary Raynaud.
• It is not known if iloprost affects digital ulcer healing because the certainty of the evidence was estimated as very low.

Matrix of evidence: Iloprost vs placebo for secondary Raynaud’s phenomenon
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
Follow the link to access the interactive version Iloprost vs placebo for secondary Raynaud’s phenomenon
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary.
Even though the project considers the periodical update of these summaries, users are invited to comment in the website of Medwave or to contact the authors through email if they realize there is new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce this summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organisation aiming to bring information closer to those making health decisions, through the use of technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.

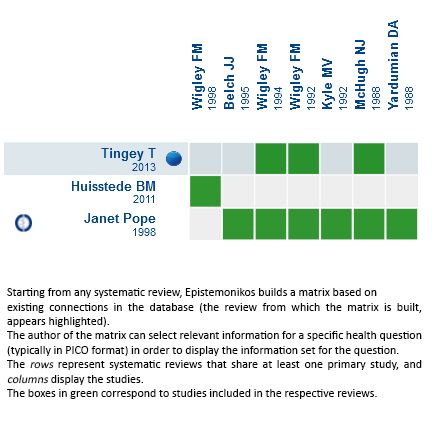 Matrix of evidence
Matrix of evidence
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Patients with systemic sclerosis frequently have Raynaud's phenomenon and digital ischemic ulcers. Iloprost, a synthetic prostacyclin analogue, may be effective in these cases. Searching in Epistemonikos database, which is maintained by screening 20 databases, we identified three systematic reviews including seven randomized trials. We combined the evidence using meta-analysis and generated a summary of findings table following the GRADE approach. We concluded iloprost may lead to little or no difference in the frequency or severity of secondary Raynaud, and it is associated to adverse effects and important costs.
 Autores:
Nicole Lustig[1,2], Gabriel Rada[1,2,3,4,5]
Autores:
Nicole Lustig[1,2], Gabriel Rada[1,2,3,4,5]

Citación: Lustig N, Rada G. Is iloprost effective in secondary Raynaud's phenomenon?. Medwave 20015;15(2):e6082 doi: 10.5867/medwave.2015.02.6082
Fecha de publicación: 9/3/2015

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Huisstede BM, Hoogvliet P, Paulis WD, van Middelkoop M, Hausman M, Coert JH, et al. Effectiveness of interventions for secondary Raynaud's phenomenon: a systematic review. Arch Phys Med Rehabil. 2011 Jul;92(7):1166-80. | CrossRef | PubMed |
Huisstede BM, Hoogvliet P, Paulis WD, van Middelkoop M, Hausman M, Coert JH, et al. Effectiveness of interventions for secondary Raynaud's phenomenon: a systematic review. Arch Phys Med Rehabil. 2011 Jul;92(7):1166-80. | CrossRef | PubMed | Pope J, Fenlon D, Thompson A, Shea B, Furst D, Wells G, et al. Iloprost and cisaprost for Raynaud's phenomenon in progressive systemic sclerosis. Cochrane Database Syst Rev. 2000;(2):CD000953. | CrossRef | PubMed |
Pope J, Fenlon D, Thompson A, Shea B, Furst D, Wells G, et al. Iloprost and cisaprost for Raynaud's phenomenon in progressive systemic sclerosis. Cochrane Database Syst Rev. 2000;(2):CD000953. | CrossRef | PubMed | Tingey T, Shu J, Smuczek J, Pope J. Meta-analysis of healing and prevention of digital ulcers in systemic sclerosis. Arthritis Care Res (Hoboken). 2013 Sep;65(9):1460-71. | CrossRef | PubMed |
Tingey T, Shu J, Smuczek J, Pope J. Meta-analysis of healing and prevention of digital ulcers in systemic sclerosis. Arthritis Care Res (Hoboken). 2013 Sep;65(9):1460-71. | CrossRef | PubMed | Belch JJ, Capell HA, Cooke ED, Kirby JD, Lau CS, Madhok R, et al. Oral iloprost as a treatment for Raynaud's syndrome: a double blind multicentre placebo controlled study. Ann Rheum Dis. 1995 Mar;54(3):197-200. | PubMed | Link |
Belch JJ, Capell HA, Cooke ED, Kirby JD, Lau CS, Madhok R, et al. Oral iloprost as a treatment for Raynaud's syndrome: a double blind multicentre placebo controlled study. Ann Rheum Dis. 1995 Mar;54(3):197-200. | PubMed | Link | Kyle MV, Belcher G, Hazleman BL. Placebo controlled study showing therapeutic benefit of iloprost in the treatment of Raynaud's phenomenon. J Rheumatol. 1992 Sep;19(9):1403-6. | PubMed |
Kyle MV, Belcher G, Hazleman BL. Placebo controlled study showing therapeutic benefit of iloprost in the treatment of Raynaud's phenomenon. J Rheumatol. 1992 Sep;19(9):1403-6. | PubMed | McHugh NJ, Csuka M, Watson H, Belcher G, Amadi A, Ring EF, et al. Infusion of iloprost, a prostacyclin analogue, for treatment of Raynaud's phenomenon in systemic sclerosis. Ann Rheum Dis. 1988 Jan;47(1):43-7. | PubMed | Link |
McHugh NJ, Csuka M, Watson H, Belcher G, Amadi A, Ring EF, et al. Infusion of iloprost, a prostacyclin analogue, for treatment of Raynaud's phenomenon in systemic sclerosis. Ann Rheum Dis. 1988 Jan;47(1):43-7. | PubMed | Link | Wigley FM, Korn JH, Csuka ME, Medsger TA Jr, Rothfield NF, Ellman M, et al. Oral iloprost treatment in patients with Raynaud's phenomenon secondary to systemic sclerosis: a multicenter, placebo-controlled, double-blind study. Arthritis Rheum. 1998 Apr;41(4):670-7. | PubMed |
Wigley FM, Korn JH, Csuka ME, Medsger TA Jr, Rothfield NF, Ellman M, et al. Oral iloprost treatment in patients with Raynaud's phenomenon secondary to systemic sclerosis: a multicenter, placebo-controlled, double-blind study. Arthritis Rheum. 1998 Apr;41(4):670-7. | PubMed | Wigley FM, Seibold JR, Wise RA, McCloskey DA, Dole WP. Intravenous iloprost treatment of Raynaud's phenomenon and ischemic ulcers secondary to systemic sclerosis. J Rheumatol. 1992 Sep;19(9):1407-14. | PubMed |
Wigley FM, Seibold JR, Wise RA, McCloskey DA, Dole WP. Intravenous iloprost treatment of Raynaud's phenomenon and ischemic ulcers secondary to systemic sclerosis. J Rheumatol. 1992 Sep;19(9):1407-14. | PubMed | Wigley FM, Wise RA, Seibold JR, McCloskey DA, Kujala G, Medsger TA Jr, et al. Intravenous iloprost infusion in patients with Raynaud phenomenon secondary to systemic sclerosis. A multicenter, placebo-controlled, double-blind study. Ann Intern Med. 1994 Feb 1;120(3):199-206. | CrossRef | PubMed |
Wigley FM, Wise RA, Seibold JR, McCloskey DA, Kujala G, Medsger TA Jr, et al. Intravenous iloprost infusion in patients with Raynaud phenomenon secondary to systemic sclerosis. A multicenter, placebo-controlled, double-blind study. Ann Intern Med. 1994 Feb 1;120(3):199-206. | CrossRef | PubMed | Yardumian DA, Isenberg DA, Rustin M, Belcher G, Snaith ML, Dowd PM, et al. Successful treatment of Raynaud's syndrome with Iloprost, a chemically stable prostacyclin analogue. Br J Rheumatol. 1988 Jun;27(3):220-6. | CrossRef | PubMed |
Yardumian DA, Isenberg DA, Rustin M, Belcher G, Snaith ML, Dowd PM, et al. Successful treatment of Raynaud's syndrome with Iloprost, a chemically stable prostacyclin analogue. Br J Rheumatol. 1988 Jun;27(3):220-6. | CrossRef | PubMed | Khanna D, Lovell DJ, Giannini E, Clements PJ, Merkel PA, Seibold JR, et al. Development of a provisional core set of response measures for clinical trials of systemic sclerosis. Ann Rheum Dis. 2008 May;67(5):703-9.
| PubMed |
Khanna D, Lovell DJ, Giannini E, Clements PJ, Merkel PA, Seibold JR, et al. Development of a provisional core set of response measures for clinical trials of systemic sclerosis. Ann Rheum Dis. 2008 May;67(5):703-9.
| PubMed | Gladue H, Maranian P, Paulus HE, Khanna D. Evaluation of test characteristics for outcome measures used in Raynaud's phenomenon clinical trials. Arthritis Care Res (Hoboken). 2013 Apr;65(4):630-6. | CrossRef | PubMed |
Gladue H, Maranian P, Paulus HE, Khanna D. Evaluation of test characteristics for outcome measures used in Raynaud's phenomenon clinical trials. Arthritis Care Res (Hoboken). 2013 Apr;65(4):630-6. | CrossRef | PubMed | Kowal-Bielecka O, Landewé R, Avouac J, Chwiesko S, Miniati I, Czirjak L, et al. EULAR recommendations for the treatment of systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group (EUSTAR). Ann Rheum Dis. 2009 May;68(5):620-8. | CrossRef | PubMed |
Kowal-Bielecka O, Landewé R, Avouac J, Chwiesko S, Miniati I, Czirjak L, et al. EULAR recommendations for the treatment of systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group (EUSTAR). Ann Rheum Dis. 2009 May;68(5):620-8. | CrossRef | PubMed | Aberer E. Offene, monozentrische, randomisierte, placebo-kontrollierte, einfach blinde und Observer-blinde klinische Studie bei PatientInnen mit Raynaud Syndrom mit / ohne progressiver systemischer Sklerodermie zur systemischen Therapie mit Iloprost versus Placebo - iloprost in raynaud. 2006. | Link |
Aberer E. Offene, monozentrische, randomisierte, placebo-kontrollierte, einfach blinde und Observer-blinde klinische Studie bei PatientInnen mit Raynaud Syndrom mit / ohne progressiver systemischer Sklerodermie zur systemischen Therapie mit Iloprost versus Placebo - iloprost in raynaud. 2006. | Link | (NCRR) NCfRR. Phase III Randomized, Double-Blind, Placebo-Controlled Study of Oral Iloprost for Raynaud's Phenomenon Secondary to Systemic Sclerosis. 2000. [on line] | Link |
(NCRR) NCfRR. Phase III Randomized, Double-Blind, Placebo-Controlled Study of Oral Iloprost for Raynaud's Phenomenon Secondary to Systemic Sclerosis. 2000. [on line] | Link | ITALFARMACO. Chronic iloprost administration in scleroderma patients effect on disease progression, as assessed by skin fibrosis evaluation. A randomized, controlled, blind-observer, multicenter phase III study. 2005. [on line] | Link |
ITALFARMACO. Chronic iloprost administration in scleroderma patients effect on disease progression, as assessed by skin fibrosis evaluation. A randomized, controlled, blind-observer, multicenter phase III study. 2005. [on line] | Link | (UK) UoD. Cardiovascular events and mortality in systemic sclerosis (SSc): a study of the effect of iloprost on these and on disease progression. ISRCTN Registry 2002. [on line] | Link |
(UK) UoD. Cardiovascular events and mortality in systemic sclerosis (SSc): a study of the effect of iloprost on these and on disease progression. ISRCTN Registry 2002. [on line] | Link |