 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Palabras clave: acute myeloid leukemia, azacitidine, palliative care
INTRODUCTION
Acute myeloid leukemia has a high mortality if untreated. Hematopoietic stem cell transplantation is the only curative treatment so far. Patients who are not eligible to receive a transplant can be treated with hypomethylating agents that have shown to improve disease-free and overall survival.
OBJECTIVE
Retrospective description of the clinical characteristics of patients suffering from advanced myelodysplastic syndrome and acute myeloid leukemia that were treated with a hypomethylating agent as well as its adverse effects and response to treatment.
METHODS
This report shows our experience in 38 patients with acute myeloid leukemia treated with azacitidine or palliative treatment.
RESULTS
Azacitidine was able to prolong survival in 80% of patients with a high incidence of adverse effects and negative impact on quality of life. Most of the patients treated with palliative intent died in the first month after diagnosis.
CONCLUSIONS
Azacitidine can prolong survival but with significant adverse effects. Untreated patients had a high early mortality.
Acute myeloid leukemia is one of the most common hematological malignancies in adults especially in the elderly [1],[2]. In young patients with good performance status hematopoiesis restoration with intense chemotherapy and allogeneic hematopoietic cell transplantation is mandatory, while in patients with poor performance status and elderly, not candidates for transplantation or intensive chemotherapy, less toxic therapies such as hypomethylating agents are a valid alternative [3],[4].
Azacitidine is a cytidine analog used to treat patients with myelodysplasia and acute leukemia, with a potent hypomethylating action of oncogenes. This drug is an alternative for patients with acute myeloid leukemia who are not candidates for more intensive therapies improving quality of life and progression free survival compared with standard therapy and exclusive transfusion support [5],[6].
In our country, there is no information available about hypomethylating agents in the treatment of acute myeloid leukemia in patients not candidates for hematopoietic cell transplantation. In this study, we present our experience in acute myeloid leukemia patients not candidates for treatment of high intensity, treated with azacitidine or support. Our aim is to describe retrospectively the clinical characteristics of patients with acute myeloid leukemia, the quality of responses achieved and adverse effects of treatment with azacitidine or palliative care.
Study design
Between 2010 and 2014, 79 patients with acute myeloid leukemia were treated at our institution. A retrospective and descriptive analysis of all patients who were not candidates for transplantation was made. Of these, 38 were not candidates for standard chemotherapy with high-dose cytarabine and transplantation, 22 were treated with azacitidine and 16 with palliative treatment with blood transfusions and end of life care. Treatment strategy was determined taking into account the decision of the patient, comorbidities and economic capacity of access to treatment. A retrospective comparison between these two groups was performed. All patients signed an informed consent document.
Diagnosis
The diagnosis of acute myeloid leukemia was suspected in those patients with altered blood count or immature cells with dysplastic changes, and confirmed by cytology and flow cytometry. The diagnosis of acute myeloid leukemia in bone marrow cytology was performed in the presence of immature myeloid cells according to WHO criteria [1] the presence of more than 20% bone marrow blasts. The diagnosis with multiparameter flow cytometry was performed using a panel of antibodies against myeloid clusters of differentiation (CD) CD13, CD14, CD15, CD16, CD33, CD34, CD36, CD38, CD45, CD56, CD117, CD11b, CD10 and MPO. In patients who underwent azacitidine treatment karyotype processing was made.
Treatment
There were two treatment modalities with subcutaneous or intravenous azacitidine: a) dose of 75mg / m2 daily for 7 days, starting on Monday b) dose of 100 mg / m2 daily for 5 days to patients with difficulties in applying the treatment on weekends. Patients who were treated with azacitidine and those with palliative care received transfusions to maintain platelet counts greater than 20,000 / uL and hemoglobin levels greater than 8gr / dL.
All patients received prophylactic antibiotic treatment with 400 mg oral fluconazole, 500 mg daily oral levofloxacin and trimethoprim / sulfamethoxazole 160/800 mg on Mondays, Wednesdays and Fridays. In those who had episodes of febrile neutropenia, in-hospital treatment was made according to the local guidelines. In patients who had symptoms of difficult handling at home, hospital treatment and care of end of life was performed.
According to the institutional policy of our hospital and faculty, all patients received clear and explicit explanations about the disease and treatment options. Both patients and physicians signed an informed consent form. The ethics committee of the Faculty of Medicine of the Catholic University of Chile approved the study.
Response criteria
Response to treatment was defined using flow cytometry and cytology of bone marrow blood sample. The complete response (CR) was defined by the presence of less than 5% leukemic cells. Partial remission was defined as the persistence of leukemic cells greater than 5% but not exceeding the value of diagnosis. Progressive disease was defined by the increase of leukemic cells. Hematologic remission was defined in patients with persistence of marrow leukemic cells but with improvement in the peripheral blood counts. The average response time was delimited as the elapsed time from the start of treatment with azacitidine and the moment of decrease of leukemic cells in peripheral blood or bone marrow. The duration of the response was determined as the time between initiation of treatment and relapse or disease progression [3],[4].
Statistical analysis
This is a retrospective descriptive study. Information from clinical records were collected for age at diagnosis, gender, blood count, LDH, bone marrow cytology, flow cytometry, karyotype, type of treatment indicated (azacitidine versus support), toxicity, bacterial, viral or fungal infections, survival time and date of death. Analysis was performed using the program Graphpad V.6.2. As for the descriptive statistics, demographic data are shown by median and range, while the survival analysis were performed using the Kaplan Meier method. Significance level was p <0.05.
Patient characteristics
Patient characteristics are summarized in Table 1. Briefly, the majority was male, with an average age of 65 years old for patients treated with azacitidine and 72 years old for patients treated with palliative support. In 63% of patients some high-risk genetic alteration was found in the karyotype.
Table 1. Patients´characteristiscs
Overall response.
As described in Table 2, complete remission was not achieved in any patient treated with azacitidine, partial remission was obtained in 60% and hematologic remission in 20%. In the remaining 20%, there was a progressive disease. The median time to treatment response was 2 months (range 0.5 to 4 months) and response duration was 7 months (range 2-24 months).
Table 2. Response to azacitidine treatment
Toxicity
Toxicity associated with azacitidine treatment is described in Table 3. All patients had episodes of pancytopenia. Forty percent of patients had episodes of febrile neutropenia, mostly caused by fungi, with prolonged requirement of antifungal treatment. In patients treated with palliative intent, transfusion dependence was observed in 16 (100%), serious infections in 15 (96%) and major bleeding (central nervous system) in four (25%).
Survival
Two years overall survival was 30% in patients with partial response and 5% in those who had progressive or refractory disease. The median survival was seven months for patients treated with azacitidine and 1 month for patients who had palliative treatment (p <0.001). Ninety five percent of patients treated with palliative intent died within the first month after diagnosis
Patients with acute myeloid leukemia not candidates for standard treatment and elderly with major comorbidities are challenging in hematology. It is clear that a rapid progression to death is guaranteed without treatment. Hypomethylating agents have shown better survival in these patients. Early reports showed that azacitidine prolonged survival in 6-12 months in patients not candidates for treatment and achieved overall response rates of around 50% [7],[8]. More recently the European multicenter acute myeloid leukemia treatment group confirm that azacitidine allows extended overall survival by 9.6 months and 40% of patients were alive after one year of treatment with an average of 4 cycles of treatment (range 1-29) [9].
Our data show that treatment with azacitidine prolonged survival of patients in the same time period as reported previously [9],[10],[11],[12]. The limitations of our study are the retrospective nature of the analysis and the small sample size. It should also be noticed that our patients treated with azacitidine received less cycles of treatment compared with previous reports. This can be explained by the high toxicity and the difficulty of access to the drug.
Moreover, patients who were not treated with curative intent had a high early mortality with a very poor quality of life. This was mainly due to severe infection and transfusion dependence, which reaffirms that azacitidine is a good treatment option to improve survival. The following process must be directed to implement adequate and appropriate hypomethilating agents’ schemes for our population allowing prolonged survival in patients with acute myeloid leukemia and high grade myelodysplastic syndrome, but with less impact on the quality of life.
Patients with acute myeloid leukemia not transplant candidates have an ominous prognosis with a high early mortality. Our retrospective analysis showed that azacitidine allows significantly prolonged survival at expense of moderate toxicity.
Ethical aspects
The Journal is aware that the scientific ethics committee of the Faculty of Medicine of the Catholic University of Chile learned about this study and its possible publication in a national journal.
Financial issues.
The authors declare no external funding.
Declaration of conflicts of interest
The authors have completed the ICMJE conflicts of Interest form and have no relevant interests to declare. Forms can be requested to the responsible author or the editorial direction of the Journal.

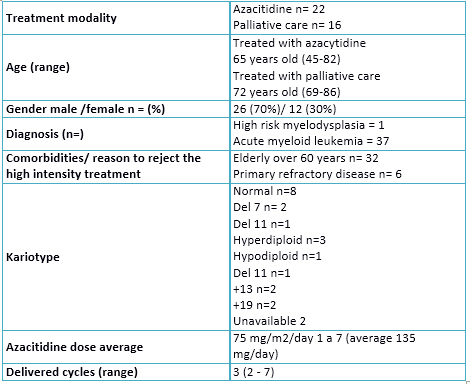 Table 1. Patients´characteristiscs
Table 1. Patients´characteristiscs

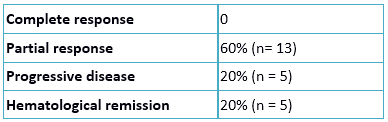 Table 2. Response to azacitidine treatment
Table 2. Response to azacitidine treatment

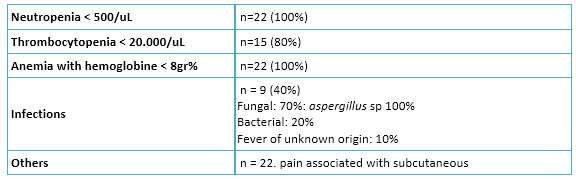 Table 3. Azacitidine toxicity
Table 3. Azacitidine toxicity

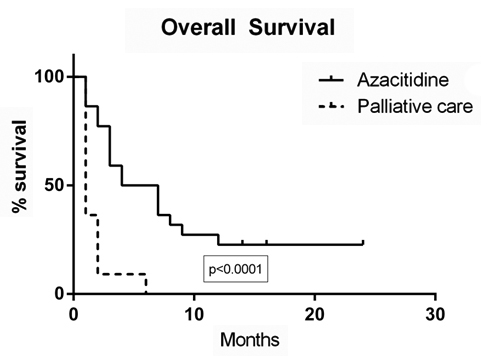 Figure 1. Overall survival
Figure 1. Overall survival
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCTION
Acute myeloid leukemia has a high mortality if untreated. Hematopoietic stem cell transplantation is the only curative treatment so far. Patients who are not eligible to receive a transplant can be treated with hypomethylating agents that have shown to improve disease-free and overall survival.
OBJECTIVE
Retrospective description of the clinical characteristics of patients suffering from advanced myelodysplastic syndrome and acute myeloid leukemia that were treated with a hypomethylating agent as well as its adverse effects and response to treatment.
METHODS
This report shows our experience in 38 patients with acute myeloid leukemia treated with azacitidine or palliative treatment.
RESULTS
Azacitidine was able to prolong survival in 80% of patients with a high incidence of adverse effects and negative impact on quality of life. Most of the patients treated with palliative intent died in the first month after diagnosis.
CONCLUSIONS
Azacitidine can prolong survival but with significant adverse effects. Untreated patients had a high early mortality.
 Autores:
Mauricio Sarmiento Maldonado[1], Mauricio Ocqueteau Tachini[1], Javier Pilcante[1], Pablo Ramírez Villanueva[1]
Autores:
Mauricio Sarmiento Maldonado[1], Mauricio Ocqueteau Tachini[1], Javier Pilcante[1], Pablo Ramírez Villanueva[1]

Citación: Sarmiento Maldonado M, Ocqueteau Tachini M, Pilcante J, Ramírez Villanueva P. Response and survival in acute myeloid leukemia patients not candidates to transplantation treated with azacitidine versus palliative treatment: a retrospective study. Medwave 2015 Ago;15(7):e6207 doi: 10.5867/medwave.2015.07.6207
Fecha de envío: 9/6/2015
Fecha de aceptación: 30/7/2015
Fecha de publicación: 10/8/2015
Origen: no solicitado
Tipo de revisión: con revisión estadística y con revisión por dos pares revisores externos

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009 Jul 30;114(5):937-51. | CrossRef | PubMed |
Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009 Jul 30;114(5):937-51. | CrossRef | PubMed | Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012 Sep 20;120(12):2454-65.
| PubMed |
Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012 Sep 20;120(12):2454-65.
| PubMed | Malcovati L, Hellström-Lindberg E, Bowen D, Adès L, Cermak J, Del Cañizo C, et al. Diagnosis and treatment of primary myelodysplastic syndromes in adults: recommendations from the European LeukemiaNet. Blood. 2013 Oct 24;122(17):2943-64. | CrossRef | PubMed |
Malcovati L, Hellström-Lindberg E, Bowen D, Adès L, Cermak J, Del Cañizo C, et al. Diagnosis and treatment of primary myelodysplastic syndromes in adults: recommendations from the European LeukemiaNet. Blood. 2013 Oct 24;122(17):2943-64. | CrossRef | PubMed | Smith BD, Beach CL, Mahmoud D, Weber L, Henk HJ. Survival and hospitalization among patients with acute myeloid leukemia treated with azacitidine or decitabine in a large managed care population: a real-world, retrospective, claims-based, comparative analysis. Exp Hematol Oncol. 2014 Mar 25;3(1):10. | CrossRef | PubMed |
Smith BD, Beach CL, Mahmoud D, Weber L, Henk HJ. Survival and hospitalization among patients with acute myeloid leukemia treated with azacitidine or decitabine in a large managed care population: a real-world, retrospective, claims-based, comparative analysis. Exp Hematol Oncol. 2014 Mar 25;3(1):10. | CrossRef | PubMed | Fenaux P, Mufti GJ, Hellström-Lindberg E, Santini V, Gattermann N, Germing U, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010 Feb 1;28(4):562-9. | CrossRef | PubMed |
Fenaux P, Mufti GJ, Hellström-Lindberg E, Santini V, Gattermann N, Germing U, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010 Feb 1;28(4):562-9. | CrossRef | PubMed | Thépot S, Itzykson R, Seegers V, Recher C, Raffoux E, Quesnel B, et al. Azacitidine in untreated acute myeloid leukemia: a report on 149 patients. Am J Hematol. 2014 Apr;89(4):410-6. | CrossRef | PubMed |
Thépot S, Itzykson R, Seegers V, Recher C, Raffoux E, Quesnel B, et al. Azacitidine in untreated acute myeloid leukemia: a report on 149 patients. Am J Hematol. 2014 Apr;89(4):410-6. | CrossRef | PubMed | Wheatley K, Brookes CL, Howman AJ, Goldstone AH, Milligan DW, Prentice AG, et al. Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br J Haematol. 2009 Jun;145(5):598-605. | CrossRef | PubMed |
Wheatley K, Brookes CL, Howman AJ, Goldstone AH, Milligan DW, Prentice AG, et al. Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br J Haematol. 2009 Jun;145(5):598-605. | CrossRef | PubMed | Al-Ali HK, Jaekel N, Junghanss C, Maschmeyer G, Krahl R, Cross M, et al. Azacitidine in patients with acute myeloid leukemia medically unfit for or resistant to chemotherapy: a multicenter phase I/II study. Leuk Lymphoma. 2012 Jan;53(1):110-7. | CrossRef | PubMed |
Al-Ali HK, Jaekel N, Junghanss C, Maschmeyer G, Krahl R, Cross M, et al. Azacitidine in patients with acute myeloid leukemia medically unfit for or resistant to chemotherapy: a multicenter phase I/II study. Leuk Lymphoma. 2012 Jan;53(1):110-7. | CrossRef | PubMed | Ramos F, Thépot S, Pleyer L, Maurillo L, Itzykson R, Bargay J, et al. Azacitidine frontline therapy for unfit acute myeloid leukemia patients: clinical use and outcome prediction. Leuk Res. 2015 Mar;39(3):296-306. | CrossRef | PubMed |
Ramos F, Thépot S, Pleyer L, Maurillo L, Itzykson R, Bargay J, et al. Azacitidine frontline therapy for unfit acute myeloid leukemia patients: clinical use and outcome prediction. Leuk Res. 2015 Mar;39(3):296-306. | CrossRef | PubMed | Huls G. Azacitidine in AML: a treatment option? Blood. 2015 Jul 16;126(3):283-4. | CrossRef | PubMed |
Huls G. Azacitidine in AML: a treatment option? Blood. 2015 Jul 16;126(3):283-4. | CrossRef | PubMed | Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015 Jul 16;126(3):291-9. | CrossRef | PubMed |
Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015 Jul 16;126(3):291-9. | CrossRef | PubMed | Cabrero M, Jabbour E, Ravandi F, Bohannan Z, Pierce S, Kantarjian HM, et al. Discontinuation of hypomethylating agent therapy in patients with myelodysplastic syndromes or acute myelogenous leukemia in complete remission or partial response: retrospective analysis of survival after long-term follow-up. Leuk Res. 2015 May;39(5):520-4. | CrossRef | PubMed |
Cabrero M, Jabbour E, Ravandi F, Bohannan Z, Pierce S, Kantarjian HM, et al. Discontinuation of hypomethylating agent therapy in patients with myelodysplastic syndromes or acute myelogenous leukemia in complete remission or partial response: retrospective analysis of survival after long-term follow-up. Leuk Res. 2015 May;39(5):520-4. | CrossRef | PubMed |