 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Palabras clave: cost-benefit analysis, multiple myeloma, lenalidomide, bortezomib
BACKGROUND
Multiple myeloma is a hematologic malignancy affecting bone marrow derived plasma cells. Current therapies are not able to eradicate the disease and most patients become refractory to the treatment. Lenalidomide and bortezomib have proved effective in the second-line treatment of these patients.
OBJECTIVE
To evaluate the cost-effectiveness of lenalidomide in combination with dexamethasone compared to bortezomib in combination with dexamethasone in patients with multiple myeloma previously treated with bortezomib, from the perspective of the Chilean National Health Service.
METHODOLOGY
A four-state Markov model (preprogression on treatment; preprogression off treatment, progression and death) was used to simulate the evolution of a cohort of multiple myeloma patients over a 25-year time horizon. Efficacy data, resource use and frequency of adverse events were extracted from MM009/010 studies and a retrospective analysis of retreatment with bortezomib. All inputs were validated by experts. A 3% annual discount rate was used for costs and health outcomes. The robustness of the results was evaluated through univariate and probabilistic sensitivity analyses.
RESULTS
Lenalidomide in combination with dexamethasone treatment provided 1.41 incremental life years and 0.83 incremental quality-adjusted life years in comparison with bortezomib in combination with dexamethasone, with an incremental cost of 11 864 597.86 CLP (19 589.86 US$). The incremental cost-effectiveness and cost-utility ratio were estimated at 8 410 266.92 CLP (13 886,35 US$) / incremental life year and 14 271 896.16 CLP (23 564,59 US$)/incremental quality-adjusted life years, respectively.
CONCLUSIONS
Lenalidomide in combination with dexamethasone represents a potentially cost-effective alternative for the second-line treatment of patients with multiple myeloma who are not eligible for transplantation, from the perspective of the Chilean National Health Service.
Multiple myeloma is a malignancy characterized by the clonal proliferation of B cells-derived plasma cells [1]. It is often preceded by an asymptomatic pre-malignant stage called monoclonal gammopathy of undetermined significance that may progress directly to symptomatic multiple myeloma or to an intermediate stage referred to as smoldering myeloma. In the latter case, approximately 10% of the patients progress to symptomatic multiple myeloma over the first five years [2]. The course of the disease is associated with a significant loss of quality of life, mostly due to the onset of complications. Of these, the most common include: anemia, thrombocytopenia, leukopenia, bone fracture, hypercalcemia and renal failure [1],[3].
Despite advances in the treatment of multiple myeloma, the mortality rate for these patients is still high, resulting in more than 260 000 deaths/year worldwide [4]. In Chile, the incidence of the disease is estimated to be 2.3 cases per 100 000 population/year with a 5-year prevalence of 1% [4].
In the absence of definitive treatment, the introduction of agents such as thalidomide, lenalidomide and bortezomib has significantly improved survival for patients with multiple myeloma [5]. Selection of the most appropriate treatment depends on the patient's risk stratification and eligibility for autologous stem cell transplantation [6],[7].
Depending on their overall clinical status, a two- or three-drug combination regimen based on bortezomib, lenalidomide or thalidomide is recommended for patients who not eligible for transplantation [6],[7],[8]. In case of relapse or progression, most clinical practice guidelines recommend repeating the same treatment or to consider switching to another therapy (lenalidomide/dexamethasone or bortezomib/pegylated doxorubicin) [6],[7],[8]. Against this background, the objective of this study is to estimate the incremental cost-effectiveness ratio of lenalidomide in combination with dexamethasone versus bortezomib in combination with dexamethasone for the second line treatment of patients with multiple myeloma who have received previous treatment with bortezomib, from the perspective of the Chilean National Health Service.
Methods
A Markov model, previously submitted to the National Institute for Health and Care Excellence (NICE), was adapted to the Chilean setting [9].
The model estimates the costs and clinical benefits of lenalidomide plus dexamethasone versus bortezomib plus dexamethasone for the treatment of multiple myeloma patients not eligible for transplantation, who were previously treated with bortezomib [9]. All inputs used in the model were validated by two hematologists with extensive experience in the treatment of multiple myeloma in Chile (CP and CG). The present work was developed in accordance with the Consolidated Health Economic Evaluation Reporting Standards reporting guidelines [10].
Model structure
The present Markov model simulate the clinical course of a hypothetical cohort of patients from treatment assignment until the end of the time horizon (25 years).
The model design was selected based on the recommendations of the International Society for Pharmacoeconomics and Outcomes Research, according to which, the use of state-transitioning models is appropriate when the conceptualization of the disease requires the use of a long-time horizon and implies the transition between different health states [11]. Likewise, Markov models should be preferred when the analysis requires the simulation of several consecutive treatment lines [12].
From the time they are they are entered into the model, patients may transition between four possible health states (Figure 1).
Briefly, all patients start in the preprogression on-treatment state, corresponding to stable patients on treatment (lenalidomide in combination with dexamethasone or bortezomib in combination with dexamethasone). In each model cycle, patients may remain in the state they are in, or transition to one of the following health states:
Transition probabilities between health states were obtained from the progression-free survival, time to failure, and overall survival curves reported by the MM-009 and MM-010 trials, which evaluated the efficacy of lenalidomide plus dexamethasone versus placebo plus dexamethasone in previously treated patients, and by a retrospective study on retreatment with bortezomib [13],[14],[15].
Figure 1. Health states included in the model
Compared treatments
The present analysis compares the costs and clinical outcomes associated with lenalidomide plus dexamethasone versus bortezomib plus dexamethasone in the second line treatment of multiple myeloma patients. The model assumes that all patients have received a first line of treatment with bortezomib. Patients are then assigned to receive lenalidomide plus dexamethasone or bortezomib plus dexamethasone until failure (following the recommendations of the Summary of Product Characteristics, treatment with bortezomib was limited to a maximum of 8 cycles) [16]. In the third line, patients treated with bortezomib receive lenalidomide plus dexamethasone, while patients treated with lenalidomide plus dexamethasone receive Best Supportive Care, a mix of treatments consisting of chemotherapeutic agents (cyclophosphamide, melphalan, cisplatin, doxorubicin, etoposide) and, eventually, lenalidomide (Table 1)[17],[18]. In the fourth line, both arms receive Best Supportive Care.
Table 1. Components of BSC used in the model.
Population
As mentioned above, first-line treatment in patients with multiple myeloma who are not eligible for transplantation consists of a two- or three- drugs regimen based on bortezomib, lenalidomide or thalidomide. In case of relapse or progression, either repeating the same treatment or switching to another therapy (lenalidomide/dexamethasone or bortezomib/pegylated doxorubicin) should be considered [6],[7],[8]. In this light, the population considered in the present analysis consists of patients with multiple myeloma not eligible for transplantation who have previously been treated with bortezomib.
The sociodemographic and clinical characteristics of the population were obtained from MM-009 and MM-010 clinical trials [13],[14]. However, in order to adjust survival curves and calculations of body surface area (BSA) to the Chilean population, the proportion of men and women with multiple myeloma and their average weight and height were taken from the literature (Table 2) [19],[20].
Table 2. Parameters used in the model.
Time horizon and study perspective
Cycle length was set at 28 days, in accordance with the length of the cycles of the main treatments. This choice is in line with the recommendations of the International Society for Pharmacoeconomics and Outcomes Research, according to which “Cycle length should be short enough to represent the frequency of clinical events and interventions” [11]. Altogether, the model is composed of 326 cycles corresponding to a 25-year time horizon, long enough to capture the relevant costs and clinical outcomes.
The Chilean health system comprises a public and a private component: the former corresponding to the National Health Fund (Fondo Nacional de Salud) and the latter mainly consisting of private health insurance plans referred to as Health Provision Institutions (Instituciones de Salud Previsionales) [24]. In the present analysis, the perspective of the Chilean public health system was adopted.
Discount rate
A discount rate of 3% was applied to the costs and benefits [22].
Efficacy
The model uses transition probabilities between the different health states to estimate life-years gained over the time horizon. These probabilities are obtained from the overall survival and progression-free survival curves for each of the treatments. A systematic review of the literature was conducted to identify available evidence regarding the efficacy of the treatments evaluated [25]. The lenalidomide plus dexamethasone curves were modeled using pooled results from the MM-009 and MM-010 trials and parameterized using Gompertz (overall survival) and Gamma (progression-free survival) distributions [13],[14],[15]. In the absence of a direct comparison between bortezomib plus dexamethasone and lenalidomide plus dexamethasone, the overall survival and progression-free survival curves for bortezomib plus dexamethasone were constructed using the hazard ratios for the indirect comparison between the MM-009/MM-010 trials and the retrospective study of bortezomib re-treatment [13],[14],[15]. The hazard ratios were calculated as the ratio between the median survival for both treatments, after adjusting the efficacy of lenalidomide plus dexamethasone to the characteristics of the comparator study population (Table 2).
The quality-adjusted life-years are estimated as the sum of the years of life spent in each health state, weighted by its utility value. Utility data, together with disutility data associated with adverse events, were taken from the literature [22],[26],[27],[28],[29],[30].
Costs
Total costs were calculated as the sum of pharmacological (Table 3), resource use (Table 4) and adverse events costs (Table 5) [16],[17],[18],[31],[32],[33],[34],[35]. Resource use costs include the costs associated with follow-up testing and monitoring, administration, visits and palliative care (Table 6).
Table 3. Pharmacological costs.
Table 4. Frequency of resource use and associated costs.
Table 5. Frequency of and cost associated with the management of adverse events (grade 3-4).
Table 6. Costs and benefits associated with Len/Dex and Bor/Dex treatment.
All costs were estimated in Chilean pesos ($) and adjusted to 2018. Exchange and conversion rates were obtained from the Central Bank of Chile website on the 28 of March 2018 [37].
Pharmacological costs were obtained from the Chilean Transparent Public Procurement (Mercado Público de Chile Compra) database [31]. For each of the drugs considered, the costs of different bids were obtained to calculate an average cost per milligram.
Doses and frequency of administration were obtained from the Summary of Product Characteristics for each drug and were adjusted according to the experts’ recommendations. Resource use costs were based on the National Health Fund (Fondo Nacional de Salud) fees for 2018 [32],[33]. The frequency of resource use was obtained from the MM-009 and MM-010 trials and validated by the experts [13],[14].
Outcomes variables
The incremental cost-effectiveness and cost-utility ratios were calculated as the ratio between the incremental cost and the incremental life-years/quality adjusted life years gained of lenalidomide plus dexamethasone versus bortezomib plus dexamethasone.
Sensitivity analysis
A One-Way Sensitivity Analysis and a Probabilistic Sensitivity Analysis were conducted to evaluate the consistency of the results under the uncertainty or variability in the input data. The former shows the results obtained when varying each of the parameters, one at a time, between a defined minimum and maximum value. These values were obtained from the 95% confidence interval or by incorporating a ±20% variation in the base case value. With respect to the discount rate, the One-Way Sensitivity Analysis allows a variation between 0 and 6%, in accordance with the Chilean Ministry of Health’s guidance on the conduct of economic evaluations [22]. The Probabilistic Sensitivity Analysis performed corresponds to a Monte Carlo simulation encompassing 1000 iterations, in which random values are assigned to each of the model’s parameters according to their own probability distribution (Table 2).
Table 6 shows the benefits and costs for the two treatment cohorts. Over the time horizon, the cumulative number of life-years gained for the patients receiving lenalidomide plus dexamethasone was 4.69 (3.09 quality adjusted life-years) versus 3.28 (2.26 quality adjusted life-years) for those receiving bortezomib plus dexamethasone. The cost of treatment with lenalidomide plus dexamethasone was 68 818 192.92 CLP (113 627.00 US$) versus 56 953 595.06 CLP (94 037.14 US$) for the comparator. The cumulative costs for the two treatment arms are presented in Table 6. The results show that lenalidomide plus dexamethasone is more effective and more expensive compared to bortezomib retreatment, providing 1.41 incremental life-years gained (0.83 quality adjusted life-years) at an incremental cost of 11 864 597.86 CLP (19 589.86 US$) (Table 6).
The World Health Organization [38] suggests that interventions are highly cost-effective, provided that their incremental cost-effectiveness ratio lie below the threshold of one Gross Domestic Product (GDP) per capita (in the Chilean setting: 9 136 835.90 CLP [15 086 US$]) per quality adjusted life-year gained [39]. By contrast, it classifies as not cost-effective those exceeding three times the GDP per capita (in the Chilean setting: 27 410 507.70 CLP [45 258 US$]) per quality adjusted life-year gained [38],[39]. In this light, lenalidomide plus dexamethasone can be regarded as a potentially cost-effective alternative to bortezomib retreatment, resulting in an incremental cost-effectiveness ratio lower than 1 GDP per capita (8 410 266.92 CLP [13 886.35 US$]/life-year gained) and an incremental cost-utility ratio below 3 GDP (14 271 896.16 CLP [23 564.59 US$]/quality adjusted life-year gained).
The One-Way Sensitivity Analysis shows that the variables with the greatest impact on the results of the analysis are time to treatment failure and overall survival in patients who have received two or more prior therapies. To be specific, an increase or decrease of 20% in time to treatment failure would cause the incremental cost-utility ratio (baseline value) to fluctuate between 4 579 321 CLP (7 561.00 US$) and 22 799 751 CLP (37 645.09 US$). Moreover, a ±20% variation in OS would result in the incremental cost-utility ratio fluctuating between 982 787 CLP (16 482.77 US$) and 23 990 535 CLP (39 611.22 US$) (Figure 2). The results of the probabilistic sensitivity analysis indicate that, in 98.4% of the simulations, lenalidomide plus dexamethasone would be cost-effective versus bortezomib plus dexamethasone, with an incremental cost-utility ratio below the threshold of 27 410 507.70 CLP (45 258 US$) (Figure 3).
Figure 2. One-way sensitivity analysis.
Analyzing the costs and benefits of the currently available options for multiple myeloma treatment is essential to ensure an efficient resource allocation. This study shows that, from the perspective of the Chilean national health system, lenalidomide plus dexamethasone is a cost-effective option for the second line treatment of patients with multiple myeloma who are not eligible for transplantation, as it provides a significant increase in survival without exceeding the willingness-to-pay threshold of 3 times the GDP per capita per quality adjusted life-year gained [38].
Our results are consistent with those reported by other studies that have assessed the cost-effectiveness of lenalidomide plus dexamethasone in other settings [40],[41]. In a study conducted in Norway, lenalidomide plus dexamethasone was shown to be cost-effective versus bortezomib, providing a greater number of life-years gained (4.06 versus 3.11) and quality adjusted life-years gained (2.95 versus 2.19) at an incremental cost of 188 245 NOK2011, with an estimated incremental cost-effectiveness ratio of 198 714 NOK2011/ life-year gained and an incremental cost-utility ratio of 247 078 NOK2011/ quality adjusted life-year gained [40].
Similarly, a study conducted in the Greek setting estimated an increase of 0.99 in life-year gained and 0.79 in quality adjusted life-year gained in favor of lenalidomide plus dexamethasone, with an incremental cost of 28 741 € and estimated incremental cost-effectiveness and cost-utility ratios of 29 415 €/life-year gained and 38 268 €/quality-adjusted life-year gained, respectively [41]. Based on these results, the authors concluded that treatment with lenalidomide plus dexamethasone would be cost-effective versus bortezomib, considering a willingness-to-pay threshold of three times the GDP per capita in Greece (60 000 €2013/ quality-adjusted life-year gained).
It should be noted that, in contrast to the present study, the data on the efficacy of bortezomib used in the Norwegian and Greek analyses were taken from the APEX clinical trial, that compared the efficacy of bortezomib versus high-dose dexamethasone in patients with previously treated multiple myeloma [42]. However, in the APEX trial, prior treatment with bortezomib was an exclusion criterion for the study, thus, in our analysis it was not deemed appropriate to use this source since the population included in that study differs substantially from the population of interest (patients previously treated with bortezomib).
By contrast, in a study conducted in the Swedish setting, treatment with bortezomib was deemed to be dominant with respect to lenalidomide plus dexamethasone, as it was associated with a greater number of quality adjusted life-year gained (2.95 versus 2.91 months) and generated savings of 546 126 SEK2010. The divergence between these results and those of our analysis is probably due to methodological differences between the studies [43]. To be specific, the Swedish analysis only considers three possible health states (preprogression, progression and death) and uses parametric survival analysis techniques (partitioned survival model) in which the proportion of patients in each state is calculated using parametric equations [43].
Conversely, a state-transition model (i.e. Markov model) was chosen in our analysis. This choice is justified by the extension of the time horizon and the nature of the disease, which entails that patients may experience phases of stability (preprogression with or without treatment) and progression (progression).
This choice is also in line with what is recommended by the International Society for Pharmacoeconomics and Outcomes Research in its Task Force, which states that "if the conceptualization of the health problem involves representing the disease or the treatment process as a series of health conditions, state transition models are appropriate"[11]. Likewise, state transition model are particularly useful when long time horizons are used, as is the case in the present analysis [11]. Finally, a recent study suggests that, compared to other designs, Markov's models are more appropriate when the analysis requires the simulation of several consecutive treatment lines [12].
This study is not free of limitations. In the absence of controlled clinical trials that assess the efficacy of retreatment with bortezomib in patients with previously treated multiple myeloma, the data on the efficacy of bortezomib plus dexamethasone have been obtained from a retrospective study of re-treatment with bortezomib conducted in multiple myeloma patients from Germany and Switzerland [15]. The sample size (n = 42) and the study design may have introduced some uncertainty into the analysis; nevertheless, the study provides data regarding the effectiveness of bortezomib in routine clinical practice, giving additional external validity to the analysis and allowing the generalization of the results. Likewise, the consistency of the results of this study with those obtained in the Greek and Norwegian settings indicates that the choice of the retrospective study for the estimation of the efficacy of bortezomib does not entail an underestimation of the clinical benefits associated with this treatment. Another possible limitation of this analysis is related to the characteristics of the population included in the MM-009/010 trials, from which the efficacy data for lenalidomide plus dexamethasone treatment were taken [13],[14]. In the mentioned studies, a lower proportion of patients had received only one prior treatment regimen before receiving lenalidomide treatment, so that the population included in these trials may differ from the population of interest. Nevertheless, after reviewing the efficacy sources used in the model, the NICE Evidence Review Group determined that, in spite of these differences, the results of the MM-009/10 trials could be extrapolated to the population of interest without compromising the generalization of results [9]. All things considered, despite possible uncertainties associated with the efficacy data used, the sensitivity analyses indicate that the results obtained are robust. In particular, the one-way sensitivity analysis shows that, for the majority of the variables assessed, the variation brought into the base case values does not affect the cost-effectiveness of lenalidomide plus dexamethasone versus bortezomib. Likewise, the probabilistic sensitivity analysis, which evaluated the effect of simultaneous variation of all of the model’s parameters on the results of the analysis, showed that treatment with Len/Dex had a 98.4% probability of being cost-effective versus bortezomib when a willingness-to-pay threshold of 27 410 507.70 CLP (45 258 US$) was taken into account.
In conclusion this study suggests that, from the perspective of the Chilean national health system, lenalidomide offers a potentially efficient alternative for the second-line treatment of patients with multiple myeloma who are not eligible for transplantation, as it provides greater survival and involves a cost/life-year gained lower than 1 GDP per capita and a cost/quality adjusted life-year gained lower than three times the Chilean GDP per capita.
From the editor
The authors originally submitted this article in Spanish and subsequently translated it into English. The Journal has not copyedited this version.
Authors’ contribution
MA: Data Collection, Methodology, Validation, Visualization, Original Drafting, Drafting-Reviewing and Editing. IG: Data Collection, Methodology, Validation, Original Drafting, Drafting-Reviewing and Editing. LL: Methodology, Project Management, Supervision, Validation, Writing, Proofreading and Editing. SA: Methodology, Project Management, Supervision, Validation, Writing, Proofreading and Editing.
Acknowledgements
We thank Dr. Camila Peña and Dr. Carolina Guerra for their participation in this project, for their validation and contribution of new clinical and economic data, which have allowed the adaptation of this analysis to the Chilean setting.
Funding
The present research work was financed by Tecnofarma S.A. The company had no influence on the design of the study, analysis or interpretation of the data. Also, there was no influence on the preparation, review or approval of the manuscript.
Conflict of interests
The authors have completed the ICMJE conflict of interest declaration form and declare that they have received funding from Tecnofarma S.A. to carry out the economic evaluation study. Forms can be requested by contacting the responsible author or the editorial management of the Journal.

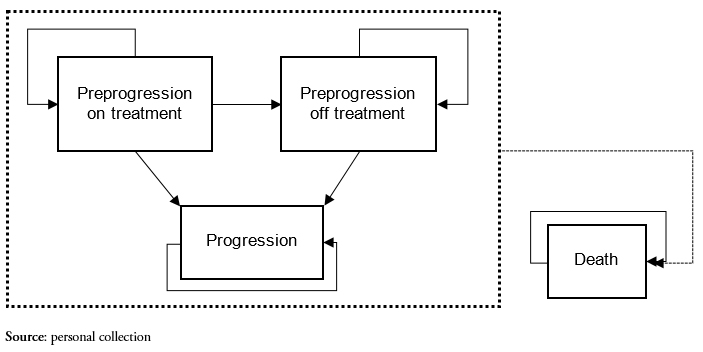 Figure 1. Health states included in the model
Figure 1. Health states included in the model

 Table 1. Components of BSC used in the model.
Table 1. Components of BSC used in the model.

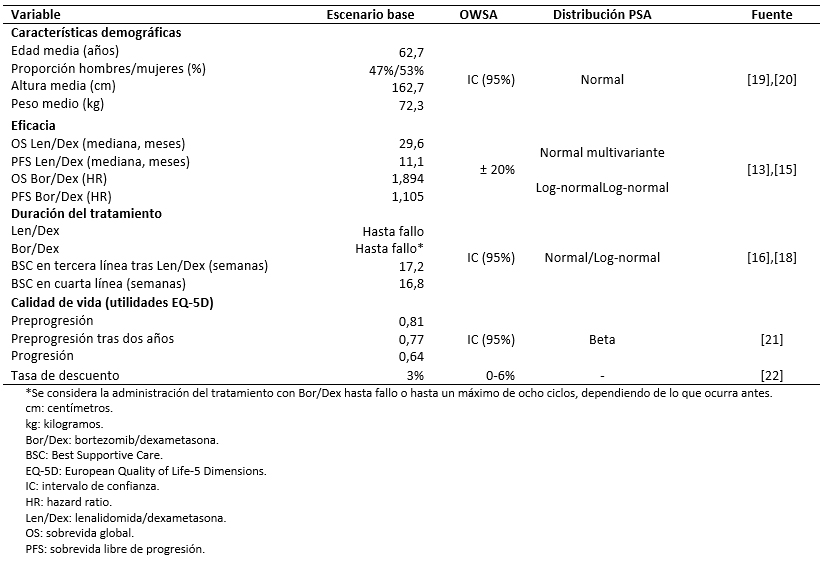 Table 2. Parameters used in the model.
Table 2. Parameters used in the model.

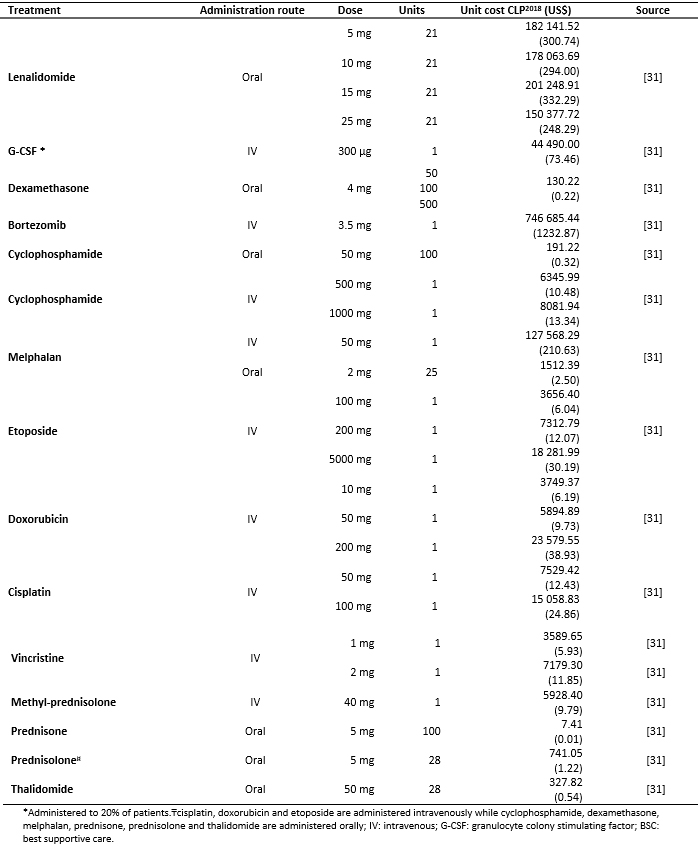 Table 3. Pharmacological costs.
Table 3. Pharmacological costs.

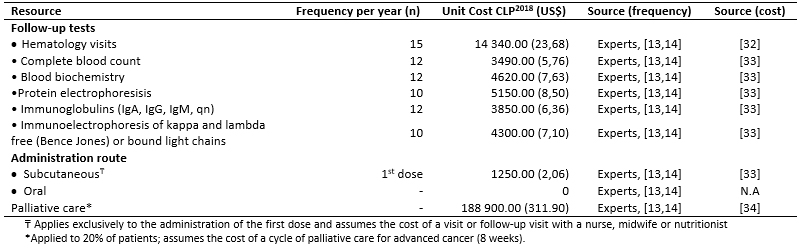 Table 4. Frequency of resource use and associated costs.
Table 4. Frequency of resource use and associated costs.

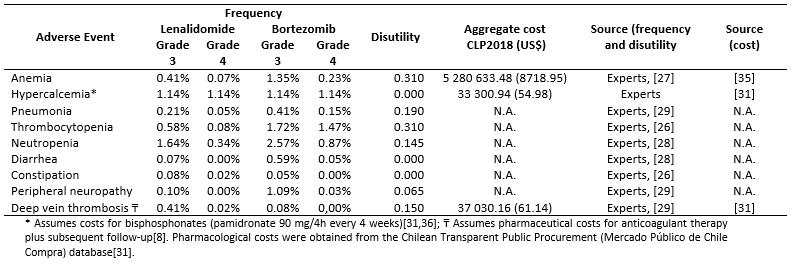 Table 5. Frequency of and cost associated with the management of adverse events (grade 3-4).
Table 5. Frequency of and cost associated with the management of adverse events (grade 3-4).

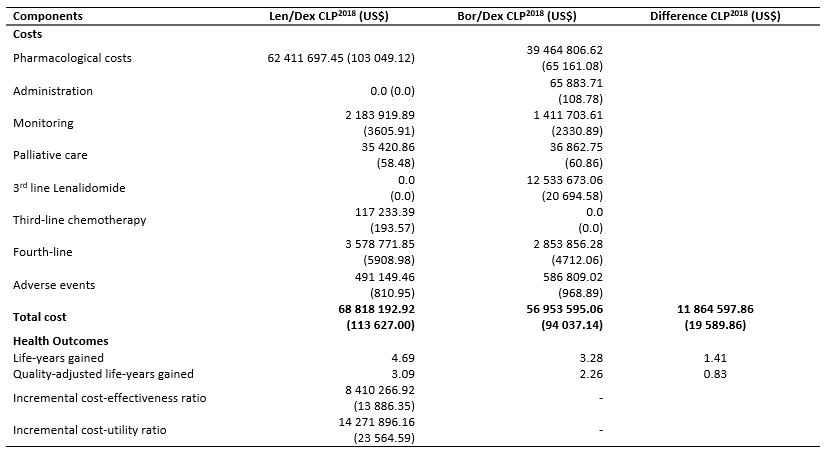 Table 6. Costs and benefits associated with Len/Dex and Bor/Dex treatment.
Table 6. Costs and benefits associated with Len/Dex and Bor/Dex treatment.

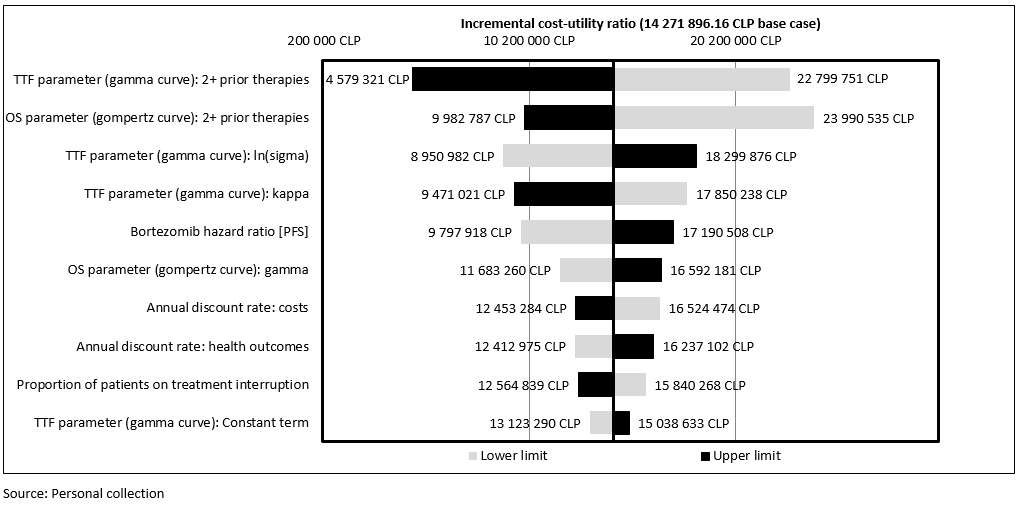 Figure 2. One-way sensitivity analysis.
Figure 2. One-way sensitivity analysis.

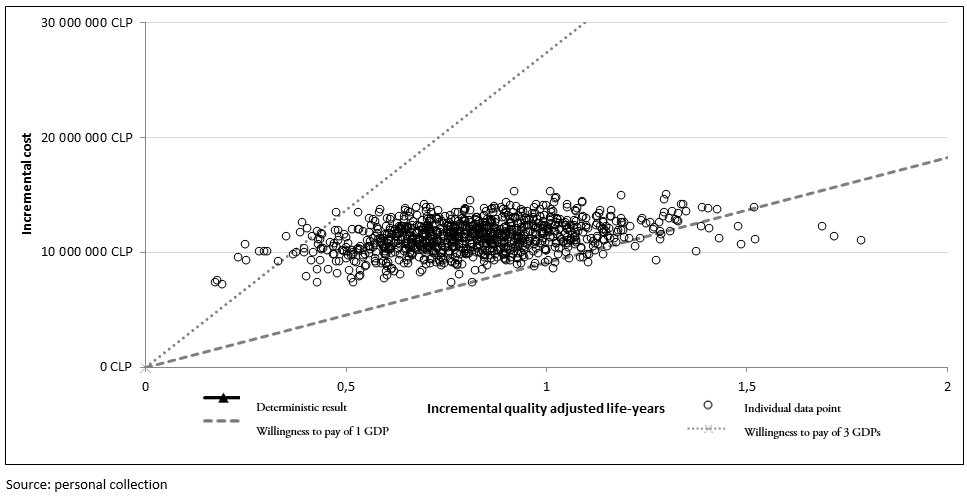 Figure 3. Results of the probabilistic sensitivity analysis: incremental cost-effectiveness scatter plot.
Figure 3. Results of the probabilistic sensitivity analysis: incremental cost-effectiveness scatter plot.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

BACKGROUND
Multiple myeloma is a hematologic malignancy affecting bone marrow derived plasma cells. Current therapies are not able to eradicate the disease and most patients become refractory to the treatment. Lenalidomide and bortezomib have proved effective in the second-line treatment of these patients.
OBJECTIVE
To evaluate the cost-effectiveness of lenalidomide in combination with dexamethasone compared to bortezomib in combination with dexamethasone in patients with multiple myeloma previously treated with bortezomib, from the perspective of the Chilean National Health Service.
METHODOLOGY
A four-state Markov model (preprogression on treatment; preprogression off treatment, progression and death) was used to simulate the evolution of a cohort of multiple myeloma patients over a 25-year time horizon. Efficacy data, resource use and frequency of adverse events were extracted from MM009/010 studies and a retrospective analysis of retreatment with bortezomib. All inputs were validated by experts. A 3% annual discount rate was used for costs and health outcomes. The robustness of the results was evaluated through univariate and probabilistic sensitivity analyses.
RESULTS
Lenalidomide in combination with dexamethasone treatment provided 1.41 incremental life years and 0.83 incremental quality-adjusted life years in comparison with bortezomib in combination with dexamethasone, with an incremental cost of 11 864 597.86 CLP (19 589.86 US$). The incremental cost-effectiveness and cost-utility ratio were estimated at 8 410 266.92 CLP (13 886,35 US$) / incremental life year and 14 271 896.16 CLP (23 564,59 US$)/incremental quality-adjusted life years, respectively.
CONCLUSIONS
Lenalidomide in combination with dexamethasone represents a potentially cost-effective alternative for the second-line treatment of patients with multiple myeloma who are not eligible for transplantation, from the perspective of the Chilean National Health Service.
 Autores:
Susana Aceituno[1], Irmina Gozalbo[1], Marilena Appierto[1], Luis Lizán[1,2]
Autores:
Susana Aceituno[1], Irmina Gozalbo[1], Marilena Appierto[1], Luis Lizán[1,2]

Citación: Aceituno S, Gozalbo I, Appierto M, Lizán L. Cost-effectiveness of lenalidomide in combination with dexamethasone compared to bortezomib in combination with dexamethasone for the second-line treatment of multiple myeloma in Chile. Medwave 2018 May-Jun;18(3):e7220 doi: 10.5867/medwave.2018.03.7220
Fecha de envío: 7/11/2017
Fecha de aceptación: 13/5/2018
Fecha de publicación: 29/6/2018
Origen: no solicitado
Tipo de revisión: con revisión por tres pares revisores externos, a doble ciego

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009 Jan;23(1):3-9.
| CrossRef | PubMed |
Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009 Jan;23(1):3-9.
| CrossRef | PubMed | Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014 Nov;15(12):e538-48. | CrossRef | PubMed |
Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014 Nov;15(12):e538-48. | CrossRef | PubMed | GLOBOCAN 2012. Estimated cancer Incidence, Mortality, Prevalence and Disability-adjusted life years (DALYs) Worldwide in 2012. [on line] | Link |
GLOBOCAN 2012. Estimated cancer Incidence, Mortality, Prevalence and Disability-adjusted life years (DALYs) Worldwide in 2012. [on line] | Link | Sonneveld P, De Wit E, Moreau P. How have evolutions in strategies for the treatment of relapsed/refractory multiple myeloma translated into improved outcomes for patients? Crit Rev Oncol Hematol. 2017 Apr;112:153-170. | CrossRef | PubMed |
Sonneveld P, De Wit E, Moreau P. How have evolutions in strategies for the treatment of relapsed/refractory multiple myeloma translated into improved outcomes for patients? Crit Rev Oncol Hematol. 2017 Apr;112:153-170. | CrossRef | PubMed | Moreau P, San Miguel J, Sonneveld P, Mateos MV, Zamagni E, Avet-Loiseau H, et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017 Jul 1;28(suppl_4):iv52-iv61. | CrossRef | PubMed |
Moreau P, San Miguel J, Sonneveld P, Mateos MV, Zamagni E, Avet-Loiseau H, et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017 Jul 1;28(suppl_4):iv52-iv61. | CrossRef | PubMed | Anderson KC, Alsina M, Atanackovic D, Biermann JS, Chandler JC, Costello C, Djulbegovic B, et al. NCCN Guidelines Insights: Multiple Myeloma, Version 3.2016. J Natl Compr Canc Netw. 2016 Apr;14(4):389-400. | PubMed |
Anderson KC, Alsina M, Atanackovic D, Biermann JS, Chandler JC, Costello C, Djulbegovic B, et al. NCCN Guidelines Insights: Multiple Myeloma, Version 3.2016. J Natl Compr Canc Netw. 2016 Apr;14(4):389-400. | PubMed | Sociedad Chilena de Hematología SOCHIHEM 2014. Guías prácticas clínicas para diagnóstico y tratamiento de mieloma múltiple. 2014. hematologia.org [On line] | Link |
Sociedad Chilena de Hematología SOCHIHEM 2014. Guías prácticas clínicas para diagnóstico y tratamiento de mieloma múltiple. 2014. hematologia.org [On line] | Link | National Institute for Health and Care Excellence (NICE). Lenalidomide for treating multiple myeloma after 1 prior treatment with bortezomib (part-review of TA171): appraisal consultation document. nice.org.uk [on line]. | Link |
National Institute for Health and Care Excellence (NICE). Lenalidomide for treating multiple myeloma after 1 prior treatment with bortezomib (part-review of TA171): appraisal consultation document. nice.org.uk [on line]. | Link | Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Eur J Health Econ. 2013 Jun;14(3):367-72. | CrossRef | PubMed |
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Eur J Health Econ. 2013 Jun;14(3):367-72. | CrossRef | PubMed | Roberts M, Russell LB, Paltiel AD, Chambers M, McEwan P, Krahn M; ISPOR-SMDM Modeling Good Research Practices Task Force. Conceptualizing a model: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-2. Med Decis Making. 2012 Sep-Oct;32(5):678-89. | PubMed |
Roberts M, Russell LB, Paltiel AD, Chambers M, McEwan P, Krahn M; ISPOR-SMDM Modeling Good Research Practices Task Force. Conceptualizing a model: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-2. Med Decis Making. 2012 Sep-Oct;32(5):678-89. | PubMed | Minacori R, Bonastre J, Lueza B, Marguet S, Levy P. How to Model Survival In Cost-Effectiveness Analysis? Differences Between Markov and Partitioned Survival Analysis Models. Value Health. 2015 Nov;18(7):A704. | CrossRef | PubMed |
Minacori R, Bonastre J, Lueza B, Marguet S, Levy P. How to Model Survival In Cost-Effectiveness Analysis? Differences Between Markov and Partitioned Survival Analysis Models. Value Health. 2015 Nov;18(7):A704. | CrossRef | PubMed | Weber DM, Chen C, Niesvizky R, Wang M, Belch A, Stadtmauer EA, Siegel D, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007 Nov 22;357(21):2133-42. | PubMed |
Weber DM, Chen C, Niesvizky R, Wang M, Belch A, Stadtmauer EA, Siegel D, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007 Nov 22;357(21):2133-42. | PubMed | Dimopoulos M, Spencer A, Attal M, Prince HM, Harousseau JL, Dmoszynska A, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007 Nov 22;357(21):2123-32. | PubMed |
Dimopoulos M, Spencer A, Attal M, Prince HM, Harousseau JL, Dmoszynska A, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007 Nov 22;357(21):2123-32. | PubMed | Taverna C, Voegeli J, Trojan A, Olie RA, von Rohr A. Effective response with bortezomib retreatment in relapsed multiple myeloma--a multicentre retrospective survey in Switzerland. Swiss Med Wkly. 2012 Apr 27;142:w13562. | CrossRef | PubMed |
Taverna C, Voegeli J, Trojan A, Olie RA, von Rohr A. Effective response with bortezomib retreatment in relapsed multiple myeloma--a multicentre retrospective survey in Switzerland. Swiss Med Wkly. 2012 Apr 27;142:w13562. | CrossRef | PubMed | Myeloma Pathways 2007-9 data. Haematological Malignancy Research Network, York UK.
Myeloma Pathways 2007-9 data. Haematological Malignancy Research Network, York UK.  Ministerio de Salud (MINSAL) Gobierno de Chile. Encuesta Nacional de Salud ENS Chile 2009-2010. [on line] | Link |
Ministerio de Salud (MINSAL) Gobierno de Chile. Encuesta Nacional de Salud ENS Chile 2009-2010. [on line] | Link | Conté LG, Figueroa MG, Lois VV, Cabrera C ME, León RA, García LH, Rojas R H. [Clinical features and survival of Chilean patients with multiple myeloma]. Rev Med Chil. 2007 Sep;135(9):1111-7. | PubMed |
Conté LG, Figueroa MG, Lois VV, Cabrera C ME, León RA, García LH, Rojas R H. [Clinical features and survival of Chilean patients with multiple myeloma]. Rev Med Chil. 2007 Sep;135(9):1111-7. | PubMed | van Agthoven M, Segeren CM, Buijt I, Uyl-de Groot CA, van der Holt B, Lokhorst HM, et al. A cost-utility analysis comparing intensive chemotherapy alone to intensive chemotherapy followed by myeloablative chemotherapy with autologous stem-cell rescue in newly diagnosed patients with stage II/III multiple myeloma; a prospective randomised phase III study. Eur J Cancer. 2004 May;40(8):1159-69. | PubMed |
van Agthoven M, Segeren CM, Buijt I, Uyl-de Groot CA, van der Holt B, Lokhorst HM, et al. A cost-utility analysis comparing intensive chemotherapy alone to intensive chemotherapy followed by myeloablative chemotherapy with autologous stem-cell rescue in newly diagnosed patients with stage II/III multiple myeloma; a prospective randomised phase III study. Eur J Cancer. 2004 May;40(8):1159-69. | PubMed | Ministerio de Salud, Gobierno de Chile. Guía Metodológica para la Evaluación Económica de intervenciones en Salud en Chile. Santiago, Chile: Minsal;2013:16-36. | Link |
Ministerio de Salud, Gobierno de Chile. Guía Metodológica para la Evaluación Económica de intervenciones en Salud en Chile. Santiago, Chile: Minsal;2013:16-36. | Link | Roberts M1, Russell LB, Paltiel AD, Chambers M, McEwan P, Krahn M. Conceptualizing a model: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force--2. Value Health. 2012 Sep-Oct;15(6):804-11. | CrossRef | PubMed |
Roberts M1, Russell LB, Paltiel AD, Chambers M, McEwan P, Krahn M. Conceptualizing a model: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force--2. Value Health. 2012 Sep-Oct;15(6):804-11. | CrossRef | PubMed | Becerril-Montekio V, de Dios Reyes J, Manuel A. Sistema de salud de Chile. Salud Publica Mex. 2011;53(SUPPL.2):s132-42. | Link |
Becerril-Montekio V, de Dios Reyes J, Manuel A. Sistema de salud de Chile. Salud Publica Mex. 2011;53(SUPPL.2):s132-42. | Link | National Institute for health and Care Excellence (NICE). Single Technology Appraisal (STA) of lenalidomide for the treatment of multiple myeloma in people who have received at least one prior therapy with bortezomib (partial review of TA171). Celgene submission of evidence. nice.org.uk [on line]. | Link |
National Institute for health and Care Excellence (NICE). Single Technology Appraisal (STA) of lenalidomide for the treatment of multiple myeloma in people who have received at least one prior therapy with bortezomib (partial review of TA171). Celgene submission of evidence. nice.org.uk [on line]. | Link | Coffey JT, Brandle M, Zhou H, Marriott D, Burke R, Tabaei BP, et al. Valuing health-related quality of life in diabetes. Diabetes Care. 2002 Dec;25(12):2238-43. | PubMed |
Coffey JT, Brandle M, Zhou H, Marriott D, Burke R, Tabaei BP, et al. Valuing health-related quality of life in diabetes. Diabetes Care. 2002 Dec;25(12):2238-43. | PubMed | Ossa DF, Briggs A, McIntosh E, Cowell W, Littlewood T, Sculpher M. Recombinant erythropoietin for chemotherapy-related anaemia: economic value and health-related quality-of-life assessment using direct utility elicitation and discrete choice experiment methods. Pharmacoeconomics. 2007;25(3):223-37. | PubMed |
Ossa DF, Briggs A, McIntosh E, Cowell W, Littlewood T, Sculpher M. Recombinant erythropoietin for chemotherapy-related anaemia: economic value and health-related quality-of-life assessment using direct utility elicitation and discrete choice experiment methods. Pharmacoeconomics. 2007;25(3):223-37. | PubMed | Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J. Health state utilities for metastatic breast cancer. Br J Cancer. 2006 Sep 18;95(6):683-90. | PubMed |
Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J. Health state utilities for metastatic breast cancer. Br J Cancer. 2006 Sep 18;95(6):683-90. | PubMed | Cykert S, Joines JD, Kissling G, Hansen CJ. Racial differences in patients' perceptions of debilitated health states. J Gen Intern Med. 1999 Apr;14(4):217-22. | PubMed |
Cykert S, Joines JD, Kissling G, Hansen CJ. Racial differences in patients' perceptions of debilitated health states. J Gen Intern Med. 1999 Apr;14(4):217-22. | PubMed | Mathias SD, Prebil LA, Putterman CG, Chmiel JJ, Throm RC, Comerota AJ. A Health-Related Quality of Life Measure in Patients with Deep Vein Thrombosis: A Validation Study. Ther Innov Regul Sci 1999 Oct 1;33(4):1173–87. | CrossRef |
Mathias SD, Prebil LA, Putterman CG, Chmiel JJ, Throm RC, Comerota AJ. A Health-Related Quality of Life Measure in Patients with Deep Vein Thrombosis: A Validation Study. Ther Innov Regul Sci 1999 Oct 1;33(4):1173–87. | CrossRef | FONASA. Alivio del Dolor y Cuidados Paliativos por Cáncer Avanzado. Disponible en: Alivio del Dolor y Cuidados Paliativos por Cáncer Avanzado. [on line]
FONASA. Alivio del Dolor y Cuidados Paliativos por Cáncer Avanzado. Disponible en: Alivio del Dolor y Cuidados Paliativos por Cáncer Avanzado. [on line]  Vargas CL, Espinoza MA, Giglio A, Soza A. Cost Effectiveness of Daclatasvir/Asunaprevir Versus Peginterferon/Ribavirin and Protease Inhibitors for the Treatment of Hepatitis c Genotype 1b Naïve Patients in Chile. PLoS One. 2015 Nov 6;10(11):e0141660. | CrossRef | PubMed |
Vargas CL, Espinoza MA, Giglio A, Soza A. Cost Effectiveness of Daclatasvir/Asunaprevir Versus Peginterferon/Ribavirin and Protease Inhibitors for the Treatment of Hepatitis c Genotype 1b Naïve Patients in Chile. PLoS One. 2015 Nov 6;10(11):e0141660. | CrossRef | PubMed | Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015 Feb 1;93(2):118-24. | CrossRef | PubMed |
Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015 Feb 1;93(2):118-24. | CrossRef | PubMed | Möller J, Nicklasson L, Murthy A. Cost-effectiveness of novel relapsed-refractory multiple myeloma therapies in Norway: lenalidomide plus dexamethasone vs bortezomib. J Med Econ. 2011;14(6):690-7. | CrossRef | PubMed |
Möller J, Nicklasson L, Murthy A. Cost-effectiveness of novel relapsed-refractory multiple myeloma therapies in Norway: lenalidomide plus dexamethasone vs bortezomib. J Med Econ. 2011;14(6):690-7. | CrossRef | PubMed | Fragoulakis V, Kastritis E, Psaltopoulou T, Maniadakis N. Economic evaluation of therapies for patients suffering from relapsed-refractory multiple myeloma in Greece. Cancer Manag Res. 2013 Apr 10;5:37-48. | CrossRef | PubMed |
Fragoulakis V, Kastritis E, Psaltopoulou T, Maniadakis N. Economic evaluation of therapies for patients suffering from relapsed-refractory multiple myeloma in Greece. Cancer Manag Res. 2013 Apr 10;5:37-48. | CrossRef | PubMed | Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005 Jun 16;352(24):2487-98. | PubMed |
Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005 Jun 16;352(24):2487-98. | PubMed |