 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Palabras clave: vascular hemichorea
Chorea rarely complicates ischemic or hemorrhagic cerebral vascular lesions. Clinical symptoms usually involve one side of the body while the injury is situated on the contralateral cerebral hemisphere. Spontaneous remission is the norm, but sometimes symptomatic treatment is required. A 58-year-old male patient who suffers from untreated high blood pressure, type II obesity, smokes 6 packs of cigarettes per year and has a moderate intake of alcohol is presented. The patient’s recent history began three days before he appeared at the Emergency Department. His symptoms were ceaseless, involuntary movements in his left arm and foot during day and night with no restriction of voluntary movements. Physical examination and laboratory tests revealed no other findings. Magnetic resonance imaging of the brain showed hyperintensity in the right posterolateral thalamic region consistent with ischemic cerebrovascular disease. Symptomatic therapy was indicated and his underlying conditions were addressed. The importance of this case lies on the low prevalence as well as the scarcity of publications regarding vascular causes of hemichorea, including diagnosis, therapy and prognosis.
Vascular chorea is a movement disorder characterized by rapid, involuntary, undulating or crawling, random movements. The movements tend to be aimless, continuous, and non-stereotypical [1]. This disorder can be caused by either ischemic or hemorrhagic vascular cerebral disease. Chorea rarely complicates cerebral vascular lesions, and even though they are highly variable, clinical symptoms usually involve one side of the body while the injury is situated on the contralateral cerebral hemisphere. Spontaneous remission is the norm, but therapy with neuroleptic and dopamine antagonist is usually needed during acute phases [2]. Thalamotomy has been described as an efficient treatment when basal ganglia damage does not resolve spontaneously.
Basal ganglia, through direct and indirect pathways, are responsible for the regulation of movement. Any alteration in their structure or their pathways impacts a highly complex positive and negative feedback system that has its endpoint in the thalamus. This structure can be uninhibited, which will result in facilitating movement, or movement can be reduced by increasing basal inhibition by the globus pallidus or by the pars reticulata of the sustancia nigra. The thalamus also participates in the input and output of information coming from the cerebral cortex, which integrates, modulates, and distributes information from the somatomotor system, reticular formation and limbic systems. Bearing in mind this level of complexity, when an ischemic event occurs, it is necessary to consider the vascular areas of the four arteries that irrigate the thalamus (thalamo-subthalamic paramedian artery, thalamo-geniculated artery, posterior choroidal artery, and polar artery) and contrast them with clinical findings in order to make a timely and accurate diagnosis.
The patient is a 58-year-old male with a previous history of untreated high blood pressure stage II diagnosed 15 years ago; a type II obese (BMI 35.5 kg/ m2) who smokes six packs of cigarettes per year, and a heavy alcohol drinker (239.76 g ethanol/week). His recent history began three days before he appeared at the Emergency Department of Miguel Perez Carreño Hospital presenting with ceaseless choreiform movements in the left arm and foot that occur throughout day and night, whether resting or active, and did not limit voluntary movement.
His family history revealed no findings; however, in addition to his untreated hypertension, he had a tibio-fibular fracture three years ago, that required surgical repair and permanently altered his gait.
Physical examination showed the following: BP 160/100 mmHg, HR 86 bpm, RR 16 bpm, and a temperature of 36.5°C. The patient appeared relaxed, alert, and cooperative (GCS 15/15). He was coherent and oriented. Cranial nerve pair I was not explored but the remaining cranial nerves had normal function. His fundoscopic examination showed Keith-Wegener stage II/IV hypertensive retinopathy. Motor exam showed normal muscle mass and movement with a global strength score of 5/5. His cerebellum, tested with rapid alternating movements and pointing tests (finger to nose, toe to knee), was normal. Gait was altered by the aforementioned fracture with an incomplete left plantar step. During Romberg test the patient was able to keep his balance with eyes closed. He did not deviate on pronation. Deep-tendon reflexes were normal and symmetric, as well as his sensory system exploration (pain, light touch, postural, vibration, stereognosis, deep touch, and temperature).
Laboratory testing showed no alterations in complete blood count, glycemia, uremia, or creatinine. Liver function tests, thyroid tests, and serum electrolytes were also normal.
Non contrast-enhanced cranial CAT showed no abnormalities, but a cerebral diffusion MRI showed a hyperintense image in the posterolateral thalamic area, which was present in two axial and two cross-sectional views. The image was 5.33 mm wide, 3.45 mm high and 6.74 mm deep, and was consistent with cerebral vascular ischemia of the posterior choroidal artery. Figures 1 and 2.
Finally, a carotid Doppler sonogram showed atheromatous disease in both internal carotid arteries and a high-embolic risk soft atheroma plaque in the common carotid artery.
Figure 1. Cerebral MRI axial cut. Fast Spin Echo (FSE) T2 enhanced.
Figure 2. Cerebral MRI frontal cut. Fast Spin Echo (FSE) T2 enhanced.
Choreic movements can be generalized, focal (for example, masticatory buccolingual dyskinesia), or involve one side of the body, as the case presented. In severe cases, it can involve posture or gait. There are movement patterns in chorea, but more specific to hemichorea are an exaggerated balancing of the upper limb with a tendency to extend the limb beyond the torso and opening the hand while walking. When sustained muscular contractions are also present, the term choreoathetosis is used. Furthermore, when the choreoathetotic movements are strikingly wide and exaggerated, they are termed ballism. Generalized chorea is a diagnostic challenge because it can be a symptom of multiple diseases such as neurodegenerative, neurometabolic, toxic, immunological, pharmacological, and infectious diseases. Unlike generalized chorea, hemichorea usually has a vascular origin or is caused by a metabolic disturbance like hyperglycemia [4].
In the case reported, a sum of factors (hypertension, smoking habit, obesity and heavy drinking) facilitated the appearance of arterial disease. This combination resulted in atherosclerosis, which favored the appearance of cerebral vascular disease. In addition, the carotid Doppler sonogram revealed atheromatous disease, and a soft plaque that had a high-embolic risk in the common carotid artery. Such an embolic event might have been the cause of this patient’s ischemic vascular stroke.
Arteriosclerosis is a generic term for hardening and thickening of arterial walls, whatever their diameter. When it affects mid- and large-caliber arteries it is termed atherosclerosis.
Atherosclerosis is a chronic inflammatory process that involves arteries in various vascular beds. It thickens the intima, and the media layer producing a loss of elasticity. The basic lesion is the atheromatous plaque, mostly made of lipids, fibrous tissue, and inflammatory cells. The atheromatous plaque progresses through different stages.
Atherosclerosis is complicated by a fissure, erosion or breakage of the plaque, which triggers thrombus formation on its surface. The plaque then enlarges, becoming ischemic or necrotic, which causes some of its clinical features. The term atherothrombotic disease attempts to include both processes in a single entity.
Atherosclerosis is a systemic disease that involves arteries in different areas simultaneously, but to different degrees. It tends to settle in the arteries that irrigate the heart (coronary arteries), the brain (carotid, vertebral, and cerebral arteries), and the lower limbs (iliac and femoral arteries). Therefore, the presence of vascular disease in one location of the body is associated with a higher risk of presenting it in other vascular beds.
Its clinical features depend on the location of the atheromatous vascular bed. In the area irrigated by the coronary arteries, it can cause acute coronary syndrome, acute myocardial infarction, or sudden death. If it involves the peripheral arteries, the symptoms can be intermittent claudication or lower limb acute ischemia. In the brain, it can cause acute cerebral vascular stroke, transient ischemic attack, or repeated episodes can end in multi-infarct dementia. Atherosclerosis can present as a chronic disease, with gradual stenosis of the arterial lumen, or can present acutely if the plaque breaks suddenly and a thrombus develops on its surface, in an acute coronary event or cerebral ischemic stroke as described in this case report [5].
The patient’s underlying pathology produced atherosclerosis, which in turn led to an ischemic event in the area irrigated by the posterior choroidal artery, compromising the posterolateral region of the thalamus. It can be assumed that this event diminished the inhibition exerted upon the thalamus by the internal globus pallidus and the pars reticulata of the sustancia nigra, offsetting direct and indirect circuits and manifesting clinically as choreiform movements limited to the left side of the body.
As much as the clinical symptoms of this patient pointed to vascular chorea, it was still necessary to perform imaging studies (sonogram, scans and/or magnetic resonance imaging) to corroborate the diagnosis, as was done in this patient. Metabolic causes such as hyperglycemia need to be ruled out as well. The patient in this case report had normal glycemic values, but the same hyperdense (in sonograms), or hyperintense (in MRI) images can appear in non-ketotic hyperglycemia, even without choreiform neurological signs [6],[7].
Hyperglycemia is a frequent cause of hemichorea in long-term poorly-controlled diabetic patients, even though it resolves quickly once glucose levels are stabilized.
Database searches were conducted in MEDLINE, Lilacs, and Cochrane, which corroborated the lack of recent publications on vascular hemichorea. In addition, the reports found are geared toward other causes of hemichorea. Most frequent among these causes are non-ketotic hyperglycemia [8], structural changes of cerebral blood vessels like stenosis [9], Moyamoya disease [10], and demyelinating diseases. These differential diagnoses need to be addressed and were clinically ruled out in the case reported without the need to undertake neuroimaging.
The most recent specific vascular hemichorea studies are case reports and case series. The Dutch study by Zijlmans [11], reports that magnetic resonance imaging is the preferred imaging study in cases of vascular hemichorea. Lesions can be found mostly in the thalamus, lenticular nucleus, and less frequently in the subthalamic nucleus.
Handley [12], in the United Kingdom, reviewed case series reports up to 2008. He points out that vascular disorders presenting as abnormal movements are mostly hyperkinetic, are self-limited and are caused by lesions in the basal ganglia or the thalamus.
The oldest reports come from Spain. The first, by Galiano [13], is a study performed between 1993 and 1999 that found ten cases with an average age of 72 years, which differs from our reported patient, who is much younger. In these patients, 80% of lesions presented in basal ganglia; half were ischemic, and half were hemorrhagic. Twenty percent were undetectable in CAT scans. The second study, by Redondo [14], reports a case of contralateral ischemic hemichorea caused by an embolism in the putamen that originated in a thrombosed intracavernous aneurysm in the carotid artery.
In Latin America, the study by Pareés [15] has the most extensive series of patients (conducted between 2004 and 2009). The hemichorea patients (15 patients) were 0.2% of the total stroke patients in the studied period. Average age was 73 years. Twelve patients (80%) had an ischemic stroke, and three patients (20%) a hemorrhagic stroke. Cerebral lesions were located in different areas; the most frequent location was the lenticular nucleus, followed by those in the cortex, thalamic, and subthalamic. Symptoms resolved rapidly with no pharmacologic treatment in eight patients (53%). The drug most frequently prescribed was haloperidol. Follow up was carried out for a mean period of 17 months. In the follow up period, hemichorea disappeared in eleven patients (73%), mostly before the second month from the onset of symptoms.
As for treatment, since neuroimaging was delayed for more than 48 hours, fibrinolysis with tissue plasminogen activator (tPA) was not an available option. This treatment has been reported to resolve symptoms and leave no complications.
The patient was initially treated with valproate, which showed a slight improvement on involuntary movements. However, given the patient’s alcohol consumption, valproate was discontinued and the patient was released on haloperidol (to treat the acute phase of chorea), enalapril, nifedipin, acetylsalicylic acid, and statins (to treat his underlying pathology) as recommended by studies about regression of atheromatous plaques [17]. In addition, he was put on physical therapy, instructed on lifestyle changes, and scheduled for follow up in three months.
Other case series and reviews report success in resolution of acute-phase choreiform movements with haloperidol as a first-line drug. Cases resistant to haloperidol can be managed with atypical antipsychotic drugs such as olanzapine or use clonazepam as an alternative [18] in patients with ischemic lesions located in basal ganglia. Other studies report the success of thalamotomy for refractory cases.
The prognosis of hemichorea can be benign, but its long-term prognosis is not specifically determined by the hemichorea but by the cerebral vascular stroke and underlying atherosclerosis.
The prognosis for our patient, based on the reviews presented, is of a high probability of complete remission of his symptoms approximately within a year if he agrees to change his lifestyle and address his underlying pathology. In the already mentioned Pareés series [15], follow up for 17 months showed complete remission in 73% of his patients. The other series that included follow up reported a complete remission in 96.43% of cases. All patients received only neuroleptic drugs on the acute phase of the disorder [11],[12],[13],[14],[15].
Vascular hemichorea has a low prevalence and papers addressing it are scarce. The purpose of this publication is to present available information in a brief and precise form regarding diagnosis, prognosis and treatment of this disorder.
Potential conflicts of interest
All authors have completed the ICMJE uniform disclosure form translated into Spanish by Medwave and declare no relationships or activities that could appear to have influenced the submitted work.
Ethics
The patient signed the informed consent form provided by Medwave, which may be requested from the corresponding author or journal.

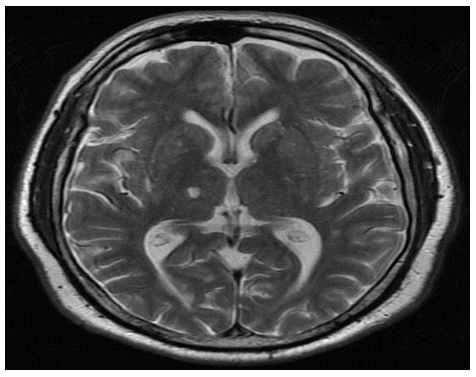 Figure 1. Cerebral MRI axial cut. Fast Spin Echo (FSE) T2 enhanced.
Figure 1. Cerebral MRI axial cut. Fast Spin Echo (FSE) T2 enhanced.

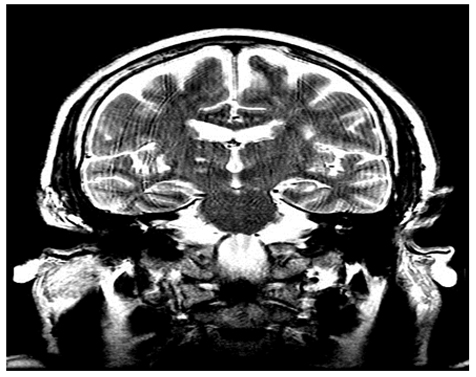 Figure 2. Cerebral MRI frontal cut. Fast Spin Echo (FSE) T2 enhanced.
Figure 2. Cerebral MRI frontal cut. Fast Spin Echo (FSE) T2 enhanced.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Chorea rarely complicates ischemic or hemorrhagic cerebral vascular lesions. Clinical symptoms usually involve one side of the body while the injury is situated on the contralateral cerebral hemisphere. Spontaneous remission is the norm, but sometimes symptomatic treatment is required. A 58-year-old male patient who suffers from untreated high blood pressure, type II obesity, smokes 6 packs of cigarettes per year and has a moderate intake of alcohol is presented. The patient’s recent history began three days before he appeared at the Emergency Department. His symptoms were ceaseless, involuntary movements in his left arm and foot during day and night with no restriction of voluntary movements. Physical examination and laboratory tests revealed no other findings. Magnetic resonance imaging of the brain showed hyperintensity in the right posterolateral thalamic region consistent with ischemic cerebrovascular disease. Symptomatic therapy was indicated and his underlying conditions were addressed. The importance of this case lies on the low prevalence as well as the scarcity of publications regarding vascular causes of hemichorea, including diagnosis, therapy and prognosis.
 Autores:
Bárbara Martínez Alfonzo[1,2], Andrés Enrique Blanco[1,2], Jairo Rojano[1], José Luis Calleja[3,4]
Autores:
Bárbara Martínez Alfonzo[1,2], Andrés Enrique Blanco[1,2], Jairo Rojano[1], José Luis Calleja[3,4]

Citación: Martínez Alfonzo B, Blanco AE, Rojano J, Calleja JL. Vascular hemichorea: case report and review. Medwave 2014;14(3):e5936 doi: 10.5867/medwave.2014.03.5936
Fecha de envío: 21/2/2014
Fecha de aceptación: 27/3/2014
Fecha de publicación: 9/4/2014
Origen: no solicitado
Tipo de revisión: con revisión por tres pares revisores externos, a doble ciego

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Borges J, De Bastos M, Cotúa M. Situaciones clínicas en neurología. Caracas, Venezuela: CDCH, 2007:182-183.
Borges J, De Bastos M, Cotúa M. Situaciones clínicas en neurología. Caracas, Venezuela: CDCH, 2007:182-183.  Sáez de Ocariz MM, Nader JA, Santos JA, Bautista M. Thalamic vascular lesions. Risk factors and clinical course for infarcts and hemorrhages. Stroke. 1996;27(9):1530-6. | CrossRef | PubMed |
Sáez de Ocariz MM, Nader JA, Santos JA, Bautista M. Thalamic vascular lesions. Risk factors and clinical course for infarcts and hemorrhages. Stroke. 1996;27(9):1530-6. | CrossRef | PubMed | Puelles L. Martinez P. Neuroanatomia. Buenos Aires, Argentina: Editorial Médica Panamericana, 2008:310-325.
Puelles L. Martinez P. Neuroanatomia. Buenos Aires, Argentina: Editorial Médica Panamericana, 2008:310-325.  Michelli F, Fernandez M. Tratado de neurología clínica. Buenos Aires, Argentina: Editorial Médica Panamericana, 2010:489-520.
Michelli F, Fernandez M. Tratado de neurología clínica. Buenos Aires, Argentina: Editorial Médica Panamericana, 2010:489-520.  Manjila S, Masri T, Shams T, Chowdhry SA, Sila C, Selman WR. Evidence-based review of primary and secondary ischemic stroke prevention in adults: a neurosurgical perspective. Neurosurg Focus. 2011;30(6):E1. | CrossRef | PubMed |
Manjila S, Masri T, Shams T, Chowdhry SA, Sila C, Selman WR. Evidence-based review of primary and secondary ischemic stroke prevention in adults: a neurosurgical perspective. Neurosurg Focus. 2011;30(6):E1. | CrossRef | PubMed | Hansford BG, Albert D, Yang E. Classic neuroimaging findings of nonketotic hyperglycemia on computed tomography and magnetic resonance imaging with absence of typical movement disorder symptoms (hemichorea-hemiballism). J Radiol Case Rep. 2013;7(8):1-9. | CrossRef | PubMed |
Hansford BG, Albert D, Yang E. Classic neuroimaging findings of nonketotic hyperglycemia on computed tomography and magnetic resonance imaging with absence of typical movement disorder symptoms (hemichorea-hemiballism). J Radiol Case Rep. 2013;7(8):1-9. | CrossRef | PubMed | Chung SJ, Im JH, Lee MC, Kim JS. Hemichorea after stroke: clinical-radiological correlation. J Neurol. 2004;251(6):725-9. | CrossRef | PubMed |
Chung SJ, Im JH, Lee MC, Kim JS. Hemichorea after stroke: clinical-radiological correlation. J Neurol. 2004;251(6):725-9. | CrossRef | PubMed | Carrion DM, Carrion AF. Non-ketotic hyperglycaemia hemichorea-hemiballismus and acute ischaemic stroke. BMJ Case Rep. 2013 Mar 6;pii: bcr2012008359.
| CrossRef | PubMed |
Carrion DM, Carrion AF. Non-ketotic hyperglycaemia hemichorea-hemiballismus and acute ischaemic stroke. BMJ Case Rep. 2013 Mar 6;pii: bcr2012008359.
| CrossRef | PubMed | Pareés I, Pujadas F, Hernández-Vara J, Lorenzo-Bosquet C, Cuberas G, Munuera J, et al. Reversible hemichorea associated with extracranial carotid artery stenosis. J Neurol Sci. 2011;300(1-2):185-6. | CrossRef | PubMed |
Pareés I, Pujadas F, Hernández-Vara J, Lorenzo-Bosquet C, Cuberas G, Munuera J, et al. Reversible hemichorea associated with extracranial carotid artery stenosis. J Neurol Sci. 2011;300(1-2):185-6. | CrossRef | PubMed | Kinboshi M, Inoue M, Kojima Y, Nakagawa T, Kanda M, Shibasaki H. Elderly case of moyamoya disease presenting with hemichorea. Rinsho Shinkeigaku. 2012;52(1):25-9. | CrossRef | PubMed |
Kinboshi M, Inoue M, Kojima Y, Nakagawa T, Kanda M, Shibasaki H. Elderly case of moyamoya disease presenting with hemichorea. Rinsho Shinkeigaku. 2012;52(1):25-9. | CrossRef | PubMed | Zijlmans JC. Vascular chorea in adults and children. Handb Clin Neurol. 2011;100:261-70. | CrossRef | PubMed |
Zijlmans JC. Vascular chorea in adults and children. Handb Clin Neurol. 2011;100:261-70. | CrossRef | PubMed | Handley A, Medcalf P, Hellier K, Dutta D. Movement disorders after stroke. Age Ageing. 2009;38(3):260-6. | CrossRef | PubMed |
Handley A, Medcalf P, Hellier K, Dutta D. Movement disorders after stroke. Age Ageing. 2009;38(3):260-6. | CrossRef | PubMed | Galiano R, Juni J, Castillo A, Parra J, Peiró C, Sancho J. Vascular. hemichorea: clinical-radiological correlation. Rev Neurol. 2000;30(5):409-11. | PubMed |
Galiano R, Juni J, Castillo A, Parra J, Peiró C, Sancho J. Vascular. hemichorea: clinical-radiological correlation. Rev Neurol. 2000;30(5):409-11. | PubMed | Redondo L, Chacón J, Valencia J, Viñuelas F, Pérez Alonso JL, García Flores C. Symptomatic chronic hemichorea of a vascular lesion in the contralateral putamen. Rev Neurol. 1996;24(127):303-5. | PubMed |
Redondo L, Chacón J, Valencia J, Viñuelas F, Pérez Alonso JL, García Flores C. Symptomatic chronic hemichorea of a vascular lesion in the contralateral putamen. Rev Neurol. 1996;24(127):303-5. | PubMed | Pareés I, Hernández-Vara J, Álvarez-Sabín J. Post-stroke hemichorea: observation-based study of 15 cases. Rev Neurol. 2010;51:460-464. | PubMed | Link |
Pareés I, Hernández-Vara J, Álvarez-Sabín J. Post-stroke hemichorea: observation-based study of 15 cases. Rev Neurol. 2010;51:460-464. | PubMed | Link | McCollum D, Silvers S, Dawson SB, Barrett KM. Resolution of acute onset hemichorea-hemiballismus after treatment with intravenous tissue plasminogen activator. Neurohospitalist. 2013;3(3):131-4. | CrossRef | PubMed |
McCollum D, Silvers S, Dawson SB, Barrett KM. Resolution of acute onset hemichorea-hemiballismus after treatment with intravenous tissue plasminogen activator. Neurohospitalist. 2013;3(3):131-4. | CrossRef | PubMed | Noyes AM, Thompson PD. A systematic review of the time course of atherosclerotic plaque regression. Atherosclerosis. 2014;234(1):75-84. | CrossRef | PubMed |
Noyes AM, Thompson PD. A systematic review of the time course of atherosclerotic plaque regression. Atherosclerosis. 2014;234(1):75-84. | CrossRef | PubMed | Ghika-Schmid F, Ghika J, Regli F, Bogousslavsky J. Hyperkinetic movement disorders during and after acute stroke: the Lausanne Stroke Registry. J Neurol Sci. 1997;146(2):109-16. | CrossRef | PubMed |
Ghika-Schmid F, Ghika J, Regli F, Bogousslavsky J. Hyperkinetic movement disorders during and after acute stroke: the Lausanne Stroke Registry. J Neurol Sci. 1997;146(2):109-16. | CrossRef | PubMed | Astradsson A, Schweder P, Joint C, Forrow B, Thevathasan W, Pereira EA, et al. Thalamotomy for postapoplectic hemiballistic chorea in older adults. J Am Geriatr Soc. 2010;58(11):2240-1. | CrossRef | PubMed |
Astradsson A, Schweder P, Joint C, Forrow B, Thevathasan W, Pereira EA, et al. Thalamotomy for postapoplectic hemiballistic chorea in older adults. J Am Geriatr Soc. 2010;58(11):2240-1. | CrossRef | PubMed | Terapia de espejo para mejorar la función motora en sujetos con accidente vascular cerebral: CAT
Terapia de espejo para mejorar la función motora en sujetos con accidente vascular cerebral: CAT