 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Palabras clave: acute uncomplicated diverticulitis, antibiotics, observational treatment, inpatient, Epistemonikos, GRADE
Introducción
La diverticulitis aguda es una de las complicaciones de la enfermedad diverticular. En la actualidad, ha habido un cambio de paradigma sobre el uso de antibióticos en el manejo de la diverticulitis aguda no complicada en pacientes hospitalizados, existiendo evidencia controversial al respecto.
Métodos
Se realizó una búsqueda en Epistemonikos, la mayor base de datos de revisiones sistemáticas en salud, la cual es mantenida mediante el tamizaje de múltiples fuentes de información, incluyendo MEDLINE/PubMed, EMBASE, Cochrane, entre otras. Se extrajeron los datos desde las revisiones identificadas, se analizaron los datos de los estudios primarios, que en este trabajo consideraron solo ensayos clínicos aleatorizados, se realizó un metanálisis y se preparó una tabla de resumen de los resultados utilizando el método GRADE.
Resultados y conclusiones
Se identificaron 11 revisiones sistemáticas que en conjunto incluyeron siete estudios primarios, de los cuales dos correspondieron a ensayos aleatorizados. Se concluyó que el uso de antibióticos en la diverticulitis aguda no complicada podría aumentar levemente las complicaciones y podría resultar en nula o poca diferencia en el riesgo de recurrencia y la necesidad de cirugía de urgencia. Sin embargo, la certeza de la evidencia es baja. Respecto a la estadía hospitalaria y la readmisión no se pudo evaluar un efecto claro de esta intervención, dado que la certeza de la evidencia fue evaluada como muy baja.
Acute diverticulitis is a complication of diverticular disease in which inflammation of one or more diverticula occurs. It occurs in about 5% of individuals with diverticula[1], of which around 75% of cases correspond to acute uncomplicated diverticulitis, which refers to the presence of diverticular inflammation and the absence of complications such as intestinal perforation, abscesses, obstruction, or fistulas[2]. The clinical condition is composed of abdominal pain, changes in bowel habits, abdominal distention, nausea, fever, and pain on palpation of the left lower quadrant[3].
In cases of acute uncomplicated diverticulitis, hospital management is preferred when dealing with patients with poor oral tolerance, severe pain, comorbidities, those who are elderly, immunocompromised, or who failed with outpatient treatment[2]. In these cases the standard treatment consists of administering antibiotics and dietary restriction, and symptom control[3].
In recent years, the use of antibiotics as a cornerstone of treatment has been challenged, arguing that antibiotics would not change the clinical outcomes presented by patients. Furthermore, the fact that the antibiotics can lead to adverse effects or poor tolerance by patients must also be considered[4],[5].
A search in Epistemonikos, the largest health-related systematic review database, was done by filtering multiple sources of information, including MEDLINE/PubMed, EMBASE, Cochrane, among others. For the search, the keywords were “acute diverticulitis”, “colonic diverticulitis”, “diverticular disease”, “antibiotic”, “antibacterial” and “bactericide agent”. Along with this, a search was carried out in Google Scholar to evaluate the existence of gray literature.
Data were extracted from the identified systematic reviews that answered the clinical question posed. The data from the primary studies were examined, which in this study only corresponded to randomized clinical trials since they are considered the best source of evidence, excluding observational studies.
With this information, a structured summary called FRISBEE (Friendly Summaries of Body of Evidence using Epistemonikos) was created, by following a pre-established format, including key messages, a summary of the body of evidence (presented as a matrix of evidence in Epistemonikos), a meta-analysis of all studies when possible, a summary table of results using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) method, and a section on other considerations for decision-making.
|
Main messages
|
|
What is the evidence?
|
We found 11 systematic reviews[6],[7],[8],[9],[10],[11],[12],[13],[14],[15],[16] which included seven primary studies that answer the clinical question, two of them being randomized trials[17],[18]. This table and the general summary are based on the latter trials since the observational studies did not increase the certainty of the existing evidence, nor did they provide additional relevant information. |
|
What type of patients were recruited in the studies? * |
All trials included patients with acute uncomplicated diverticulitis, clinically diagnosed using computed tomography. All patients included in the trials were older than 18 years old[17],[18]. The age range in one trial was between 48 and 64 years old[17], while in the other trial, the mean age of patients was 57 years old[18]. One trial recruited patients with a medical history of previous acute diverticulitis[18]. Patients diagnosed with or suspected of other diseases on computed tomography, such as colon cancer and inflammatory bowel disease, were excluded. |
|
What types of interventions were used in the studies? * |
In both trials, patients were managed as inpatients. One trial used the administration of intravenous antibiotics, amoxicillin plus clavulanic acid, for two days and then switched to an oral scheme for eight days. In allergic patients, ciprofloxacin was used in addition to metronidazole for ten days[17]. The other trial used the administration of intravenous antibiotics as intervention, cefuroxime or cefotaxime, in addition to metronidazole, carbapenems, or piperacillin-tazobactam. Then an oral scheme with ciprofloxacin or cefadroxil in addition to metronidazole. Antibiotic therapy lasted for at least seven days[18]. As a comparison, both trials used a symptomatic treatment without antibiotics, with intravenous fluids and supportive measures[17] or only fluids[18]. |
|
What kind of outcomes were measured? |
The outcomes measured were the following:
|
* Information on primary studies was collected from identified systematic reviews, not directly from studies unless otherwise specified.
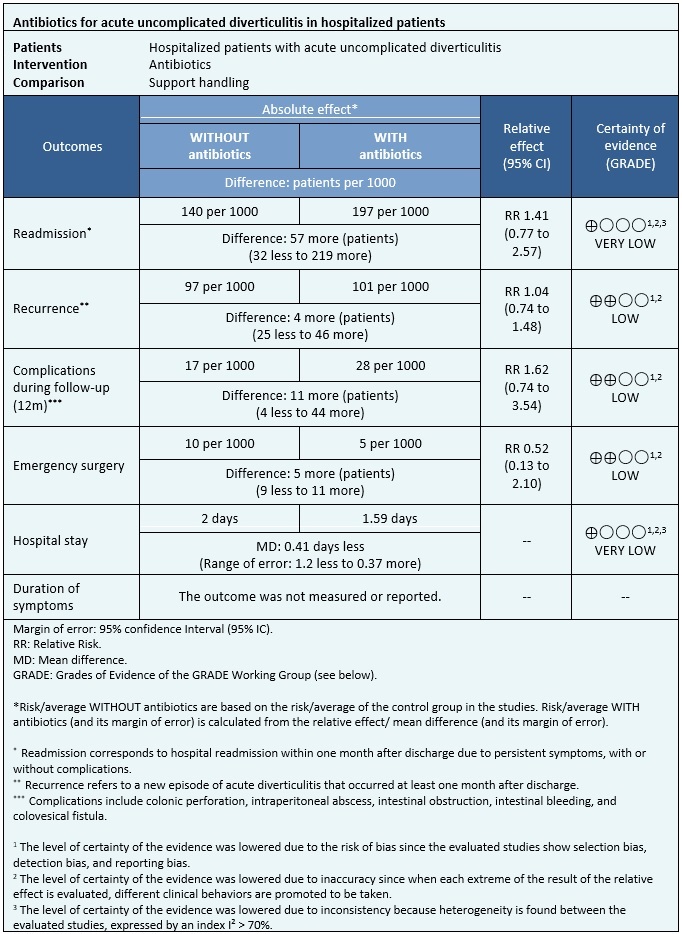
Information on the effects of antibiotic use for acute uncomplicated diverticulitis in hospitalized patients is based on two randomized trials that included 1151 patients.
In both trials, the outcomes of readmission, length of hospital stay, complications during follow-up, need for emergency surgery (1151 patients), and recurrence (1110 patients) were measured[17],[18].
The summary of the results is as follows:
|
Follow the link to access the interactive version of this table (Interactive Summary of Findings - iSoF) |
|
Whom does this evidence apply to? |
|
|
In relation to the outcomes included in this summary |
|
|
Harm/benefit balance and certainty of the evidence |
|
|
Resource considerations |
|
|
What do patients and their caregivers think? |
|
|
Differences between this summary and other sources |
|
|
Could this information change in the future? |
|
Using automated and collaborative methods, we collected all the relevant evidence for the question of interest which was presented in an evidence matrix.
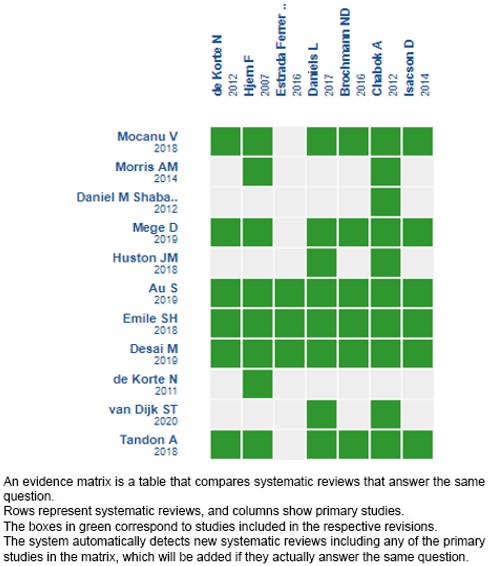
Please, follow the link to access the interactive version: Antibióticos versus manejo sintomático para el tratamiento de diverticulitis aguda no complicada en pacientes hospitalizados.
If new systematic reviews on this topic are published after the publication of this summary, a notice of “new evidence” will be displayed at the top of the matrix. Although the project contemplates the periodic updating of these summaries, users are invited to comment on the Medwave website or contact the authors by email if they believe that there is evidence that motivates an earlier update.
After creating an account on Epistemonikos, when saving the matrices, you will receive automatic notifications every time there is new evidence that may potentially answer this question.
This paper is part of the Epistemonikos Evidence Synthesis Project. It is prepared with a pre-established methodology, following rigorous methodological standards and an internal peer review process. Each of these papers corresponds to a summary, called FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the set of evidence of a specific question in a friendly format for clinical professionals. Its main resources are based on the Epistemonikos evidence matrix and the analysis of results using the GRADE methodology. Further details of the methods to make this FRISBEE are described here: (http://dx.doi.org/10.5867/medwave.2014.06.5997)
The Epistemonikos Foundation is an organization that seeks to bring information to those who make decisions in health through the use of technologies. Its main development is the Epistemonikos database.(www.epistemonikos.org).
Contribution roles
CAQ: conceptualization, methodology, software, validation, formal analysis, research, resources, data management, manuscript, manuscript revision, visualization, project administration and acquisition of funds. LTB: conceptualization, methodology, validation, formal analysis, research, resources, data management, manuscript, visualization, project administration and acquisition of funds. GGB: conceptualization, methodology, validation, formal analysis, research, resources, data management, manuscript, visualization and acquisition of funds. AZC: conceptualization, validation, manuscript revision, visualization, monitoring, project administration and acquisition of funds.
Acknowledgments
We would like to thank doctors Francisca Verdugo and Camila Avila for introducing and instructing the work team in the FRISBEE method
Competing interests
The authors declare that they have no conflicts of interest with the subject of this manuscript.
Ethical statement
Given that this research is a study on secondary sources of information, it does not need approval by an ethics committee.
Funding
School of Medicine, Universidad Finis Terrae, Santiago, Chile.
Data repository declaration
The data are available upon request and evaluation of the rationale by the authors.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Introduction
Acute diverticulitis is one of the complications of diverticular disease. Nowadays, there is a paradigm shift regarding the use of antibiotics to manage acute uncomplicated diverticulitis in hospitalized patients, with controversial information about it.
Methods
A search was done in Epistemonikos, the most comprehensive health-related systematic review database, maintained by screening multiple information sources including MEDLINE/PubMed, EMBASE, Cochrane, among others. Data were extracted from the identified systematic reviews, data from primary studies were analyzed, which in this work considered only randomized clinical trials, a meta-analysis was done, and a summary table of results was created using GRADE methodology.
Results and conclusions
Eleven systematic reviews were identified that included seven primary studies in total, of which two were randomized control trials. We concluded that the use of antibiotics in acute uncomplicated diverticulitis could slightly increase complications and result in a minor or no difference in the risk of recurrence and need for urgent surgery. However, the certainty of the evidence is low. Regarding hospital stay and readmission, it was not possible to evaluate the effect due to a low certainty of evidence.
 Authors:
Cristóbal Araya-Quezada[1], Lídice Torres-Bavestrello[1], Gustavo Gómez-Barbieri[1], Alejandro Zárate-Castillo[2]
Authors:
Cristóbal Araya-Quezada[1], Lídice Torres-Bavestrello[1], Gustavo Gómez-Barbieri[1], Alejandro Zárate-Castillo[2]

Citación: Araya-Quezada C, Torres-Bavestrello L, Gómez-Barbieri G, Zárate-Castillo A. Antibiotics for acute uncomplicated diverticulitis in hospitalized patients. Medwave 2021;21(2):e8140 doi: 10.5867/medwave.2021.02.8140
Fecha de envío: 11/9/2020
Fecha de aceptación: 1/3/2021
Fecha de publicación: 26/3/2021
Origen: Not commissioned
Tipo de revisión: Externally peer-reviewed by two reviewers, double-blind

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Shahedi K, Fuller G, Bolus R, Cohen E, Vu M, Shah R, et al. Long-term risk of acute diverticulitis among patients with incidental diverticulosis found during colonoscopy. Clin Gastroenterol Hepatol. 2013 Dec;11(12):1609-13. | CrossRef | PubMed |
Shahedi K, Fuller G, Bolus R, Cohen E, Vu M, Shah R, et al. Long-term risk of acute diverticulitis among patients with incidental diverticulosis found during colonoscopy. Clin Gastroenterol Hepatol. 2013 Dec;11(12):1609-13. | CrossRef | PubMed | Crovari F, Manzor M. Capítulo 25: Enfermedad Diverticular. en: Manual de Patología Quirúrgica. 1° ed. Chile: Ediciones UC; 2014. p. 313 – 326.
Crovari F, Manzor M. Capítulo 25: Enfermedad Diverticular. en: Manual de Patología Quirúrgica. 1° ed. Chile: Ediciones UC; 2014. p. 313 – 326.  Siddiqui J, Zahid A, Hong J, Young CJ. Colorectal surgeon consensus with diverticulitis clinical practice guidelines. World J Gastrointest Surg. 2017 Nov 27;9(11):224-232. | CrossRef | PubMed |
Siddiqui J, Zahid A, Hong J, Young CJ. Colorectal surgeon consensus with diverticulitis clinical practice guidelines. World J Gastrointest Surg. 2017 Nov 27;9(11):224-232. | CrossRef | PubMed | Hall J, Hardiman K, Lee S, Lightner A, Stocchi L, Paquette IM, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Treatment of Left-Sided Colonic Diverticulitis. Dis Colon Rectum. 2020 Jun;63(6):728-747. | CrossRef | PubMed |
Hall J, Hardiman K, Lee S, Lightner A, Stocchi L, Paquette IM, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Treatment of Left-Sided Colonic Diverticulitis. Dis Colon Rectum. 2020 Jun;63(6):728-747. | CrossRef | PubMed | Schultz JK, Azhar N, Binda GA, Barbara G, Biondo S, Boermeester MA, et al. European Society of Coloproctology: guidelines for the management of diverticular disease of the colon. Colorectal Dis. 2020 Sep;22 Suppl 2:5-28. | CrossRef | PubMed |
Schultz JK, Azhar N, Binda GA, Barbara G, Biondo S, Boermeester MA, et al. European Society of Coloproctology: guidelines for the management of diverticular disease of the colon. Colorectal Dis. 2020 Sep;22 Suppl 2:5-28. | CrossRef | PubMed | van Dijk ST, Chabok A, Dijkgraaf MG, Boermeester MA, Smedh K. Observational versus antibiotic treatment for uncomplicated diverticulitis: an individual-patient data meta-analysis. Br J Surg. 2020 Jul;107(8):1062-1069. | CrossRef | PubMed |
van Dijk ST, Chabok A, Dijkgraaf MG, Boermeester MA, Smedh K. Observational versus antibiotic treatment for uncomplicated diverticulitis: an individual-patient data meta-analysis. Br J Surg. 2020 Jul;107(8):1062-1069. | CrossRef | PubMed | Mege D, Yeo H. Meta-analyses of Current Strategies to Treat Uncomplicated Diverticulitis. Dis Colon Rectum. 2019 Mar;62(3):371-378. | CrossRef | PubMed |
Mege D, Yeo H. Meta-analyses of Current Strategies to Treat Uncomplicated Diverticulitis. Dis Colon Rectum. 2019 Mar;62(3):371-378. | CrossRef | PubMed | Au S, Aly EH. Treatment of Uncomplicated Acute Diverticulitis Without Antibiotics: A Systematic Review and Meta-analysis. Dis Colon Rectum. 2019 Dec;62(12):1533-1547. | CrossRef | PubMed |
Au S, Aly EH. Treatment of Uncomplicated Acute Diverticulitis Without Antibiotics: A Systematic Review and Meta-analysis. Dis Colon Rectum. 2019 Dec;62(12):1533-1547. | CrossRef | PubMed | Desai M, Fathallah J, Nutalapati V, Saligram S. Antibiotics Versus No Antibiotics for Acute Uncomplicated Diverticulitis: A Systematic Review and Meta-analysis. Dis Colon Rectum. 2019 Aug;62(8):1005-1012. | CrossRef | PubMed |
Desai M, Fathallah J, Nutalapati V, Saligram S. Antibiotics Versus No Antibiotics for Acute Uncomplicated Diverticulitis: A Systematic Review and Meta-analysis. Dis Colon Rectum. 2019 Aug;62(8):1005-1012. | CrossRef | PubMed | Mocanu V, Dang JT, Switzer N, Tavakoli I, Tian C, de Gara C, et al. The role of antibiotics in acute uncomplicated diverticulitis: A systematic review and meta-analysis. Am J Surg. 2018 Sep;216(3):604-609. | CrossRef | PubMed |
Mocanu V, Dang JT, Switzer N, Tavakoli I, Tian C, de Gara C, et al. The role of antibiotics in acute uncomplicated diverticulitis: A systematic review and meta-analysis. Am J Surg. 2018 Sep;216(3):604-609. | CrossRef | PubMed | Huston JM, Zuckerbraun BS, Moore LJ, Sanders JM, Duane TM. Antibiotics versus No Antibiotics for the Treatment of Acute Uncomplicated Diverticulitis: Review of the Evidence and Future Directions. Surg Infect (Larchmt). 2018 Oct;19(7):648-654. | CrossRef | PubMed |
Huston JM, Zuckerbraun BS, Moore LJ, Sanders JM, Duane TM. Antibiotics versus No Antibiotics for the Treatment of Acute Uncomplicated Diverticulitis: Review of the Evidence and Future Directions. Surg Infect (Larchmt). 2018 Oct;19(7):648-654. | CrossRef | PubMed | Emile SH, Elfeki H, Sakr A, Shalaby M. Management of acute uncomplicated diverticulitis without antibiotics: a systematic review, meta-analysis, and meta-regression of predictors of treatment failure. Tech Coloproctol. 2018 Jul;22(7):499-509. | CrossRef | PubMed |
Emile SH, Elfeki H, Sakr A, Shalaby M. Management of acute uncomplicated diverticulitis without antibiotics: a systematic review, meta-analysis, and meta-regression of predictors of treatment failure. Tech Coloproctol. 2018 Jul;22(7):499-509. | CrossRef | PubMed | Tandon A, Fretwell VL, Nunes QM, Rooney PS. Antibiotics versus no antibiotics in the treatment of acute uncomplicated diverticulitis - a systematic review and meta-analysis. Colorectal Dis. 2018 Jan 11. | CrossRef | PubMed |
Tandon A, Fretwell VL, Nunes QM, Rooney PS. Antibiotics versus no antibiotics in the treatment of acute uncomplicated diverticulitis - a systematic review and meta-analysis. Colorectal Dis. 2018 Jan 11. | CrossRef | PubMed | Morris AM, Regenbogen SE, Hardiman KM, Hendren S. Sigmoid diverticulitis: a systematic review. JAMA. 2014 Jan 15;311(3):287-97. | CrossRef | PubMed |
Morris AM, Regenbogen SE, Hardiman KM, Hendren S. Sigmoid diverticulitis: a systematic review. JAMA. 2014 Jan 15;311(3):287-97. | CrossRef | PubMed | Shabanzadeh DM, Wille-Jørgensen P. Antibiotics for uncomplicated diverticulitis. Cochrane Database Syst Rev. 2012 Nov 14;11:CD009092. | CrossRef | PubMed |
Shabanzadeh DM, Wille-Jørgensen P. Antibiotics for uncomplicated diverticulitis. Cochrane Database Syst Rev. 2012 Nov 14;11:CD009092. | CrossRef | PubMed | de Korte N, Unlü C, Boermeester MA, Cuesta MA, Vrouenreats BC, Stockmann HB. Use of antibiotics in uncomplicated diverticulitis. Br J Surg. 2011 Jun;98(6):761-7. | CrossRef | PubMed |
de Korte N, Unlü C, Boermeester MA, Cuesta MA, Vrouenreats BC, Stockmann HB. Use of antibiotics in uncomplicated diverticulitis. Br J Surg. 2011 Jun;98(6):761-7. | CrossRef | PubMed | Daniels L, Ünlü Ç, de Korte N, van Dieren S, Stockmann HB, Vrouenraets BC, et al. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017 Jan;104(1):52-61. | CrossRef | PubMed |
Daniels L, Ünlü Ç, de Korte N, van Dieren S, Stockmann HB, Vrouenraets BC, et al. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017 Jan;104(1):52-61. | CrossRef | PubMed | Chabok A, Påhlman L, Hjern F, Haapaniemi S, Smedh K; AVOD Study Group. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012 Apr;99(4):532-9. | CrossRef | PubMed |
Chabok A, Påhlman L, Hjern F, Haapaniemi S, Smedh K; AVOD Study Group. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012 Apr;99(4):532-9. | CrossRef | PubMed | National Institute for Health and Core Excellence (NICE). Diverticular disease: diagnosis and management. 2019. [On line]. | Link |
National Institute for Health and Core Excellence (NICE). Diverticular disease: diagnosis and management. 2019. [On line]. | Link | Sartelli M, Weber DG, Kluger Y, Ansaloni L, Coccolini F, Abu-Zidan F, et al. 2020 update of the WSES guidelines for the management of acute colonic diverticulitis in the emergency setting. World J Emerg Surg. 2020 May 7;15(1):32. | CrossRef | PubMed |
Sartelli M, Weber DG, Kluger Y, Ansaloni L, Coccolini F, Abu-Zidan F, et al. 2020 update of the WSES guidelines for the management of acute colonic diverticulitis in the emergency setting. World J Emerg Surg. 2020 May 7;15(1):32. | CrossRef | PubMed | Jaung R, Nisbet S, Gosselink MP, Di Re A, Keane C, Lin A, et al. Antibiotics Do Not Reduce Length of Hospital Stay for Uncomplicated Diverticulitis in a Pragmatic Double-Blind Randomized Trial. Clin Gastroenterol Hepatol. 2021 Mar;19(3):503-510.e1. | CrossRef | PubMed |
Jaung R, Nisbet S, Gosselink MP, Di Re A, Keane C, Lin A, et al. Antibiotics Do Not Reduce Length of Hospital Stay for Uncomplicated Diverticulitis in a Pragmatic Double-Blind Randomized Trial. Clin Gastroenterol Hepatol. 2021 Mar;19(3):503-510.e1. | CrossRef | PubMed | Garnfinkle R, Salama E. Non-Antibiotic Therapy for Acute Uncomplicated Diverticulitis: A Non-Inferiority Meta-Analysis Based on Delphi Consensus. PROSPERO 2020 CRD42020167804. [On line]. | Link |
Garnfinkle R, Salama E. Non-Antibiotic Therapy for Acute Uncomplicated Diverticulitis: A Non-Inferiority Meta-Analysis Based on Delphi Consensus. PROSPERO 2020 CRD42020167804. [On line]. | Link | Au S. The role of antibiotics in mild uncomplicated acute diverticulitis: a systematic review. PROSPERO 2018 CRD42018086228. [On line]. | Link |
Au S. The role of antibiotics in mild uncomplicated acute diverticulitis: a systematic review. PROSPERO 2018 CRD42018086228. [On line]. | Link |