 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Palabras clave: SARS-CoV-2, COVID-19, genetic variants, COVID-19 vaccines, COVID-19 treatment, COVID-19 diagnosis
Coronavirus disease 2019 (COVID-19), caused by the SARS-CoV-2 virus discovered in December 2019 in Wuhan, China, has had an enormous impact on public health worldwide due to its rapid spread and pandemic behavior, challenges in its control and mitigation, and few therapeutic alternatives. In this review, we summarize the pathophysiological mechanisms, clinical presentation, and diagnostic techniques. In addition, the main lineages and the different strategies for disease prevention are reviewed, with emphasis on the development of vaccines and their different platforms. Finally, some of the currently available therapeutic strategies are summarized. Throughout the article, we point out the current knowns and unknowns at the time of writing this article.
|
Main messages
|
On December 31, 2019, the World Health Organization (WHO) reported a series of pneumonia cases caused by an unknown agent in Wuhan City, Hubei Province, China. In January 2020, the cause of this infection was reported to be a novel coronavirus, initially named 2019-nCoV (novel coronavirus 2019). WHO subsequently named SARS-CoV-2 and the disease caused by it COVID-19 (coronavirus disease 2019). The infection spread rapidly through China and its neighboring countries, spreading throughout the world in a few weeks, probably facilitated by inter-city travel and tourist arrivals in the context of the Lunar New Year being celebrated in China at that time. The global repercussions of the epidemic were quickly evident, which is why on January 30, 2020, WHO declared COVID-19 a public health emergency of international concern and in March of the same year declared it a pandemic[1],[2],[3],[4].
Since the publication of the first cases reported in Wuhan, China, in December 2019[5], the disease caused by SARS-CoV-2 (COVID-19) has resulted in more than 138 million cases and 3 million deaths worldwide to date[6], and is one of the most devastating pandemics of recent times.
This article will review the main epidemiological and clinical features of the disease caused by SARS-CoV-2 and some virological, diagnostic, and therapeutic aspects.
Coronaviruses are members of the subfamily Coronavirinae in the family Coronaviridae and the order Nidovirales. This subfamily consists of four genera: alphacoronavirus, betacoronavirus, gammacoronavirus, and deltacoronavirus, based on their phylogenetic relationships and genomic structures. Alphacoronaviruses and betacoronaviruses infect only mammals, whereas gammacoronaviruses and deltacoronaviruses infect birds, but some of them can also infect mammals. Coronaviruses are large, enveloped, single-stranded RNA viruses found in humans and other mammals, such as dogs, cats, chickens, cows, pigs, and birds, and can cause respiratory, gastrointestinal, and neurological diseases. The most common coronaviruses affecting humans are 229E, OC43, NL63, and HKU1, which usually cause common cold symptoms in immunocompetent individuals. In 2002, an outbreak of a new coronavirus was described in the Guandong province of China called SARS (Severe Acute Respiratory Syndrome). Ten years later, in 2012, another highly pathogenic coronavirus, MERS-CoV (Middle East Respiratory Syndrome Coronavirus), affected several countries in the Middle East. We are currently experiencing a third coronavirus outbreak, this time due to SARS-CoV-2, a virus that shares 79% of the genomic sequence with SARS and 50% with MERS, and with pandemic behavior. SARS was transmitted to humans from civets, MERS from dromedary camels, and SARS-CoV-2 from pangolins. All three viruses are thought to have originated in bats[7],[8],[9]. Domestic animals may play important roles as intermediate hosts that allow transmission of the virus from natural hosts to humans, supporting the theory that the virus originated in the Wuhan market. However, this has been disputed given the finding in France of SARS-CoV-2 by PCR in a stored sample from a patient who had pneumonia in late 2019, suggesting that the virus may have been circulating earlier than currently believed[10].
SARS-CoV-2 has a diameter of 60 nm to 140 nm and distinctive spikes, ranging from 9 nm to 12 nm, giving the virions the appearance of a solar corona. Coronaviruses can adapt and infect new hosts through recombination and genetic variation[8], which has led to the emergence of several variants of worldwide interest, the most recognized to date being B.1.1.7 (UK), B.1.351 (South Africa), B.1.1.28 (Brazil), B.1.427/B.1.429 (USA) and, more recently, B.1.617 (India)[11],[12].
SARS coronaviruses use angiotensin-converting enzyme 2 (ACE2) as a receptor and invade mainly bronchial ciliated epithelial cells and type II pneumocytes. On the other hand, MERS-CoV uses dipeptidyl peptidase-4 (DPP4) as a receptor and invades non-ciliated bronchial epithelium and type II pneumocytes[9].
Once the virus comes into contact with the respiratory mucosa, the SARS-CoV-2 spike glycoprotein (S) binds to surface receptors on the host cells and mediates viral entry by interacting with the angiotensin-converting enzyme 2 receptors (ACE2), resulting in disease transmission and pathogenesis. The transmembrane protease serine 2 (TMPRSS2) in the host cell further promotes viral uptake by cleaving ACE2 and activating the SARS-CoV-2 S protein. Upon binding to respiratory tract epithelial cells, SARS-CoV-2 begins to replicate and migrate into the airways and enters the alveolar epithelial cells of the lungs. Rapid replication of SARS-CoV-2 in the lungs can trigger a strong immune response, mediated by inflammatory signaling molecules, T lymphocytes, monocytes and neutrophils released from infected cells and alveolar macrophages. Cytokine storm syndrome causes acute respiratory distress syndrome and respiratory failure, which is considered the leading cause of death in patients with COVID-19[7],[8],[13],[14].
| What we know | What we don't know |
|
|
The disease caused by SARS-CoV-2, COVID-19, is characterized mainly by respiratory involvement with various signs and symptoms that have been described as the pandemic unfolded.
The viral incubation period varies between 2 to 14 days, with an average of 4 days[15],[16], while viral shedding varies by clinical severity. It has been shown that in mild to moderate cases, the highest risk of transmission or contagion begins two days before the onset of symptoms until approximately five days after the onset of symptoms. However, viable viruses have been found in cell cultures up to 7 to 10 days after the onset of symptoms[17]. This period can last up to 20 days in severe cases and immunocompromised patients[18]. It is important to note that the finding of viral RNA by polymerase chain reaction (PCR) does not equate to a viable virus with infecting capacity being present and can be found in significant quantities up to several months after illness[18].
The most frequently reported symptoms are cough, myalgia, headache, and fever, but dyspnea, odynophagia, diarrhea, nausea and vomiting, anosmia, ageusia, nasal congestion, fatigue, and chest pain have also been reported[15],[19],[20],[21].
It is estimated that one-third of SARS-CoV-2 infections are asymptomatic[22], although this is difficult to determine since a percentage of patients considered asymptomatic at the time of confirming the diagnosis with a positive PCR test may present symptoms during the following days. This group of patients may have other manifestations of the disease, such as laboratory alterations or abnormalities on chest CT scan[23]. These individuals can infect others and may not isolate due to the lack of symptoms that would warn of the disease in time[24],[25].
The disease can have a mild (80%), severe (15%), or critical (5%) course, with an estimated case fatality rate of 2.3% in the first reports[26]. Mild disease corresponds to the absence of pneumonia or mild pneumonia; severe disease corresponds to dyspnea, hypoxia, or involvement of more than 50% of the lung parenchyma on chest imaging; and critical disease is characterized by respiratory failure, shock, or multiorgan dysfunction[26],[27].
The recovery time of the disease is variable, depending on various factors such as the severity of the condition, age, and comorbidities. Most mild cases recover within the first two weeks of illness; however, prolonged manifestations of COVID-19 of variable duration averaging three months have been described. This form of presentation is most frequently seen in people who have had a severe or critical illness, and the most frequent clinical manifestations are fatigue (53%), dyspnea (43%), joint pain (27%), and chest pain (22%)[28],[29]. Neuropsychiatric symptoms such as anxiety disorder, dementia, and insomnia have also been described[30]. It is estimated that over 80% of patients maintain at least one symptom 60 days after overcoming acute infection[28].
Although severe cases can occur at any age, according to published data[26],[31],[32],[33],[34],[35],[36],[37],[38], older age, male sex, obesity, cardiovascular morbidities such as hypertension, diabetes, dyslipidemia, and coronary heart disease; chronic obstructive pulmonary disease and other lung conditions; chronic kidney disease, cancer, smoking, and a history of solid organ and hematopoietic precursors transplantation; have been associated with a higher risk of developing severe COVID and even higher mortality; have been associated with an increased risk of developing severe COVID and increased mortality. Another factor that has been associated with increased risk of COVID-19 complications is inequity in the social determinants of health, including socioeconomic status[39] and belonging to underrepresented racial/ethnic groups[40], among others.
As the pandemic progresses, a deeper understanding of the transmission mechanisms has been achieved, starting with the zoonosis theory from the Wuhan animal market to the current evidence supporting person-to-person transmission, which is now considered the main route of transmission of the disease[41].
The primary mode of transmission is through close contact (less than 1.5 to 2 meters distance) by inhaling air with respiratory particles from the respiratory tract of a SARS-CoV-2 infected person. The large droplets produced by talking, coughing, or sneezing can contact the respiratory mucosa of a susceptible host, invade it, and cause disease. Infection can also occur if these respiratory secretions contaminate a person’s hands (e.g., by touching contaminated surfaces) and then touching the eyes, nose, or mouth. Nonetheless, more recent evidence suggests contact with contaminated surfaces is not a significant route of transmission[41],[42]. Therefore, maintaining a physical distance of at least 1.5 meters between people, the widespread use of masks and timely and frequent handwashing have become the main measures to prevent the transmission and spread of SARS-CoV-2[43]. Another transmission route is aerosols—particles smaller than droplets that remain suspended in the air for a longer time and travel longer distances. Recently, increasing evidence has been published regarding this transmission route in the spread of the pandemic, making more evident the role of indoor ventilation as a fundamental measure to reduce the risk of transmission[43],[44],[45]. Although SARS-CoV-2 has been detected in non-respiratory samples (stool, blood, semen, urine), it has not been shown to play a significant role in viral transmission[18],[46].
As the pandemic has progressed, different variants have been identified as a result of genetic mutations, a common process inherent to viruses, which is exacerbated by the high rates of community transmission worldwide (> 100 new cases of COVID-19 per million population per day)[47]. Compared to other viruses, such as influenza and HIV, SARS-CoV-2 has a lower mutation rate, and most mutations do not impact viral function.
To date, five variants of SARS-CoV-2 have generated interest worldwide because of the potential impact on transmission dynamics and disease severity[11],[12],[48],[49]. These variants, termed “variants of concern,” have in common an amino acid substitution in the spike protein (D614G, glycine for aspartic acid), which translates into increased replication and transmission capacity, explained by a higher viral load in the upper respiratory tract and stronger affinity for the ACE2 receptor. Data are still lacking to affirm that these variants increase the risk of hospitalization by COVID-19 or affect the neutralizing capacity of anti-spike antibodies[50],[51],[52],[53], but some studies have shown that some of these variants of interest are associated with a higher rate of complications[54],[55] and lower susceptibility to the immune response generated by some vaccines[56],[57],[58] (Table 1).
Table 1. SARS-CoV-2 variants of concern.
Lineage B.1.1.7 (a.k.a. 20I/501Y.V1 VOC 202012/01): Identified in December 2020 in the United Kingdom. Retrospectively, it was found that circulation began in September 2020, so its dissemination to other countries during that period is unknown. Variant B.1.1.7 has a mutation in the receptor-binding region of protein S. Some studies suggest that this new variant is significantly more transmissible than others, with an estimated potential increase in the reproductive number of 0.4 or more and with estimated increased transmissibility of up to 90%[59]. It has also been observed to increase the mortality risk, although the research is still unfolding[54],[55]. Variant B.1.1.7 is associated with a slight decrease in neutralizing antibody titers but above the levels associated with protection, with a low risk of reinfection[60]. Regarding the impact on vaccine-generated immunity, this variant does not appear to significantly reduce the efficacy of the Sinovac, Pfizer/BioNTech, Moderna, Novavax, and AstraZeneca vaccines[59]. To date, B.1.1.7 has been identified in 137 countries according to the WHO epidemiological report of April 20, 2021[49].
Lineage B.1.351 (a.k.a. 20H/501Y.V2): identified in South Africa in August 2020 and reported in 85 countries[49]. This variant has multiple mutations in the spike protein, several of them in common with variant B.1.1.7. There is now evidence that the response to neutralizing antibodies and vaccines may be diminished compared to other variants[61],[62] because of a specific mutation in the spike protein (E484K), although the evidence is still unfolding[63],[64],[65]. It has also been described that mutations present in this variant may help the virus evade the immune response triggered by previous SARS-CoV-2 infection, with a consequent risk of reinfection. This variant presents a higher risk of transmission, with the possibility of generating a more severe clinical picture compared to the original variant[66].
Lineage B.1.1.28 (a.k.a. 20J/501Y.V3 or P.1): first identified in December 2020 in Japan in travelers’ samples from Brazil. This variant contains three mutations in the receptor-binding domain of the spike protein. So far, there is no clear evidence regarding the impact of this mutation on the transmissibility and aggressiveness of the disease. Cases of reinfection with this variant and a reduction in the neutralizing capacity of antibodies have been reported, with the possibility of presenting reinfections or, eventually, lower response to vaccines[57]. However, there is also evidence supporting the efficacy of at least one vaccine (Sinovac) in a high-circulation environment of this lineage[67],[68],[69]. According to the WHO epidemiological report, as of April 20, 2021, this variant had been reported in 52 countries[49].
Lineage B.1.627 (a.k.a. G/452R.V3): first detected in India in October 2020. It has gained special interest given the rapid increase of COVID-19 cases in India with a significant epidemiological impact in this country, even though there is no evidence regarding the involvement of this variant in transmission or morbidity and mortality associated with the infection. The limited evidence currently available has not shown a decrease in the effectiveness of vaccines against this variant. To date, the presence of this lineage has been reported in 21 countries[70].
B.1.427 and B.1.429 (a.k.a. 20C/S:452R) lineage: identified in California (USA), structurally similar to each other. They are associated with increased transmissibility and, apparently, reduced neutralization capacity[56]. These lineages have been listed as “variants of concern” by the US Centers for Disease Control and Prevention (CDC), but not by the World Health Organization[12].
In addition, other variants called “variants of interest” have been reported, with lesser but significant clinical impact than the “variants of concern.” Outstanding in this group are the B.1.526 and B.1.526.1 lineages (New York), B.1.525 (United Kingdom/Nigeria), P.2 (Brazil), among others[71].
The importance of these variants regarding transmissibility, severity, antibody neutralization capabilities, and potential impact on the effectiveness of COVID-19 vaccines is still under investigation. The detection of other mutations potentially impacting public health is also evaluated and monitored through surveillance and genetic sequencing in different countries worldwide[11],[47],[48],[49].
The possibility of reinfection with all four known human seasonal coronavirus infections, even in the presence of pre-existing antibodies, is not unusual; however, reactivated, relapsing, or latent infection appears less likely and has not been described for the coronavirus family[72]. Isolated cases of reinfection with SARS-CoV-2 have been documented in individuals with a history of previous COVID[72],[73], estimating a 0.02% risk with a 0.36 incidence rate[74]. Establishing the diagnosis of reinfection is challenging, and molecular testing alone is not of great utility due to the possibility of prolonged respiratory excretion of viral RNA after acute infection. A second positive PCR test in a patient who has recovered from COVID-19 does not necessarily indicate reinfection. Factors that increase the likelihood of reinfection include a longer time interval since the first infection, a high level of viral RNA in the repeat test, and undetectable IgG antibody at the time reinfection is considered. However, reinfection can only be confirmed by genomic sequencing to establish that the infections were caused by two different viruses[72],[73],[74].
| What we know | What we don't know |
|
|
Molecular testing
The first genetic sequencing of SARS-CoV-2 was performed in January 2020, only a few weeks after the first cases were reported, making a molecular test for diagnosis available early in the course of the pandemic[9],[75]. Detection of SARS-CoV-2 RNA based on reverse transcription-polymerase chain reaction (RT-PCR) from respiratory samples is the standard for diagnosis; however, the sensitivity of the tests varies with the timing of the test with exposure. Thus, the test’s sensitivity can range from 30% in the first four days post-exposure to 80% three days after symptom onset. Factors associated with higher sensitivity of the results include correct specimen collection technique, longer time since exposure, and the point of origin of the specimen (lower airway specimens, such as those obtained by bronchoalveolar lavage, which is more sensitive than upper airway specimens). Recently, techniques have been developed from saliva samples, which may be an alternative sample source requiring less personal protective equipment and fewer supplies. It is also possible to detect SARS-CoV 2 in stool[8].
Antigen testing
Another diagnostic test for COVID-19 is antigen testing, which can be performed rapidly and at point-of-care and, therefore, may be more accessible with a faster turnaround time to results than molecular tests, although it is less sensitive than molecular tests. Antigen testing can be useful in certain situations, provided that the possibility of false negatives is considered and results are interpreted based on pre-test probability. Frequently, the result of an antigen test must be confirmed by molecular testing (PCR)[76],[77].
Serological tests
Various serologic tests have been developed to support the diagnosis of disease and the assessment of vaccine response; however, the presence of antibodies may not confer immunity because not all antibodies produced in response to infection are neutralizing. It is not known whether the presence of antibodies changes susceptibility to subsequent infections or how long antibody protection lasts.
Different tests for anti-SARS-CoV-2 antibodies are commercially and experimentally available, using different technologies to qualitatively or quantitatively measure individual immunoglobulins (IgM, IgG, or IgA) or total antibodies (predominantly IgM, IgG, but also including other antigen-specific immunoglobulins)[78]. These serological tests detect SARS-CoV-2 antigens, specifically spike protein (S) or nucleocapsid (N). IgM antibodies are detectable within five days of infection, with the highest levels during weeks two to three of illness. In contrast, the elevation of IgG antibody titers is seen approximately 14 days after symptom onset, although, in some patients, both immunoglobulins are elevated simultaneously[78]. Secretory IgA is central to mucosal immunity, but its kinetics have not yet been elucidated, so its measurement is uncommon. Serologic assays include point-of-care assays and high-throughput enzyme immunoassays; however, test performance, accuracy, and validity are variable[8]. In general, tests that measure IgM have lower sensitivity for detecting past infection than those that detect IgG or total antibody; and those that detect IgA tend to have lower specificity[78].
Specificity is important in seroprevalence studies when the community prevalence of past infection is expected to be low. Serological tests must have high sensitivity and specificity (≥ 99.5%) to be valid[78]. Detection of IgG or total antibody at 3 to 4 weeks after symptom onset provides the highest sensitivity and, therefore, the lowest rate of false-negative results compared with other immunoglobulin classes. IgG or total antibody tests also provide high specificity and reduce the rate of false-positive results compared with other antibody types[78]. Unlike other viral infections where IgM tests show high sensitivity soon after symptom onset compared to IgG, the sensitivity of IgM against SARS-CoV-2 is relatively low initially, and there is no significant increase over time as seen with IgG or total antibodies. The use of IgM testing alone could result in increased false-negative rates compared to IgG or total antibody testing[78].
The most commonly used laboratory platforms for serological diagnosis of SARS-CoV-2 are lateral flow, enzyme-linked immunosorbent assay (ELISA), and chemiluminescence or electrochemiluminescence immunoassay (CLIA or ECLIA). Lateral flow tests require a drop of blood, serum or plasma applied to a test strip and provide rapid results (15 to 30 minutes), making it an optimal test for point-of-care diagnosis and large, population-based seroprevalence studies. ELISA and CLIA/ECLIA assays are more complex laboratory techniques than lateral flow, useful for high-throughput testing using serum, plasma, or potentially dry blood, and allow quantifying binding antibody titers and determination of neutralizing antibodies[78]. It is important to mention that different vaccines use different antigens to stimulate the immune response, so if an individual receives a spike protein-based vaccine, they will generate anti-S-protein antibodies, for example, and not necessarily against other viral antigens. In addition, there are different components of the immune response, such as the humoral response and the cellular response, and the magnitude of specific antibody production may vary between individuals[79],[80],[81],[82]. Consequently, it is not appropriate to evaluate the immune response to vaccines exclusively based on serological tests, but it is essential to determine efficacy and effectiveness using clinical outcomes[83],[84].
Implications of the humoral and cellular immune response
Multiple studies have been conducted worldwide to determine the prevalence of individuals with or without symptoms of COVID-19 and with or without a confirmatory microbiological diagnosis who show evidence of having developed antibodies to SARS-CoV-2. To date, there is no clarity regarding the relationship between severity of infection, antibody titers, and risk of reinfection among different individuals and the absence of seroconversion in some individuals nor the role of cell-mediated immunity in the immune response to COVID-19[74],[79],[81],[82],[85],[86],[87]. Neutralizing antibodies are detected in approximately 40% to 70% of infected individuals; at least 30% of patients have no detectable antibody levels, and less than 15% achieve high neutralizing titers in vitro[87]. An association between neutralizing antibody titer and severity of COVID-19 disease has been observed, and those who have mild symptoms or are asymptomatic are less prone to generate a neutralizing response[87].
Seroprevalence studies
Worldwide, reported seroprevalence varies by factors such as the population studied (e.g., health care workers vs. general population) and the impact of the pandemic in the setting where the study is conducted (the evolution of the pandemic and the incidence rate vary according to the country, the season of the year, policies implemented, among other factors). Results range from prevalences as low as 0.4% to rates of 22%[88],[89],[90]. Publicly available software tools such as the Canadian Serotracker platform[91] will be very useful to assess the evolution and impact of the pandemic worldwide.
| What we know | What we don't know |
|
|
While the management of COVID-19 is mainly based on symptomatic relief in mild to moderate cases and on ventilatory and, ultimately, hemodynamic support in severe or critical cases, the following section will describe some pharmacological interventions that have been shown to have an impact on the morbidity and mortality caused by COVID-19.
Corticosteroids
In patients with severe COVID, requiring supplemental oxygen or ventilatory support, the use of glucocorticoids, particularly dexamethasone, hydrocortisone, and methylprednisolone, has been shown to decrease 28-day mortality, as well as reduce the likelihood of requiring mechanical ventilation[92],[93],[94],[95],[96],[97],[98]. Particular caution should be taken with the adverse effects associated with the use of corticosteroids, such as hyperglycemia and bacterial or fungal superinfections[94],[99]. On the other hand, the use of inhaled budesonide has shown promising results in recent studies, accelerating recovery in early-stage cases of COVID-19 and decreasing the need for hospitalization or emergency department consultation[100],[101].
Remdesivir
The use of remdesivir—a nucleoside analog polymerase inhibitor—has been shown to decrease recovery time in adults hospitalized for COVID-19[102], as well as the need for mechanical ventilation, with no significant impact on mortality, when used in the first week of the disease[98],[99],[103],[104]. This benefit is more significant when remdesivir is associated with baricitinib[105],[106] (see below).
Interleukin-6 inhibitors
Elevation of pro-inflammatory interleukins, including IL-6, is associated with unfavorable outcomes and increased mortality in SARS-CoV-2, so their inhibition and the blockade of the inflammatory pathways may prevent disease progression[107]. Sarilumab and tocilizumab are monoclonal antibodies that neutralize the IL-6 receptor, while siltuximab is a direct inhibitor of this interleukin[108]. Some studies have shown that they could decrease the need for mechanical ventilation, the length of intensive care stay, hospitalization[99], and mortality[109],[110].
JAK inhibitors
Janus kinase 1 and 2 inhibitors are drugs that interfere with intracellular signaling of some interleukins (IL-2, 6, and 10), granulocyte and macrophage colony-stimulating factors, and interferon gamma, blocking the process of viral endocytosis by inhibiting AP2-associated protein kinase 1[105],[106]. Baricitinib and ruxolitinib have been shown to have some impact on reducing mortality, the need for mechanical ventilation, and the duration of hospitalization for COVID-19[99]. The association of remdesivir with baricitinib has been shown to reduce recovery time and accelerate clinical improvement in patients with COVID-19, especially in those with the need for high-flow oxygen or noninvasive ventilation[105],[106].
Colchicine
The use of colchicine in patients with mild to moderate disease—those who do not require hospitalization—could decrease mortality, the need for mechanical ventilation, and the duration of hospitalization for COVID-19. Nonetheless, there are ongoing studies that will provide further evidence for its usefulness in the management of this disease[98],[99],[111].
Interventions that have not consistently demonstrated usefulness in the management of COVID-19
The evidence available to date has determined that the risk of using some drugs outweighs the benefit in the treatment of COVID-19, including chloroquine, hydroxychloroquine, azithromycin, ritonavir-boosted lopinavir, favipiravir, and ivermectin[98],[99],[106],[112],[113],[114],[115],[116].
Recently, an open-label randomized study with a large sample size showed that the use of convalescent plasma has no significant impact on survival or other outcomes[117].
Empirical antibiotic treatment is not recommended in patients with COVID-19, regardless of the severity of the condition, unless there is significant evidence of bacterial infection (compatible clinical picture associated with the appearance of new infiltrates on chest imaging and positive cultures). It has been reported that more than half of patients with COVID-19 are prescribed antimicrobial therapy during the course of the disease, with bacterial infection confirmed in only 3.5% of cases[118],[119]. If antibiotic therapy is given, the indication should be re-evaluated daily, abbreviated courses of antimicrobials should be sought and adjusted to culture results[120].
| What we know | What we don't know |
|
|
Personal prevention measures
Considering that the main route of transmission of SARS-CoV-2 is through droplets and aerosols generated from respiratory secretions, the interventions most proven to prevent infection are the universal use of masks, frequent and timely handwashing, physical distancing between people, ventilation of enclosed spaces, and avoidance of non-essential gatherings indoors and in crowded outdoor spaces[121].
Vaccines
Thanks to the global interest in controlling the pandemic, different vaccine platforms have been developed rapidly. When writing this review, 93 vaccines are in clinical development and 184 in the preclinical stage, as reported by the World Health Organization; 25 are in phase 3[122].
There are different technological platforms for SARS-CoV-2 vaccines, of which the most widely used are shown in Table 2[123],[124],[125].
Live attenuated virus. The first vaccine of this type to be developed was the smallpox vaccine (1978). These vaccines are produced by generating a genetically weakened version of the virus, maintaining a limited replication capacity without producing disease, but inducing an immune response similar to that generated by natural infection. The immune response generated by this type of vaccine targets both structural and non-structural viral proteins through cellular and antibody-mediated immune responses. The disadvantage of these vaccines is safety in immunocompromised patients and the difficulty involved in modifying the virus. Examples of live virus vaccines are measles, mumps, yellow fever, and shingles. Few SARS-CoV-2 vaccines use this platform, and none of them are in phase 3 clinical trials.
Inactivated virus. The first vaccine based on an inactivated virus was typhoid in 1986. These vaccines are developed using viruses treated with chemicals (e.g., formaldehyde), heat, or radiation, thus canceling their replicative capacity but maintaining their ability to generate an immune response. When the complete virus is presented to the immune system, in addition to generating a response against the spike protein of SARS-CoV-2, it also does so against the matrix, the envelope, and the nucleoprotein of the virus. Large quantities of the virus with infectious capacity grown in cell culture are required to produce this type of vaccine. Examples are hepatitis A, polio, influenza, pertussis, and rabies. Among the vaccines against COVID-19 that use this platform are Sinovac and Sinopharm.
DNA/RNA-based. This is a platform developed in recent years, and the SARS-CoV-2 vaccine is the first of this type to be used in humans outside the experimental setting. These vaccines consist of DNA or RNA fragments that code for a target antigen, which in the case of SARS-CoV-2 is often the spike (S) protein, allowing the vaccine recipient to express these antigens and then induce a humoral and cellular immune response against them. An advantage of these vaccines is the ease and speed of large-scale production; however, their disadvantage is the need to store and maintain them at low temperatures (between -20 and -70ºC), because of the significant logistical problem for their distribution, especially to remote and difficult-to-access locations. The most widely used COVID-19 vaccines worldwide with this platform are those of the Pfizer and Moderna laboratories.
Protein subunit-based vaccines. The first vaccine of this type to be used was the anthrax vaccine in 1970. They are based on purified virus particles or protein antigens. Research is being conducted on vaccines with the spike (S) protein and the RBD (receptor binding domain) protein. An advantage of these vaccines is that they can be produced without manipulating the live virus. Examples of this type of vaccine are hepatitis B, pneumococcus, and meningococcus, among others. It is currently the most widely used platform in clinical studies (31%)[122].
Vector vaccines. This is a relatively new technology, first used on a large scale for the Ebola virus in 2019. Vector vaccines use a genetically modified virus capable of producing proteins that induce an immune response in the vaccine recipient, such as the spike (S) protein of SARS-CoV-2. To prevent the recipient’s immune system from responding against this viral vector before the proteins needed to mount the immune response against the disease to be prevented are produced, viruses that do not affect humans, such as the chimpanzee adenovirus used in the production of the AstraZeneca laboratory’s vaccine, can be used. Other vaccines using this type of platform include those from CanSino, Janssen, and Gamaleya.
Over 600 million people worldwide have received at least one dose of the COVID-19 vaccine[126]. The duration of immunity generated by the vaccines and whether there are significant differences between the different platforms is yet unknown. We still do not know to what extent the genetic variants of the SARS-CoV-2 virus will affect the secondary immune response to infection or vaccination, nor how the genetic and environmental aspects specific to each patient will determine the immune response.
Data on the real-world effectiveness of some of the vaccines being used are now increasingly available. In Israel, the Pfizer/BioNTech vaccine showed significant concordance with published efficacy in the phase 3 study with very high effectiveness despite the prevalence of the B.1.1.7 (UK) variant at an estimated 94.5% (Table 2)[127]. An unpublished study conducted by the Ministry of Health of Chile showed that 14 days after the second dose, the CoronaVac vaccine from the Sinovac laboratory was 67% effective in preventing symptomatic COVID-19, 85% in preventing hospitalizations, 89% in preventing admission to the intensive care unit and 80% in preventing death from COVID[69]. Another study conducted by the CDC in the United States showed that the Pfizer and Moderna vaccines reduced the risk of infection by 90% two or more weeks after administering the second dose and by 80% after the first dose[128].
| What we know | What we don't know |
|
|
SARS-CoV-2 and the disease it produces, COVID-19, has generated an unprecedented global health impact and is likely to be a global public health problem for a long time to come. Different questions have been raised regarding the evolution of the pandemic as part of the world’s population is undergoing vaccination, there is no clarity on the duration of the immunity generated by both vaccines and natural infection, nor if collective immunity will be effectively achieved by reaching a significant coverage of the population or if it will be necessary to vaccinate again after a while as occurs with other respiratory viruses such as influenza. Likewise, there is little clarity regarding the impact of the different genetic variants on the post-infection immune response and the possibility of reinfection. Nor do we know what the level of the immune response to the different COVID vaccine platforms that have been developed will be in the long run. Different diagnostic and therapeutic strategies have been rapidly developed during this little more than a year of pandemic, while the scientific, academic, and policy worlds continue striving to increase the knowledge and technological developments necessary to combat this infection.
Contributor roles
VCB conceived the idea for the article. ISA wrote the first extended version, and VCB critically reviewed the manuscript and contributed to its final version.
Competing interests
The authors declare that they have no competing interests with the subject matter of this article.
Funding
There was no funding for this work.
Ethics
This article uses secondary sources. No data were collected from individuals or patients, and therefore no ethics committee approval was required.

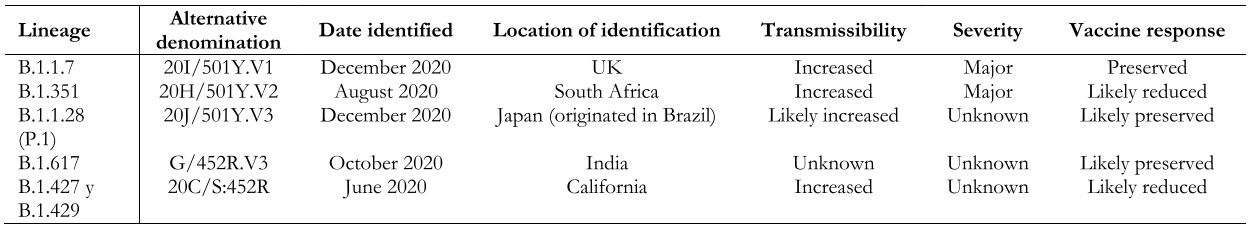 Table 1. SARS-CoV-2 variants of concern.
Table 1. SARS-CoV-2 variants of concern.

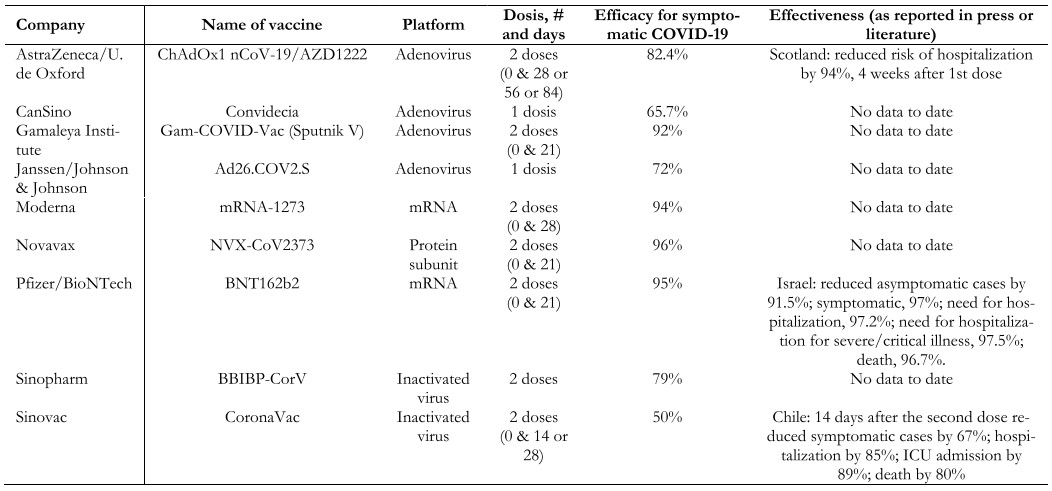 Table 2. SARS-CoV-2 vaccines.
Table 2. SARS-CoV-2 vaccines.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Coronavirus disease 2019 (COVID-19), caused by the SARS-CoV-2 virus discovered in December 2019 in Wuhan, China, has had an enormous impact on public health worldwide due to its rapid spread and pandemic behavior, challenges in its control and mitigation, and few therapeutic alternatives. In this review, we summarize the pathophysiological mechanisms, clinical presentation, and diagnostic techniques. In addition, the main lineages and the different strategies for disease prevention are reviewed, with emphasis on the development of vaccines and their different platforms. Finally, some of the currently available therapeutic strategies are summarized. Throughout the article, we point out the current knowns and unknowns at the time of writing this article.
 Autores:
Ignacio Silva-Ayarza[1,2], Vivienne C Bachelet[1]
Autores:
Ignacio Silva-Ayarza[1,2], Vivienne C Bachelet[1]

Citación: Silva-Ayarza I, Bachelet VC. What we know and don’t know on SARS-CoV-2 and COVID-19. Medwave 2021;21(4):e8198 doi: 10.5867/medwave.2021.04.8198
Fecha de envío: 18/5/2021
Fecha de aceptación: 18/5/2021
Fecha de publicación: 25/5/2021
Origen: Solicitado
Tipo de revisión: Sin revisión por pares externa

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 World Health Organization. Novel Coronavirus (2019-nCoV): Situation report - 1. Geneva: WHO; 2020. [On line]. | Link |
World Health Organization. Novel Coronavirus (2019-nCoV): Situation report - 1. Geneva: WHO; 2020. [On line]. | Link | World Health Organization. Novel Coronavirus(2019-nCoV): Situation Report - 11. Geneva: WHO; 2020. [On line]. | Link |
World Health Organization. Novel Coronavirus(2019-nCoV): Situation Report - 11. Geneva: WHO; 2020. [On line]. | Link | World Health Organization. Coronavirus disease 2019 (COVID-19): Situation Report – 51. Geneva: WHO; 2020. [On line]. | Link |
World Health Organization. Coronavirus disease 2019 (COVID-19): Situation Report – 51. Geneva: WHO; 2020. [On line]. | Link | World Health Organization. WHO Director-General’s remarks at the media briefing on 2019-nCoV on 11 February 2020. Geneva: WHO; 2020. [On line]. | Link |
World Health Organization. WHO Director-General’s remarks at the media briefing on 2019-nCoV on 11 February 2020. Geneva: WHO; 2020. [On line]. | Link | Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020 Feb 20;382(8):727-733. | CrossRef | PubMed |
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020 Feb 20;382(8):727-733. | CrossRef | PubMed | World Health Organization. Coronavirus disease (COVID-19) pandemic. Geneva: WHO; 2021. [On line]. | Link |
World Health Organization. Coronavirus disease (COVID-19) pandemic. Geneva: WHO; 2021. [On line]. | Link | Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021 Mar;19(3):141-154. | CrossRef | PubMed |
Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021 Mar;19(3):141-154. | CrossRef | PubMed | Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020 Aug 25;324(8):782-793. | CrossRef | PubMed |
Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020 Aug 25;324(8):782-793. | CrossRef | PubMed | Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019 Mar;17(3):181-192. | CrossRef | PubMed |
Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019 Mar;17(3):181-192. | CrossRef | PubMed | Deslandes A, Berti V, Tandjaoui-Lambotte Y, Alloui C, Carbonnelle E, Zahar JR, et al. SARS-CoV-2 was already spreading in France in late December 2019. Int J Antimicrob Agents. 2020 Jun;55(6):106006. | CrossRef | PubMed |
Deslandes A, Berti V, Tandjaoui-Lambotte Y, Alloui C, Carbonnelle E, Zahar JR, et al. SARS-CoV-2 was already spreading in France in late December 2019. Int J Antimicrob Agents. 2020 Jun;55(6):106006. | CrossRef | PubMed | Centers for Disease Control and Prevention. Science Brief: Emerging SARS-CoV-2 Variants. USA: CDC; 2021. [On line]. | Link |
Centers for Disease Control and Prevention. Science Brief: Emerging SARS-CoV-2 Variants. USA: CDC; 2021. [On line]. | Link | World Health Organization. Weekly epidemiological update on COVID-19 - 4 May 2021. Geneva: WHO; 2021. [On line]. | Link |
World Health Organization. Weekly epidemiological update on COVID-19 - 4 May 2021. Geneva: WHO; 2021. [On line]. | Link | Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 Mar;579(7798):270-273. | CrossRef | PubMed |
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 Mar;579(7798):270-273. | CrossRef | PubMed | Seyed Hosseini E, Riahi Kashani N, Nikzad H, Azadbakht J, Hassani Bafrani H, Haddad Kashani H. The novel coronavirus Disease-2019 (COVID-19): Mechanism of action, detection and recent therapeutic strategies. Virology. 2020 Dec;551:1-9. | CrossRef | PubMed |
Seyed Hosseini E, Riahi Kashani N, Nikzad H, Azadbakht J, Hassani Bafrani H, Haddad Kashani H. The novel coronavirus Disease-2019 (COVID-19): Mechanism of action, detection and recent therapeutic strategies. Virology. 2020 Dec;551:1-9. | CrossRef | PubMed | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 Apr 30;382(18):1708-1720. | CrossRef | PubMed |
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 Apr 30;382(18):1708-1720. | CrossRef | PubMed | Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020 Mar 26;382(13):1199-1207. | CrossRef | PubMed |
Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020 Mar 26;382(13):1199-1207. | CrossRef | PubMed | Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020 May;581(7809):465-469. | CrossRef | PubMed |
Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020 May;581(7809):465-469. | CrossRef | PubMed | Fontana LM, Villamagna AH, Sikka MK, McGregor JC. Understanding viral shedding of severe acute respiratory coronavirus virus 2 (SARS-CoV-2): Review of current literature. Infect Control Hosp Epidemiol. 2020 Oct 20:1-10. | CrossRef | PubMed |
Fontana LM, Villamagna AH, Sikka MK, McGregor JC. Understanding viral shedding of severe acute respiratory coronavirus virus 2 (SARS-CoV-2): Review of current literature. Infect Control Hosp Epidemiol. 2020 Oct 20:1-10. | CrossRef | PubMed | Centers for Disease Control and Prevention. Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19). USA: CDC; 2021. [On line]. | Link |
Centers for Disease Control and Prevention. Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19). USA: CDC; 2021. [On line]. | Link | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020 Mar 17;323(11):1061-1069. | CrossRef | PubMed |
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020 Mar 17;323(11):1061-1069. | CrossRef | PubMed | Departamento de Epidemiología, Ministerio de Salud de Chile. Informe Epidemiológico N°97 Enfermedad por SARS-CoV-2 (COVID-19) Chile 22-02-2021. Chile: MINSAL; 2021. [On line]. | Link |
Departamento de Epidemiología, Ministerio de Salud de Chile. Informe Epidemiológico N°97 Enfermedad por SARS-CoV-2 (COVID-19) Chile 22-02-2021. Chile: MINSAL; 2021. [On line]. | Link | Oran DP, Topol EJ. The Proportion of SARS-CoV-2 Infections That Are Asymptomatic : A Systematic Review. Ann Intern Med. 2021 May;174(5):655-662. | CrossRef | PubMed |
Oran DP, Topol EJ. The Proportion of SARS-CoV-2 Infections That Are Asymptomatic : A Systematic Review. Ann Intern Med. 2021 May;174(5):655-662. | CrossRef | PubMed | Hu Z, Song C, Xu C, Jin G, Chen Y, Xu X, et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020 May;63(5):706-711. | CrossRef | PubMed |
Hu Z, Song C, Xu C, Jin G, Chen Y, Xu X, et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020 May;63(5):706-711. | CrossRef | PubMed | Wang Y, He Y, Tong J, Qin Y, Xie T, Li J, et al. Characterization of an Asymptomatic Cohort of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infected Individuals Outside of Wuhan, China. Clin Infect Dis. 2020 Nov 19;71(16):2132-2138. | CrossRef | PubMed |
Wang Y, He Y, Tong J, Qin Y, Xie T, Li J, et al. Characterization of an Asymptomatic Cohort of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infected Individuals Outside of Wuhan, China. Clin Infect Dis. 2020 Nov 19;71(16):2132-2138. | CrossRef | PubMed | Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med. 2020 Mar 5;382(10):970-971. | CrossRef | PubMed |
Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med. 2020 Mar 5;382(10):970-971. | CrossRef | PubMed | Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020 Apr 7;323(13):1239-1242. | CrossRef | PubMed |
Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020 Apr 7;323(13):1239-1242. | CrossRef | PubMed | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497-506. | CrossRef | PubMed |
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497-506. | CrossRef | PubMed | Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020 Aug 11;324(6):603-605. | CrossRef | PubMed |
Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020 Aug 11;324(6):603-605. | CrossRef | PubMed | Goërtz YMJ, Van Herck M, Delbressine JM, Vaes AW, Meys R, Machado FVC, et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020 Oct 26;6(4):00542-2020. | CrossRef | PubMed |
Goërtz YMJ, Van Herck M, Delbressine JM, Vaes AW, Meys R, Machado FVC, et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020 Oct 26;6(4):00542-2020. | CrossRef | PubMed | Taquet M, Luciano S, Geddes JR, Harrison PJ. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021 Feb;8(2):130-140. | CrossRef | PubMed |
Taquet M, Luciano S, Geddes JR, Harrison PJ. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021 Feb;8(2):130-140. | CrossRef | PubMed | Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, et al. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern Med. 2020 Oct 1;180(10):1345-1355. | CrossRef | PubMed |
Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, et al. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern Med. 2020 Oct 1;180(10):1345-1355. | CrossRef | PubMed | Gupta S, Hayek SS, Wang W, Chan L, Mathews KS, Melamed ML, et al. Factors Associated With Death in Critically Ill Patients With Coronavirus Disease 2019 in the US. JAMA Intern Med. 2020 Nov 1;180(11):1436-1447. | CrossRef | PubMed |
Gupta S, Hayek SS, Wang W, Chan L, Mathews KS, Melamed ML, et al. Factors Associated With Death in Critically Ill Patients With Coronavirus Disease 2019 in the US. JAMA Intern Med. 2020 Nov 1;180(11):1436-1447. | CrossRef | PubMed | Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020 Mar 26;368:m1091. | CrossRef | PubMed |
Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020 Mar 26;368:m1091. | CrossRef | PubMed | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-1062. | CrossRef | PubMed |
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-1062. | CrossRef | PubMed | Centers for Disease Control and Prevention. People with Certain Medical Conditions. USA: CDC; 2021. [On line]. | Link |
Centers for Disease Control and Prevention. People with Certain Medical Conditions. USA: CDC; 2021. [On line]. | Link | Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020 May 22;369:m1966. | CrossRef | PubMed |
Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020 May 22;369:m1966. | CrossRef | PubMed | Harrison SL, Fazio-Eynullayeva E, Lane DA, Underhill P, Lip GYH. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: A federated electronic medical record analysis. PLoS Med. 2020 Sep 10;17(9):e1003321. | CrossRef | PubMed |
Harrison SL, Fazio-Eynullayeva E, Lane DA, Underhill P, Lip GYH. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: A federated electronic medical record analysis. PLoS Med. 2020 Sep 10;17(9):e1003321. | CrossRef | PubMed | Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020 Aug;584(7821):430-436. | CrossRef | PubMed |
Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020 Aug;584(7821):430-436. | CrossRef | PubMed | Mena GE, Martinez PP, Mahmud AS, Marquet PA, Buckee CO, Santillana M. Socioeconomic status determines COVID-19 incidence and related mortality in Santiago, Chile. Science. 2021 Apr 27:eabg5298. | CrossRef | PubMed |
Mena GE, Martinez PP, Mahmud AS, Marquet PA, Buckee CO, Santillana M. Socioeconomic status determines COVID-19 incidence and related mortality in Santiago, Chile. Science. 2021 Apr 27:eabg5298. | CrossRef | PubMed | Moore JT, Ricaldi JN, Rose CE, Fuld J, Parise M, Kang GJ, et al. Disparities in Incidence of COVID-19 Among Underrepresented Racial/Ethnic Groups in Counties Identified as Hotspots During June 5-18, 2020 - 22 States, February-June 2020. MMWR Morb Mortal Wkly Rep. 2020 Aug 21;69(33):1122-1126. | CrossRef | PubMed |
Moore JT, Ricaldi JN, Rose CE, Fuld J, Parise M, Kang GJ, et al. Disparities in Incidence of COVID-19 Among Underrepresented Racial/Ethnic Groups in Counties Identified as Hotspots During June 5-18, 2020 - 22 States, February-June 2020. MMWR Morb Mortal Wkly Rep. 2020 Aug 21;69(33):1122-1126. | CrossRef | PubMed | Meyerowitz EA, Richterman A, Gandhi RT, Sax PE. Transmission of SARS-CoV-2: A Review of Viral, Host, and Environmental Factors. Ann Intern Med. 2021 Jan;174(1):69-79. | CrossRef | PubMed |
Meyerowitz EA, Richterman A, Gandhi RT, Sax PE. Transmission of SARS-CoV-2: A Review of Viral, Host, and Environmental Factors. Ann Intern Med. 2021 Jan;174(1):69-79. | CrossRef | PubMed | USDA secretary, commissioner of Foods and Drugs - Food and Drug Administration. COVID-19 Update: USDA, FDA Underscore Current Epidemiologic and Scientific Information Indicating No Transmission of COVID-19 Through Food or Food Packaging. USA: USDA, FDA; 2021. [On line]. | Link |
USDA secretary, commissioner of Foods and Drugs - Food and Drug Administration. COVID-19 Update: USDA, FDA Underscore Current Epidemiologic and Scientific Information Indicating No Transmission of COVID-19 Through Food or Food Packaging. USA: USDA, FDA; 2021. [On line]. | Link | World Health Organization. Transmission of SARS-CoV-2: implications for infection prevention precautions. Geneva: WHO; 2021. [On line]. | Link |
World Health Organization. Transmission of SARS-CoV-2: implications for infection prevention precautions. Geneva: WHO; 2021. [On line]. | Link | Morawska L, Milton DK. It Is Time to Address Airborne Transmission of Coronavirus Disease 2019 (COVID-19). Clin Infect Dis. 2020 Dec 3;71(9):2311-2313. | CrossRef | PubMed |
Morawska L, Milton DK. It Is Time to Address Airborne Transmission of Coronavirus Disease 2019 (COVID-19). Clin Infect Dis. 2020 Dec 3;71(9):2311-2313. | CrossRef | PubMed | Chagla Z, Hota S, Khan S, Mertz D; International Hospital and Community Epidemiology Group. Airborne Transmission of COVID-19. Clin Infect Dis. 2020 Aug 11:ciaa1118. | CrossRef | PubMed |
Chagla Z, Hota S, Khan S, Mertz D; International Hospital and Community Epidemiology Group. Airborne Transmission of COVID-19. Clin Infect Dis. 2020 Aug 11:ciaa1118. | CrossRef | PubMed | Johnson H, Garg M, Shantikumar S, Thachil J, Rai B, Aboumarzouk OM, et al. COVID-19 (SARS-CoV-2) in Non-Airborne body fluids: A systematic review & Meta-analysis. Turk J Urol. 2021 Mar;47(2):87-97. | CrossRef | PubMed |
Johnson H, Garg M, Shantikumar S, Thachil J, Rai B, Aboumarzouk OM, et al. COVID-19 (SARS-CoV-2) in Non-Airborne body fluids: A systematic review & Meta-analysis. Turk J Urol. 2021 Mar;47(2):87-97. | CrossRef | PubMed | Commissioners of the Lancet COVID-19 Commission. Electronic address: sachs@columbia.edu; Task Force Chairs and members of the Lancet COVID-19 Commission; Commission Secretariat and Staff of the Lancet COVID-19 Commission; Commissioners of the Lancet COVID-19 Commission. Priorities for the COVID-19 pandemic at the start of 2021: statement of the Lancet COVID-19 Commission. Lancet. 2021 Mar 13;397(10278):947-950. | CrossRef | PubMed |
Commissioners of the Lancet COVID-19 Commission. Electronic address: sachs@columbia.edu; Task Force Chairs and members of the Lancet COVID-19 Commission; Commission Secretariat and Staff of the Lancet COVID-19 Commission; Commissioners of the Lancet COVID-19 Commission. Priorities for the COVID-19 pandemic at the start of 2021: statement of the Lancet COVID-19 Commission. Lancet. 2021 Mar 13;397(10278):947-950. | CrossRef | PubMed | Organización Panamericana de la Salud. Actualización Epidemiológica Enfermedad por coronavirus (COVID-19) - 9 de Febrero 2021. OPS; 2021. [On line]. | Link |
Organización Panamericana de la Salud. Actualización Epidemiológica Enfermedad por coronavirus (COVID-19) - 9 de Febrero 2021. OPS; 2021. [On line]. | Link | World Health Organization. Weekly epidemiological update on COVID-19 - 20 April 2021. Geneva: WHO; 2021. [On line]. | Link |
World Health Organization. Weekly epidemiological update on COVID-19 - 20 April 2021. Geneva: WHO; 2021. [On line]. | Link | Plante JA, Liu Y, Liu J, Xia H, Johnson BA, Lokugamage KG, et al. Spike mutation D614G alters SARS-CoV-2 fitness. Nature. 2021 Apr;592(7852):116-121. | CrossRef | PubMed |
Plante JA, Liu Y, Liu J, Xia H, Johnson BA, Lokugamage KG, et al. Spike mutation D614G alters SARS-CoV-2 fitness. Nature. 2021 Apr;592(7852):116-121. | CrossRef | PubMed | Zhou B, Thao TTN, Hoffmann D, Taddeo A, Ebert N, Labroussaa F, et al. SARS-CoV-2 spike D614G change enhances replication and transmission. Nature. 2021 Apr;592(7852):122-127. | CrossRef | PubMed |
Zhou B, Thao TTN, Hoffmann D, Taddeo A, Ebert N, Labroussaa F, et al. SARS-CoV-2 spike D614G change enhances replication and transmission. Nature. 2021 Apr;592(7852):122-127. | CrossRef | PubMed | Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W, et al. Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell. 2020 Aug 20;182(4):812-827.e19. | CrossRef | PubMed |
Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W, et al. Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell. 2020 Aug 20;182(4):812-827.e19. | CrossRef | PubMed | Klumpp-Thomas C, Kalish H, Hicks J, Mehalko J, Drew M, Memoli MJ, et al. Effect of D614G Spike Variant on Immunoglobulin G, M, or A Spike Seroassay Performance. J Infect Dis. 2021 Mar 3;223(5):802-804. | CrossRef | PubMed |
Klumpp-Thomas C, Kalish H, Hicks J, Mehalko J, Drew M, Memoli MJ, et al. Effect of D614G Spike Variant on Immunoglobulin G, M, or A Spike Seroassay Performance. J Infect Dis. 2021 Mar 3;223(5):802-804. | CrossRef | PubMed | Davies NG, Jarvis CI; CMMID COVID-19 Working Group, Edmunds WJ, Jewell NP, Diaz-Ordaz K, et al. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature. 2021 May;593(7858):270-274. | CrossRef | PubMed |
Davies NG, Jarvis CI; CMMID COVID-19 Working Group, Edmunds WJ, Jewell NP, Diaz-Ordaz K, et al. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature. 2021 May;593(7858):270-274. | CrossRef | PubMed | Challen R, Brooks-Pollock E, Read JM, Dyson L, Tsaneva-Atanasova K, Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ. 2021 Mar 9;372:n579. | CrossRef | PubMed |
Challen R, Brooks-Pollock E, Read JM, Dyson L, Tsaneva-Atanasova K, Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ. 2021 Mar 9;372:n579. | CrossRef | PubMed | Deng X, Garcia-Knight MA, Khalid MM, Servellita V, Wang C, Morris MK, et al. Transmission, infectivity, and antibody neutralization of an emerging SARS-CoV-2 variant in California carrying a L452R spike protein mutation. medRxiv [Preprint]. 2021 Mar 9:2021.03.07.21252647. | CrossRef | PubMed |
Deng X, Garcia-Knight MA, Khalid MM, Servellita V, Wang C, Morris MK, et al. Transmission, infectivity, and antibody neutralization of an emerging SARS-CoV-2 variant in California carrying a L452R spike protein mutation. medRxiv [Preprint]. 2021 Mar 9:2021.03.07.21252647. | CrossRef | PubMed | Wang P, Casner RG, Nair MS, Wang M, Yu J, Cerutti G, et al. Increased Resistance of SARS-CoV-2 Variant P.1 to Antibody Neutralization. bioRxiv [Preprint]. 2021 Mar 2:2021.03.01.433466. | CrossRef | PubMed |
Wang P, Casner RG, Nair MS, Wang M, Yu J, Cerutti G, et al. Increased Resistance of SARS-CoV-2 Variant P.1 to Antibody Neutralization. bioRxiv [Preprint]. 2021 Mar 2:2021.03.01.433466. | CrossRef | PubMed | Garcia-Beltran WF, Lam EC, St Denis K, Nitido AD, Garcia ZH, Hauser BM, et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021 Apr 29;184(9):2372-2383.e9. | CrossRef | PubMed |
Garcia-Beltran WF, Lam EC, St Denis K, Nitido AD, Garcia ZH, Hauser BM, et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021 Apr 29;184(9):2372-2383.e9. | CrossRef | PubMed | Davies NG, Abbott S, Barnard RC, Jarvis CI, Kucharski AJ, Munday JD, et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021 Apr 9;372(6538):eabg3055. | CrossRef | PubMed |
Davies NG, Abbott S, Barnard RC, Jarvis CI, Kucharski AJ, Munday JD, et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021 Apr 9;372(6538):eabg3055. | CrossRef | PubMed | Shen X, Tang H, McDanal C, Wagh K, Fischer W, Theiler J, et al. SARS-CoV-2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral spike vaccines. Cell Host Microbe. 2021 Apr 14;29(4):529-539.e3. | CrossRef | PubMed |
Shen X, Tang H, McDanal C, Wagh K, Fischer W, Theiler J, et al. SARS-CoV-2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral spike vaccines. Cell Host Microbe. 2021 Apr 14;29(4):529-539.e3. | CrossRef | PubMed | Mahase E. Covid-19: Novavax vaccine efficacy is 86% against UK variant and 60% against South African variant. BMJ. 2021 Feb 1;372:n296. | CrossRef | PubMed |
Mahase E. Covid-19: Novavax vaccine efficacy is 86% against UK variant and 60% against South African variant. BMJ. 2021 Feb 1;372:n296. | CrossRef | PubMed | Eyal N, Caplan A, Plotkin S. COVID vaccine efficacy against the B.1.351 ("South African") variant-The urgent need to lay the groundwork for possible future challenge studies. Hum Vaccin Immunother. 2021 Apr 27:1-2. | CrossRef | PubMed |
Eyal N, Caplan A, Plotkin S. COVID vaccine efficacy against the B.1.351 ("South African") variant-The urgent need to lay the groundwork for possible future challenge studies. Hum Vaccin Immunother. 2021 Apr 27:1-2. | CrossRef | PubMed | Wibmer CK, Ayres F, Hermanus T, Madzivhandila M, Kgagudi P, Oosthuysen B, et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. bioRxiv [Preprint]. 2021 Jan 19:2021.01.18.427166. | CrossRef | PubMed |
Wibmer CK, Ayres F, Hermanus T, Madzivhandila M, Kgagudi P, Oosthuysen B, et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. bioRxiv [Preprint]. 2021 Jan 19:2021.01.18.427166. | CrossRef | PubMed | Weisblum Y, Schmidt F, Zhang F, DaSilva J, Poston D, Lorenzi JC, et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. Elife. 2020 Oct 28;9:e61312. | CrossRef | PubMed |
Weisblum Y, Schmidt F, Zhang F, DaSilva J, Poston D, Lorenzi JC, et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. Elife. 2020 Oct 28;9:e61312. | CrossRef | PubMed | Hacisuleyman E, Hale C, Saito Y, Blachere NE, Bergh M, Conlon EG, et al. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. N Engl J Med. 2021 Apr 21. | CrossRef | PubMed |
Hacisuleyman E, Hale C, Saito Y, Blachere NE, Bergh M, Conlon EG, et al. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. N Engl J Med. 2021 Apr 21. | CrossRef | PubMed | Pearson CAB, Russell TW, Davies N, Kucharski AJ, CMMID COVID-19 working group, Edmunds WJ, et al. Estimates of severity and transmissibility of novel SARS-CoV-2 variant 501Y.V2 in South Africa. CMMID; 2021. [On line]. | Link |
Pearson CAB, Russell TW, Davies N, Kucharski AJ, CMMID COVID-19 working group, Edmunds WJ, et al. Estimates of severity and transmissibility of novel SARS-CoV-2 variant 501Y.V2 in South Africa. CMMID; 2021. [On line]. | Link | Victora C, Castro MC, Gurzenda S, Barros AJD. Estimating the early impact of immunization against COVID-19 on deaths among elderly people in Brazil: analyses of secondary data on vaccine coverage and mortality. medRxiv; 2021. | CrossRef |
Victora C, Castro MC, Gurzenda S, Barros AJD. Estimating the early impact of immunization against COVID-19 on deaths among elderly people in Brazil: analyses of secondary data on vaccine coverage and mortality. medRxiv; 2021. | CrossRef | Hitchings MDT, Ranzani OT, Scaramuzzini Torres MS, de Oliveira SB de, Almiron M, Said R, et al. Effectiveness of CoronaVac in the setting of high SARS-CoV-2 P.1 variant transmission in Brazil: A test-negative case-control study. medRxiv; 2021. | CrossRef |
Hitchings MDT, Ranzani OT, Scaramuzzini Torres MS, de Oliveira SB de, Almiron M, Said R, et al. Effectiveness of CoronaVac in the setting of high SARS-CoV-2 P.1 variant transmission in Brazil: A test-negative case-control study. medRxiv; 2021. | CrossRef | Ministerio de Salud de Chile. La vacuna CoronaVac demostró ser efectiva en un 89% para evitar hospitalizaciones UCI. Chile: MINSAL; 2021. [On line]. | Link |
Ministerio de Salud de Chile. La vacuna CoronaVac demostró ser efectiva en un 89% para evitar hospitalizaciones UCI. Chile: MINSAL; 2021. [On line]. | Link | Yadav PD, Sapkal GN, Abraham P, Ella R, Deshpande G, Patil DY, et al. Neutralization of variant under investigation B.1.617 with sera of BBV152 vaccinees. Clin Infect Dis. 2021 May 7:ciab411. | CrossRef | PubMed |
Yadav PD, Sapkal GN, Abraham P, Ella R, Deshpande G, Patil DY, et al. Neutralization of variant under investigation B.1.617 with sera of BBV152 vaccinees. Clin Infect Dis. 2021 May 7:ciab411. | CrossRef | PubMed | Centers for Disease Control and Prevention. SARS-CoV-2 Variant Classifications and Definitions. USA: CDC; 2021. [On line]. | Link |
Centers for Disease Control and Prevention. SARS-CoV-2 Variant Classifications and Definitions. USA: CDC; 2021. [On line]. | Link | Tomassini S, Kotecha D, Bird PW, Folwell A, Biju S, Tang JW. Setting the criteria for SARS-CoV-2 reinfection - six possible cases. J Infect. 2021 Feb;82(2):282-327. | CrossRef | PubMed |
Tomassini S, Kotecha D, Bird PW, Folwell A, Biju S, Tang JW. Setting the criteria for SARS-CoV-2 reinfection - six possible cases. J Infect. 2021 Feb;82(2):282-327. | CrossRef | PubMed | To KK, Hung IF, Ip JD, Chu AW, Chan WM, Tam AR, et al. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin Infect Dis. 2020 Aug 25:ciaa1275. | CrossRef | PubMed |
To KK, Hung IF, Ip JD, Chu AW, Chan WM, Tam AR, et al. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin Infect Dis. 2020 Aug 25:ciaa1275. | CrossRef | PubMed | Abu-Raddad LJ, Chemaitelly H, Malek JA, Ahmed AA, Mohamoud YA, Younuskunju S, et al. Assessment of the risk of SARS-CoV-2 reinfection in an intense re-exposure setting. Clin Infect Dis. 2020 Dec 14:ciaa1846. | CrossRef | PubMed |
Abu-Raddad LJ, Chemaitelly H, Malek JA, Ahmed AA, Mohamoud YA, Younuskunju S, et al. Assessment of the risk of SARS-CoV-2 reinfection in an intense re-exposure setting. Clin Infect Dis. 2020 Dec 14:ciaa1846. | CrossRef | PubMed | Gralinski LE, Menachery VD. Return of the Coronavirus: 2019-nCoV. Viruses. 2020 Jan 24;12(2):135. | CrossRef | PubMed |
Gralinski LE, Menachery VD. Return of the Coronavirus: 2019-nCoV. Viruses. 2020 Jan 24;12(2):135. | CrossRef | PubMed | Dinnes J, Deeks JJ, Adriano A, Berhane S, Davenport C, Dittrich S, et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2020 Aug 26;8(8):CD013705. | CrossRef | PubMed |
Dinnes J, Deeks JJ, Adriano A, Berhane S, Davenport C, Dittrich S, et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2020 Aug 26;8(8):CD013705. | CrossRef | PubMed | Weissleder R, Lee H, Ko J, Pittet MJ. COVID-19 diagnostics in context. Sci Transl Med. 2020 Jun 3;12(546):eabc1931. | CrossRef | PubMed |
Weissleder R, Lee H, Ko J, Pittet MJ. COVID-19 diagnostics in context. Sci Transl Med. 2020 Jun 3;12(546):eabc1931. | CrossRef | PubMed | Hanson KE, Caliendo AM, Arias CA, Englund JA, Hayden MK, Lee MJ, et al. Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19:Serologic Testing. Clin Infect Dis. 2020 Sep 12:ciaa1343. | CrossRef | PubMed |
Hanson KE, Caliendo AM, Arias CA, Englund JA, Hayden MK, Lee MJ, et al. Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19:Serologic Testing. Clin Infect Dis. 2020 Sep 12:ciaa1343. | CrossRef | PubMed | Plotkin SA. Updates on immunologic correlates of vaccine-induced protection. Vaccine. 2020 Feb 24;38(9):2250-2257. | CrossRef | PubMed |
Plotkin SA. Updates on immunologic correlates of vaccine-induced protection. Vaccine. 2020 Feb 24;38(9):2250-2257. | CrossRef | PubMed | Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011 Jun;12(6):509-17. | CrossRef | PubMed |
Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011 Jun;12(6):509-17. | CrossRef | PubMed | Prendecki M, Clarke C, Brown J, Cox A, Gleeson S, Guckian M, et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet. 2021 Mar 27;397(10280):1178-1181. | CrossRef | PubMed |
Prendecki M, Clarke C, Brown J, Cox A, Gleeson S, Guckian M, et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet. 2021 Mar 27;397(10280):1178-1181. | CrossRef | PubMed | Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020 Oct;586(7830):594-599. | CrossRef | PubMed |
Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020 Oct;586(7830):594-599. | CrossRef | PubMed | Lin DY, Zeng D, Mehrotra DV, Corey L, Gilbert PB. Evaluating the Efficacy of COVID-19 Vaccines. Clin Infect Dis. 2020 Dec 19:ciaa1863. | CrossRef | PubMed |
Lin DY, Zeng D, Mehrotra DV, Corey L, Gilbert PB. Evaluating the Efficacy of COVID-19 Vaccines. Clin Infect Dis. 2020 Dec 19:ciaa1863. | CrossRef | PubMed | World Health Organization. Evaluation of COVID-19 vaccine effectiveness. Geneva: WHO; 2021. [On line]. | Link |
World Health Organization. Evaluation of COVID-19 vaccine effectiveness. Geneva: WHO; 2021. [On line]. | Link | Babiker A, Marvil CE, Waggoner JJ, Collins MH, Piantadosi A. The Importance and Challenges of Identifying SARS-CoV-2 Reinfections. J Clin Microbiol. 2021 Mar 19;59(4):e02769-20. | CrossRef | PubMed |
Babiker A, Marvil CE, Waggoner JJ, Collins MH, Piantadosi A. The Importance and Challenges of Identifying SARS-CoV-2 Reinfections. J Clin Microbiol. 2021 Mar 19;59(4):e02769-20. | CrossRef | PubMed | Iwasaki A. What reinfections mean for COVID-19. Lancet Infect Dis. 2021 Jan;21(1):3-5. | CrossRef | PubMed |
Iwasaki A. What reinfections mean for COVID-19. Lancet Infect Dis. 2021 Jan;21(1):3-5. | CrossRef | PubMed | Carrillo J, Izquierdo-Useros N, Ávila-Nieto C, Pradenas E, Clotet B, Blanco J. Humoral immune responses and neutralizing antibodies against SARS-CoV-2; implications in pathogenesis and protective immunity. Biochem Biophys Res Commun. 2021 Jan 29;538:187-191. | CrossRef | PubMed |
Carrillo J, Izquierdo-Useros N, Ávila-Nieto C, Pradenas E, Clotet B, Blanco J. Humoral immune responses and neutralizing antibodies against SARS-CoV-2; implications in pathogenesis and protective immunity. Biochem Biophys Res Commun. 2021 Jan 29;538:187-191. | CrossRef | PubMed | Chess K, Jacobson TA, Smith LE, Miller C, Hirschhorn L, Huffman MD. SARS-CoV-2 Seroprevalence Studies: A Rapid Review. ResearchSquare; 2020. | CrossRef |
Chess K, Jacobson TA, Smith LE, Miller C, Hirschhorn L, Huffman MD. SARS-CoV-2 Seroprevalence Studies: A Rapid Review. ResearchSquare; 2020. | CrossRef | Stringhini S, Wisniak A, Piumatti G, Azman AS, Lauer SA, Baysson H, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020 Aug 1;396(10247):313-319. | CrossRef | PubMed |
Stringhini S, Wisniak A, Piumatti G, Azman AS, Lauer SA, Baysson H, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020 Aug 1;396(10247):313-319. | CrossRef | PubMed | Rostami A, Sepidarkish M, Leeflang M, Riahi SM, Shiadeh MN, Esfandyari S, et al. First “snap-shot” meta-analysis to estimate the prevalence of serum antibodies to SARS-CoV-2 in humans. medRxiv; 2020. | CrossRef |
Rostami A, Sepidarkish M, Leeflang M, Riahi SM, Shiadeh MN, Esfandyari S, et al. First “snap-shot” meta-analysis to estimate the prevalence of serum antibodies to SARS-CoV-2 in humans. medRxiv; 2020. | CrossRef | Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA. 2020 Oct 6;324(13):1307-1316. | CrossRef | PubMed |
Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA. 2020 Oct 6;324(13):1307-1316. | CrossRef | PubMed | WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA. 2020 Oct 6;324(13):1330-1341. | CrossRef | PubMed |
WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA. 2020 Oct 6;324(13):1330-1341. | CrossRef | PubMed | RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021 Feb 25;384(8):693-704. | CrossRef | PubMed |
RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021 Feb 25;384(8):693-704. | CrossRef | PubMed | Ranjbar K, Moghadami M, Mirahmadizadeh A, Fallahi MJ, Khaloo V, Shahriarirad R, et al. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial. BMC Infect Dis. 2021 Apr 10;21(1):337. | CrossRef | PubMed |
Ranjbar K, Moghadami M, Mirahmadizadeh A, Fallahi MJ, Khaloo V, Shahriarirad R, et al. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial. BMC Infect Dis. 2021 Apr 10;21(1):337. | CrossRef | PubMed | Corral-Gudino L, Bahamonde A, Arnaiz-Revillas F, Gómez-Barquero J, Abadía-Otero J, García-Ibarbia C, et al. Methylprednisolone in adults hospitalized with COVID-19 pneumonia : An open-label randomized trial (GLUCOCOVID). Wien Klin Wochenschr. 2021 Apr;133(7-8):303-311. | CrossRef | PubMed |
Corral-Gudino L, Bahamonde A, Arnaiz-Revillas F, Gómez-Barquero J, Abadía-Otero J, García-Ibarbia C, et al. Methylprednisolone in adults hospitalized with COVID-19 pneumonia : An open-label randomized trial (GLUCOCOVID). Wien Klin Wochenschr. 2021 Apr;133(7-8):303-311. | CrossRef | PubMed | Angus DC, Derde L, Al-Beidh F, Annane D, Arabi Y, Beane A, et al. Effect of Hydrocortisone on Mortality and Organ Support in Patients With Severe COVID-19: The REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial. JAMA. 2020 Oct 6;324(13):1317-1329. | CrossRef | PubMed |
Angus DC, Derde L, Al-Beidh F, Annane D, Arabi Y, Beane A, et al. Effect of Hydrocortisone on Mortality and Organ Support in Patients With Severe COVID-19: The REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial. JAMA. 2020 Oct 6;324(13):1317-1329. | CrossRef | PubMed | Juul S, Nielsen EE, Feinberg J, Siddiqui F, Jørgensen CK, Barot E, et al. Interventions for treatment of COVID-19: Second edition of a living systematic review with meta-analyses and trial sequential analyses (The LIVING Project). PLoS One. 2021 Mar 11;16(3):e0248132. | CrossRef | PubMed |
Juul S, Nielsen EE, Feinberg J, Siddiqui F, Jørgensen CK, Barot E, et al. Interventions for treatment of COVID-19: Second edition of a living systematic review with meta-analyses and trial sequential analyses (The LIVING Project). PLoS One. 2021 Mar 11;16(3):e0248132. | CrossRef | PubMed | Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020 Jul 30;370:m2980. | CrossRef | PubMed |
Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020 Jul 30;370:m2980. | CrossRef | PubMed | Yu L-M, Group PC, Bafadhel M, Dorward J, Hayward G, Saville BR, et al. Inhaled budesonide for COVID-19 in people at higher risk of adverse outcomes in the community: interim analyses from the PRINCIPLE trial. medRxiv; 2021. | CrossRef |
Yu L-M, Group PC, Bafadhel M, Dorward J, Hayward G, Saville BR, et al. Inhaled budesonide for COVID-19 in people at higher risk of adverse outcomes in the community: interim analyses from the PRINCIPLE trial. medRxiv; 2021. | CrossRef | Ramakrishnan S, Nicolau DV Jr, Langford B, Mahdi M, Jeffers H, Mwasuku C, et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med. 2021 Apr 9:S2213-2600(21)00160-0. | CrossRef | PubMed |
Ramakrishnan S, Nicolau DV Jr, Langford B, Mahdi M, Jeffers H, Mwasuku C, et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med. 2021 Apr 9:S2213-2600(21)00160-0. | CrossRef | PubMed | Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020 Nov 5;383(19):1813-1826. | CrossRef | PubMed |
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020 Nov 5;383(19):1813-1826. | CrossRef | PubMed | Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 May 16;395(10236):1569-1578. | CrossRef | PubMed |
Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 May 16;395(10236):1569-1578. | CrossRef | PubMed | WHO Solidarity Trial Consortium, Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V, et al. Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med. 2021 Feb 11;384(6):497-511. | CrossRef | PubMed |
WHO Solidarity Trial Consortium, Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V, et al. Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med. 2021 Feb 11;384(6):497-511. | CrossRef | PubMed | Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med. 2021 Mar 4;384(9):795-807. | CrossRef | PubMed |
Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med. 2021 Mar 4;384(9):795-807. | CrossRef | PubMed | Atzeni F, Masala IF, Rodríguez-Carrio J, Ríos-Garcés R, Gerratana E, La Corte L, et al. The Rheumatology Drugs for COVID-19 Management: Which and When? J Clin Med. 2021 Feb 16;10(4):783. | CrossRef | PubMed |
Atzeni F, Masala IF, Rodríguez-Carrio J, Ríos-Garcés R, Gerratana E, La Corte L, et al. The Rheumatology Drugs for COVID-19 Management: Which and When? J Clin Med. 2021 Feb 16;10(4):783. | CrossRef | PubMed | Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020 Mar 28;395(10229):1033-1034. | CrossRef | PubMed |
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020 Mar 28;395(10229):1033-1034. | CrossRef | PubMed | Gritti G, Raimondi F, Ripamonti D, Riva I, Landi F, Alborghetti L, et al. IL-6 signalling pathway inactivation with siltuximab in patients with COVID-19 respiratory failure: an observational cohort study. medRxiv; 2020. | CrossRef |
Gritti G, Raimondi F, Ripamonti D, Riva I, Landi F, Alborghetti L, et al. IL-6 signalling pathway inactivation with siltuximab in patients with COVID-19 respiratory failure: an observational cohort study. medRxiv; 2020. | CrossRef | RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021 May 1;397(10285):1637-1645. | CrossRef | PubMed |
RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021 May 1;397(10285):1637-1645. | CrossRef | PubMed | REMAP-CAP Investigators, Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N Engl J Med. 2021 Apr 22;384(16):1491-1502. | CrossRef | PubMed |
REMAP-CAP Investigators, Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N Engl J Med. 2021 Apr 22;384(16):1491-1502. | CrossRef | PubMed | Tardif JC, Bouabdallaoui N, L’Allier PL, Gaudet D, Shah B, Pillinger MH, et al. Efficacy of Colchicine in Non-Hospitalized Patients with COVID-19. medRxiv; 2021. | CrossRef |
Tardif JC, Bouabdallaoui N, L’Allier PL, Gaudet D, Shah B, Pillinger MH, et al. Efficacy of Colchicine in Non-Hospitalized Patients with COVID-19. medRxiv; 2021. | CrossRef | Solaymani-Dodaran M, Ghanei M, Bagheri M, Qazvini A, Vahedi E, Hassan Saadat S, et al. Safety and efficacy of Favipiravir in moderate to severe SARS-CoV-2 pneumonia. Int Immunopharmacol. 2021 Jun;95:107522. | CrossRef | PubMed |
Solaymani-Dodaran M, Ghanei M, Bagheri M, Qazvini A, Vahedi E, Hassan Saadat S, et al. Safety and efficacy of Favipiravir in moderate to severe SARS-CoV-2 pneumonia. Int Immunopharmacol. 2021 Jun;95:107522. | CrossRef | PubMed | RECOVERY Collaborative Group. Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021 Feb 13;397(10274):605-612. | CrossRef | PubMed |
RECOVERY Collaborative Group. Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021 Feb 13;397(10274):605-612. | CrossRef | PubMed | RECOVERY Collaborative Group, Horby P, Mafham M, Linsell L, Bell JL, Staplin N, et al. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020 Nov 19;383(21):2030-2040. | CrossRef | PubMed |
RECOVERY Collaborative Group, Horby P, Mafham M, Linsell L, Bell JL, Staplin N, et al. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020 Nov 19;383(21):2030-2040. | CrossRef | PubMed | RECOVERY Collaborative Group. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020 Oct 5;396(10259):1345–52. | CrossRef | PubMed |
RECOVERY Collaborative Group. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020 Oct 5;396(10259):1345–52. | CrossRef | PubMed | López-Medina E, López P, Hurtado IC, Dávalos DM, Ramirez O, Martínez E, et al. Effect of Ivermectin on Time to Resolution of Symptoms Among Adults With Mild COVID-19: A Randomized Clinical Trial. JAMA. 2021 Apr 13;325(14):1426-1435. | CrossRef | PubMed |
López-Medina E, López P, Hurtado IC, Dávalos DM, Ramirez O, Martínez E, et al. Effect of Ivermectin on Time to Resolution of Symptoms Among Adults With Mild COVID-19: A Randomized Clinical Trial. JAMA. 2021 Apr 13;325(14):1426-1435. | CrossRef | PubMed | Horby PW, Estcourt L, Peto L, Emberson JR, Staplin N, Spata E, et al. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv; 2021. | CrossRef |
Horby PW, Estcourt L, Peto L, Emberson JR, Staplin N, Spata E, et al. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv; 2021. | CrossRef | Vaughn VM, Gandhi TN, Petty LA, Patel PK, Prescott HC, Malani AN, et al. Empiric Antibacterial Therapy and Community-onset Bacterial Coinfection in Patients Hospitalized With Coronavirus Disease 2019 (COVID-19): A Multi-hospital Cohort Study. Clin Infect Dis. 2021 May 18;72(10):e533-e541. | CrossRef | PubMed |
Vaughn VM, Gandhi TN, Petty LA, Patel PK, Prescott HC, Malani AN, et al. Empiric Antibacterial Therapy and Community-onset Bacterial Coinfection in Patients Hospitalized With Coronavirus Disease 2019 (COVID-19): A Multi-hospital Cohort Study. Clin Infect Dis. 2021 May 18;72(10):e533-e541. | CrossRef | PubMed | Chedid M, Waked R, Haddad E, Chetata N, Saliba G, Choucair J. Antibiotics in treatment of COVID-19 complications: a review of frequency, indications, and efficacy. J Infect Public Health. 2021 May;14(5):570-576. | CrossRef | PubMed |
Chedid M, Waked R, Haddad E, Chetata N, Saliba G, Choucair J. Antibiotics in treatment of COVID-19 complications: a review of frequency, indications, and efficacy. J Infect Public Health. 2021 May;14(5):570-576. | CrossRef | PubMed | Levy Hara G, Kanj SS, Pagani L, Abbo L, Endimiani A, Wertheim HFL, et al. Ten key points for the appropriate use of antibiotics in hospitalised patients: a consensus from the Antimicrobial Stewardship and Resistance Working Groups of the International Society of Chemotherapy. Science Direct. 2016;48(3):239-246. | CrossRef |
Levy Hara G, Kanj SS, Pagani L, Abbo L, Endimiani A, Wertheim HFL, et al. Ten key points for the appropriate use of antibiotics in hospitalised patients: a consensus from the Antimicrobial Stewardship and Resistance Working Groups of the International Society of Chemotherapy. Science Direct. 2016;48(3):239-246. | CrossRef | Honein MA, Christie A, Rose DA, Brooks JT, Meaney-Delman D, Cohn A, et al. Summary of Guidance for Public Health Strategies to Address High Levels of Community Transmission of SARS-CoV-2 and Related Deaths, December 2020. MMWR Morb Mortal Wkly Rep. 2020 Dec 11;69(49):1860-1867. | CrossRef | PubMed |
Honein MA, Christie A, Rose DA, Brooks JT, Meaney-Delman D, Cohn A, et al. Summary of Guidance for Public Health Strategies to Address High Levels of Community Transmission of SARS-CoV-2 and Related Deaths, December 2020. MMWR Morb Mortal Wkly Rep. 2020 Dec 11;69(49):1860-1867. | CrossRef | PubMed | World Health Organization. Draft landscape and tracker of COVID-19 candidate vaccines. Geneva: WHO; 2021. [On line]. | Link |
World Health Organization. Draft landscape and tracker of COVID-19 candidate vaccines. Geneva: WHO; 2021. [On line]. | Link | Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020 Oct;586(7830):516-527. | CrossRef | PubMed |
Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020 Oct;586(7830):516-527. | CrossRef | PubMed | Callaway E. The race for coronavirus vaccines: a graphical guide. Nature. 2020 Apr;580(7805):576-577. | CrossRef | PubMed |
Callaway E. The race for coronavirus vaccines: a graphical guide. Nature. 2020 Apr;580(7805):576-577. | CrossRef | PubMed | Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021 Feb;21(2):83-100. | CrossRef | PubMed |
Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021 Feb;21(2):83-100. | CrossRef | PubMed | Ritchie H, Ortiz-Ospina E, Beltekian D, Mathieu E, Hassell J, Macdonald B, et al. Coronavirus (COVID-19) Vaccinations. England: Our World In Data; 2021. [On line]. | Link |
Ritchie H, Ortiz-Ospina E, Beltekian D, Mathieu E, Hassell J, Macdonald B, et al. Coronavirus (COVID-19) Vaccinations. England: Our World In Data; 2021. [On line]. | Link | Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021 May 15;397(10287):1819-1829. | CrossRef | PubMed |
Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021 May 15;397(10287):1819-1829. | CrossRef | PubMed | Centers for Disease Control and Prevention. CDC Real-World Study Confirms Protective Benefits of mRNA COVID-19 Vaccines. USA: CDC; 2021. [On line]. | Link |
Centers for Disease Control and Prevention. CDC Real-World Study Confirms Protective Benefits of mRNA COVID-19 Vaccines. USA: CDC; 2021. [On line]. | Link |