 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Palabras clave: hypertension, practice guidelines
The aim of this study is the methodological evaluation of Clinical Practice Guidelines (CPG) in hypertension. This is the first in a series of review articles, analysis, assessment in methodology and content of clinical practice guidelines in Cardiology. Of all clinical practice guidelines, three were selected and the Appraisal of Guidelines for Research and Evaluation (AGREE II) instrument was used to assess each guide. The guidelines obtained the lowest score in the domain of applicability (mean 43.8%); while the highest score was for clarity of presentation (mean 81.5%). The lowest percentage was found in the applicability domain (European guideline) and the highest of all scores was found in two domains: scope and purpose, and clarity of presentation (Canadian guideline). Assessing the quality of the clinical practice guidelines analyzed, the Canadian is one with the best scores obtained by applying the AGREE II instrument, and it is advised to be used without modifications.
Clinical practice guidelines are documents that emerge from the analysis of various types of studies, from primary research to synopses [1], with the aim of helping health providers and patients to make an informed decision for specific clinical circumstances [2],[3]. Generally developed by government institutions and / or medical associations in order to make recommendations, particularly supported by studies in similar populations to which this guide applies [4]. The development of a clinical practice guideline de novo is a long and expensive process that involves a systematization of information, so an efficient option is the adoption / adaptation of external guidelines, with the aim of ensuring clear recommendations according to their place of application. However, this process involves methodological and content limitations due to the external validity of the findings that support the recommendations [5].
There is controversy regarding the conceptual appreciation and use of clinical practice guidelines. For developers, these are documents that incorporate systematic searches based on the evidence, evaluating their quality and proposing recommendations based on the best available evidence, although this not being of the best quality. However, for users the evidence-based concept is misunderstood in the sense that the recommendations are based solely on high quality evidence randomized controlled trials (RCTs) [6],[7]. While there is a wide variety of clinical practice guidelines, this can create confusion in the medical and health professionals (target of clinical practice guidelines users) [8], due to the variety of recommendations, as Tisdale stated: "rather than clarify the treatment of cardiovascular diseases, these new guidelines have clouded the decision-making process" [9].
A guideline with high methodological quality during its production process is more likely to have relevant and appropriate recommendations [6]. Therefore, it is important to assess the methodology of these guidelines, since proper compliance will answer questions of external validity, applicability and clinical relevance [10].
One method to assess the methodological rigor of clinical practice guidelines is the application of the AGREE II instrument (for its acronym in English, Appraisal of Guidelines for Research & Evaluation) [11],[12],[13],[14],[15], a valid and reliable instrument for this purpose. This will allow a standardization of methodological features to ensure the quality of the recommendations. The AGREE II instrument consists of 23 items that punctuate different dimensions of the quality of the guideline using Likert scales. Each item is evaluated from one (strongly disagree) to seven (strongly agree). The instrument is organized into six separate domains and each domain score is calculated as a percentage of the maximum possible score for each domain [8],[11],[12].
The domain, scope and objective considers the overall purpose of the guideline, as well as to specific clinical PICO questions (Patient, Intervention, Comparison and Outcome). The participation of those involved analyzes whether the guide includes the points of view of users to whom it is intended. The rigor of development considering the processes involved to obtain evidence, the method for making recommendations and updates. The clarity and presentation assess the language and format of the guideline. The applicability analyzes whether the implications of clinical practice guidelines on aspects of the organization, costs and use were considered. Editorial independence takes into account possible conflicts of interest of the development team, as well as relationships with industry.
The clinical practice guidelines support proper management of various diseases prevalent in each country to provide medical support for the use of new interventions of proven benefit and identify ineffective ethods in their daily practice [6],[10]. In Peru and worldwide, hypertension is a relevant disease (31.6%) [16] for which the attributable risk for cardiovascular disease (CVD) mortality is 40.6%. This is the highest percentage compared with other risk factors [17].
Several studies have analyzed the variability of the contents and the methodological quality of clinical practice guidelines on hypertension [8],[18], reporting that of all recommendations in the treatment of hypertension, less than a third were based in high quality evidence applicable to the population where these studies [10] were conducted. Therefore, it is necessary to conduct a methodological evaluation of clinical practice guidelines on hypertension.
This is the first in a series of review articles about analysis, assessment, methodology and content of clinical practice guidelines in cardiology. A specialist conducted the search of the literature of previous studies [18],[19].
A systematic search of clinical practice guidelines was performed using keywords, generic filters and MeSH terms: hypertension, blood pressure, practice guidelines, clinical practice guidelines in databases such as Guideline Clearinghouse, Scottish Intercollegiate Guidelines Network (SIGN), The National Institute for Health and Care Excellence (NICE) and MEDLINE. Fourteen guidelines were found in adults, of which the Eighth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure [20], the European Guidelines [21] and Canada Guidelines [22], were selected due to having less than five years since last updated (annual update to the Canadian), use a grading system of recommendations by level of evidence and its frequent use in our environment [23].
A methodological evaluation was performed using the Appraisal of Guidelines for Research & Evaluation (AGREE II) instrument. Four evaluators used AGREE II instrument to measure each guide. Discrepancies were resolved by consensus, as in previous studies [8],[10],[18],[24].
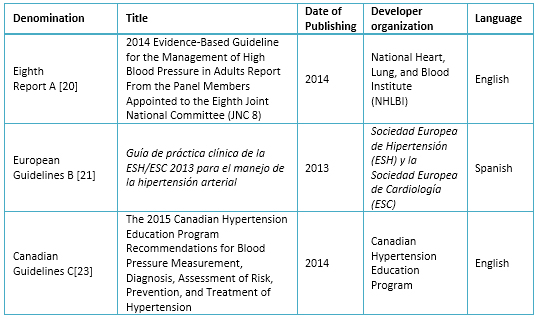
Table 1. Selected Clinical Practice Guidelines
The basic characteristics of the guidelines evaluated are presented (Tables 2 and 3), and linked their recommendations to the levels of evidence (Table 4).
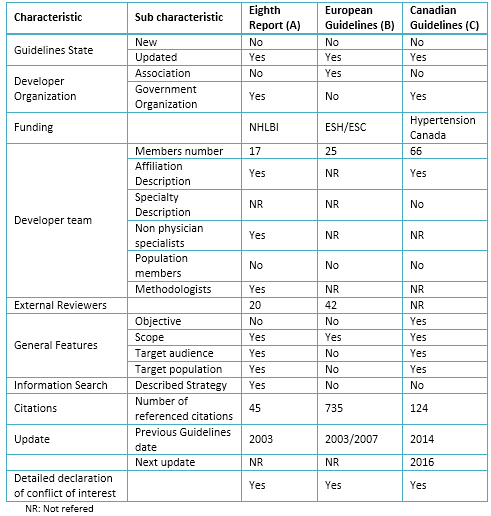
Table 2. Characteristics of selected Clinical Practice Guidelines
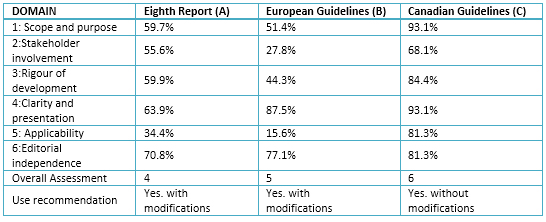
Table 3. Rating Domains (%) for selected guides
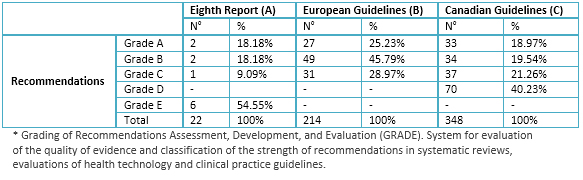
Table 4. Summary of levels of evidence according GRADE* recommendations
1. Domain 1: scope and objectives
A. 59.7%: objectives and health aspects are not clearly described, but could be considered as a central objective: "meet the needs of users, especially the needs of primary care physicians"; or answer if "the questions concerning the limits and targets for pharmacological treatment of hypertension and whether any particular antihypertensive medications improve important health prognosis compared with other drugs." The guideline considers the population that will be applied in the supplement, it details the target population, age, medical condition, but does not include the severity, comorbidities and excluded population.
B. 51.4%: the objectives are not clearly expressed and are not considered fundamental principles that inspired the previous editions of the clinical practice guidelines. Among them "Consider maximum data level derived from controlled clinical trials on the most important diagnostic and therapeutic aspects, like other guides of the European Society of Cardiology, the recommendations of this society for the development of clinical practice guidelines". The content of health aspects was not expressed clearly. New aspects compared to previous clinical practice guidelines were mentioned but they did not consider the population (patients) to which the guidelines will apply. The target population is not described, assuming the population of hypertensive patients with primary focus on disease, defining and addressing their epidemiology.
C. 93.1%: The objectives were adequately described; the main objective was to "provide updated recommendations based on evidence for prevention, diagnosis, evaluation and treatment of hypertension in adults". The health aspects were covered mostly as the primary outcome considering the health management, and decreased blood pressure. Additionally, the population was detailed, including the target population, age, clinical conditions, severity, comorbidities and excluded populations. Including comorbid conditions such as endocrine hypertension, heart failure, cerebral vascular accident, ventricular hypertrophy, chronic kidney disease, and diabetes mellitus stands out.
2. Domain 2: stakeholder involvement
A. 55.6%: The involvement in the development of the guideline in the item "Guideline process" which includes specialists and subspecialists was described; with institutional affiliation available in the supplement, no details on their specialty. We did not found in the published document or in the supplement, information on the views of the target population; although an indirect description of the target users as "primary care" is considered.
B. 27.8%: the group of developers of clinical practice guidelines had 25 specialists from different countries and 42 European reviewers, but did not describe their specialties. It also did not have a methodologist. The views of the target population or target users of this guide are not detailed.
C. 68.1%: the developer group mentioned their names, without specialty, although they include details of institutional affiliation, geographical location and role of the member in the developer group. Specialists including adherence strategies for patients, and in each of the sub-stands. The views of the target population were not considered, although it was the one from the target users.
3. Domain 3: Rigor of development
A. 59.9%: search methods were described in the supplement, including search terms, but did not report the used databases. The evidence based selection criteria were established, as well as the strengths and limitations; both they were detailed in the supplement. The methods used for formulating the recommendations were described, although these were not clear. Neither the benefits nor the risks when making the recommendations were considered. A weak relation between the recommendations and the evidence on which they were based was found. While not included the procedure to update the guide, an external review of the clinical practice guidelines was made.
B. 44.3%: systematic search methods were not described nor databases were reported, but the use of GRADE classification system was reported. References that support the levels of evidence in the recommendations are listed, but the criteria for selecting the evidence were not described. The strengths and limitations of the evidence, or the methods used to formulate the recommendations and how it had come to final decisions (consensus or other) are described. A table of levels of evidence and grades of recommendation based on the kind of study is reported. The health benefits, side effects and risks were taken into account in formulating the recommendations. There is an explicit link between recommendations and the evidence on which they are based. Forty two experts evaluated the guide, although a systematic approach to updating the guide is not included.
C. 84.4%: the developer group used some systematic methods such as MEDLINE search, but did not consider other databases. The search date was until August 2014, using MeSH terms, although not have details of the specific search. Various types of studies were considered without the schema of Patient, Intervention, Comparison and Outcome questions for each evaluation of the available evidence. The limitations, given the magnitude of benefit versus harm were considered. The development group formed subgroups, whose members were experts in their field, who made annual searches were then evaluated for methodological experts. The recommendations were sent to 70 voting members of the Canadian Hypertension Education Program (CHEP), abstaining those who presented conflicts of interest. The recommendations have been formulated considering the benefits and risks in general, not in each of the recommendations. The recommendations were accompanied by paragraphs of evidence, whose preparation by the developer group is described. The guide was reviewed by an external device and will be updated annually, including hypertension in pediatrics and in pregnancy.
4. Domain 4: clarity and presentation
A. 63.9%: the recommendations were specific and readily identifiable, although the various management options were not considered.
B. 87.5%: there is a specific description of the recommendations for each subtopic and considered the different options for screening, prevention, diagnosis or treatment of hypertension. The recommendations are easily identifiable.
C. 93.1%: the recommendations are specific and considered the management options, with identifiable key recommendations.
5. Domain 5: applicability
A. 34.4%: How to facilitate the implementation of the guide was not described and only appears an algorithm as a useful tool, nor criteria for monitoring or auditing.
B. 15.6%: Did not describe enabling factors and barriers to implementation. There was not an implementation section of the guideline, tools and application resources as summary documents, algorithms, information for patients, among others. No economic evaluations took into account neither costs.
C. 81.3%: it included an item on implementation of the guideline, describing the enabling factors as the flowchart for the diagnosis of hypertension. No detailed economic evaluations, but a demarcation is done because the recommendations didn’t perceive the associated costs. Additionally, the Canadian Hypertension Education Program (CHEP) regularly receives feedback from end users to enhance the development process of the guide.
6. Domain 6: editorial independence
A. 70.8%: the Eighth Joint National Committee report was funded by the National Heart, Lung, and Blood Institute (NHLBI). It recorded and dealt with conflicts of interest, although it was not detailed.
B. 77.1%: the financial support was provided by the European Society of Hypertension and the European Society of Cardiology, but not reaffirmed that the views of the funding entity have not influenced the content of the guideline. Conflicts of interest were well addressed (www.escardio.org/guidelines and www.eshonline.org).
C. 81.3%: it is assumed that the views of the funding entity have not influenced, as committee members "voluntarily contributed their time and experience" and the "process was independent of external influence". Conflicts of interest of the development group, as well as detailing the role and relationship fulfilled.
7. Overall guideline evaluation
The Eighth Report of the Joint National Committee provides information with marked deficiencies regarding the development and inclusion of details on the development process of the guideline. We recommended its use with changes.
B. The European Guide provides an extensive, detailed and specific recommendations that are readily identifiable, but does not describe various details about the process We recommended the use of it with changes.
C. We recommended the use of it without changes. The guideline includes a complete description of the diagnosis and management of hypertension, with an annual update.
When analyzing and comparing the guides, we found differences, strengths and weaknesses. However, the controversial nature of many of the guidelines shows the gap between studies and the need for further research, which would help the physician in choosing the right treatment for patients [9].
Of the six domains from Appraisal of Guidelines for Research & Evaluation (AGREE II), the lowest score was in the applicability domain with an average of 43.8% (between 15.6% and 81.3%); while clarity of presentation domain obtained the best scores with a mean of 81.5% (from 63.9% to 93.1%). The lowest score was in the applicability domain (European Guidelines) and the highest was in two domains: scope and purpose, and clarity of presentation (Canadian Guideline).
The Canadian Guideline was the one obtained the highest score in the various domains of AGREE II, placing it as the best with a score of 6/7. This is similar to that reported by Bonet et al result. [8] who had similar percentages in the domains, especially in methodological rigor in selecting the scientific evidence. About the use, it is recommended to apply the Canadian Guideline unchanged, unlike the European and the Eighth Report of the Joint National Committee to implement with changes.
The three guidelines studied the degree of link of their recommendations to levels of evidence in the Eighth Report of the Joint National Committee, objectives blood pressure have grade of recommendation E, the cutoff point for initiation of therapy recommendation A and therapy had recommendations of various degrees. In the European Guidelines, although the highest percentage of recommendations are grade B, these are distributed in the various aspects of the guidelines (diagnosis, treatment, and others); unlike Canadian Guide, where most therapy recommendations are grade A.
In assessing the institutional support offered by medical societies, we saw the bias that meant the loss of the National Heart, Lung, and Blood Institute support at the time of the publication of the Eighth Report of the Joint National Committee as well as the simultaneous publication of an article by the American Heart Association and the American College of Cardiology [25]. The other clinical practice guidelines were supported by medical societies and/or governmental institutions of their place of origin, and the European Guidelines being published in various languages, allowing its applicability in those countries.
The annual update of the Canadian Guide stands out, keeping many of the previous recommendations, which could explain the lower number of citations in its content, unlike the European guide with a considerably higher number of references. Needless to say is the small number of references of the Eighth Report of the Joint National Committee was because it included only randomized controlled clinical trials and the panel conducted its own systematic reviews. This may have affected the validity of the recommendations [26]. In the scope of applicability, the Canadian Guide have extensive resources for patients and users guide.
In the Eighth Report of the Joint National Committee, the points discussed were the target blood pressure after treatment, the goal in older adults and the use of drugs according to patient characteristics. To define blood pressure goals, the Eighth Report of the Joint National Committee was supported by clinical trials that met certain requirements for inclusion and exclusion [27],[28],[29],[30],[31],[32] , comparing treatment with a particular goal against placebo or no treatment; or comparing two blood pressure goals. To set a goal of systolic blood pressure in patients over 60, the Eighth Report of the Joint National Committee is based on two clinical trials, defining the J curve, where systolic blood pressure <140 mmHg provides no additional benefits compared with a target of 140 to 160 mmHg.
Regarding drugs, the Eighth Report Joint National Committee supports the choice of drugs of first and second line depending on the race of the patient and their preferences for thiazide diuretics or calcium channel blockers as first-line drugs in African-American people. Another widely discussed issue is the generalization of the risk associated with sex, not differentiating the increased risk associated with women. As a background to the Eighth Report of the Joint National Committee, the seventh report [33] based the onset of antihypertensive treatment on blood pressure level. In contrast, the European Guidelines [34] raised cardiovascular risk stratification, implying a higher cost due to the increased number of tests required, unlike the seventh report of the Joint National Committee [33].
For their part, the European Guidelines have not changed compared to its predecessors, the authors stress reducing salt intake to 2,300 mg of sodium, and the importance of ambulatory blood pressure, compared with the pressure taken in the office. This is a better predictor of outcome and should be taken for two weeks, twice a day, and consider the average of the last two of three measuring blood pressure.
The Canadian Guide restricts the combined use of inhibitors of angiotensin-converting enzyme blockers and angiotensin receptor, whatever its use (previously used as nephroprotective and combined as antihypertensive), due to increased risk of dialysis by increasing the double the serum creatinine value, compared with separate use. These warnings are based on the ONTARGET [35] study in which it was shown that telmisartan was not inferior to ramipril but the combination of both is harmful rather than beneficial.
By controlling high blood pressure, the risk of suffering a cerebral vascular accident is significantly reduced (called residual risk) but is still higher than the non-hypertensive risk. That is while the treatment lowers blood pressure, the cause of high blood pressure not always is controlled or other factors keep the risk high. You can decrease the residual risk with early-onset therapy, rapid achievement of therapeutic goals and treatment of concomitant risks.
In short, the most controversial issue for the three guidelines studied were thresholds to start blood pressure treatment. Whereas the aim of antihypertensive treatment is to reduce cardiovascular morbidity and mortality associated with elevated blood pressure and minimizing the impact of other factors or cardiovascular risk associated comorbidities; reaching the therapeutic objective requires prior risk stratification and define the blood pressure to baseline, target blood pressure and time to achieve it.
The Eighth Report of the Joint National Committee recommends (general population> 60 years) starting treatment with a systolic blood pressure of ≥150 mmHg, or diastolic blood pressure of ≥90 mmHg, and try to systolic blood pressure goals <150 mmHg and diastolic blood pressure <90 mmHg. The European Guideline postures as blood pressure goal <140/90 mmHg in patients at low or moderate risk, since patients with values> 140/90 mmHg are at increased risk of ischemic heart disease, heart failure and stroke; and goals of <130/80 mmHg in hypertensive patients at high risk (diabetes, cerebral vascular disease, cardiovascular or renal). The blood pressure goals of the European Guidelines are consistent with the issues raised by the Canadian Guide to levels of systolic <140 mmHg and diastolic blood pressure <90mmHg blood pressure. However, it believes that in patients over 80 years, the goal should be <150mmHg.
While there are differences between the guidelines evaluated, it is important to consider that before analyzing the content we should make a methodological evaluation to know the essential aspects met by the guide. Once you assessed the methodological rigor, an analysis of the content of each of the guides should be made, in order to get the best recommendations and facilitate both diagnosis and management of blood pressure. We cannot say that a guideline is better than another. However, we can say that the Canadian Guide presents a greater rigor in its preparation, which together with its annual update, you can allow better implementation in daily practice and better evaluation of the latest available research, compared with the Eighth Report the Joint National Committee and the European Guidelines. We must highlight the extensive literature review conducted by the developer of the European guideline. Despite this, for unknown reasons, they did not consider various aspects needed in a clinical practice guidelines based on scientific evidence.
In appraising the quality of the Hypertension guidelines analyzed, the Canadian is which obtained the best scores by applying the AGREE II, being recommended without modifications.
From the editor
This article was originally submitted in Spanish and was translated into English by the authors. The Journal has not copyedited this version.
Conflicts of Interests
The authors completed the conflict of interests declaration form from the ICMJE, and declared not having any conflict of interests with the matter dealt herein. Forms can be requested to the responsible author or the editorial direction of the Journal.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

The aim of this study is the methodological evaluation of Clinical Practice Guidelines (CPG) in hypertension. This is the first in a series of review articles, analysis, assessment in methodology and content of clinical practice guidelines in Cardiology. Of all clinical practice guidelines, three were selected and the Appraisal of Guidelines for Research and Evaluation (AGREE II) instrument was used to assess each guide. The guidelines obtained the lowest score in the domain of applicability (mean 43.8%); while the highest score was for clarity of presentation (mean 81.5%). The lowest percentage was found in the applicability domain (European guideline) and the highest of all scores was found in two domains: scope and purpose, and clarity of presentation (Canadian guideline). Assessing the quality of the clinical practice guidelines analyzed, the Canadian is one with the best scores obtained by applying the AGREE II instrument, and it is advised to be used without modifications.
 Autores:
Mayita Lizbeth Álvarez-Vargas[1,2], José Kelvin Galvez-Olortegui[1,2,3,4,5], Tomas Vladimir Galvez-Olortegui[1,2,6], José Manuel Sosa-Rosado[7,8], Luis Arturo Camacho-Saavedra[9,10]
Autores:
Mayita Lizbeth Álvarez-Vargas[1,2], José Kelvin Galvez-Olortegui[1,2,3,4,5], Tomas Vladimir Galvez-Olortegui[1,2,6], José Manuel Sosa-Rosado[7,8], Luis Arturo Camacho-Saavedra[9,10]

Citación: Álvarez-Vargas ML, Galvez-Olortegui JK, Galvez-Olortegui TV, Sosa-Rosado JM, Camacho-Saavedra LA. Clinical practice guidelines in hypertension: a review. Medwave 2015 Oct;15(9):e6290 doi: 10.5867/medwave.2015.09.6290
Fecha de envío: 14/8/2015
Fecha de aceptación: 11/10/2015
Fecha de publicación: 23/10/2015
Origen: no solicitado
Tipo de revisión: con revisión por dos pares revisores externos, a doble ciego

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 DiCenso A, Bayley L, Haynes RB. ACP Journal Club. Editorial: Accessing preappraised evidence: fine-tuning the 5S model into a 6S model. Ann Intern Med. 2009 Sep 15;151(6):JC3-2, JC3-3. | PubMed |
DiCenso A, Bayley L, Haynes RB. ACP Journal Club. Editorial: Accessing preappraised evidence: fine-tuning the 5S model into a 6S model. Ann Intern Med. 2009 Sep 15;151(6):JC3-2, JC3-3. | PubMed | Institute of Medicine. Clinical practice guidelines we can trust. Washington DC: The National Academies Press; 2011: 266.
Institute of Medicine. Clinical practice guidelines we can trust. Washington DC: The National Academies Press; 2011: 266.  Haynes RB, Sackett DL, Gray JM, Cook DJ, Guyatt GH. Transferring evidence from research into practice: 1. The role of clinical care research evidence in clinical decisions. ACP J Club. 1996 Nov-Dec;125(3):A14-6. | PubMed |
Haynes RB, Sackett DL, Gray JM, Cook DJ, Guyatt GH. Transferring evidence from research into practice: 1. The role of clinical care research evidence in clinical decisions. ACP J Club. 1996 Nov-Dec;125(3):A14-6. | PubMed | Pagliari C, Grimshaw J, Eccles M. The potential influence of small group processes on guideline development. J Eval Clin Pract. 2001 May;7(2):165-73. | PubMed |
Pagliari C, Grimshaw J, Eccles M. The potential influence of small group processes on guideline development. J Eval Clin Pract. 2001 May;7(2):165-73. | PubMed | Schünemann HJ, Fretheim A, Oxman AD. Improving the use of research evidence in guideline development: 13. Applicability, transferability and adaptation. Health Res Policy Syst. 2006 Dec 8;4:25. | PubMed |
Schünemann HJ, Fretheim A, Oxman AD. Improving the use of research evidence in guideline development: 13. Applicability, transferability and adaptation. Health Res Policy Syst. 2006 Dec 8;4:25. | PubMed | Chen Y, Hu S, Wu L, Fang X, Xu W, Shen G. Clinical practice guidelines for hypertension in China: a systematic review of the methodological quality. BMJ Open. 2015 Jul 15;5(7):e008099. | CrossRef | PubMed |
Chen Y, Hu S, Wu L, Fang X, Xu W, Shen G. Clinical practice guidelines for hypertension in China: a systematic review of the methodological quality. BMJ Open. 2015 Jul 15;5(7):e008099. | CrossRef | PubMed | Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004 Jun 19;328(7454):1490. | PubMed |
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004 Jun 19;328(7454):1490. | PubMed | Pla AB, Doménech CS, Baqué AD, Vilaubí JMP, Sardiña MD, Cabello MIE, et al. Revisión y valoración de 5 guías de manejo de la hipertensión arterial. Aten Primaria 2004;36(4):221-231. | Link |
Pla AB, Doménech CS, Baqué AD, Vilaubí JMP, Sardiña MD, Cabello MIE, et al. Revisión y valoración de 5 guías de manejo de la hipertensión arterial. Aten Primaria 2004;36(4):221-231. | Link | Tisdale JE. Can I get a guideline to help me interpret treatment guidelines? Can J Hosp Pharm. 2014 May;67(3):181-2. | PubMed |
Tisdale JE. Can I get a guideline to help me interpret treatment guidelines? Can J Hosp Pharm. 2014 May;67(3):181-2. | PubMed | McAlister FA, van Diepen S, Padwal RS, Johnson JA, Majumdar SR. How evidence-based are the recommendations in evidence-based guidelines? PLoS Med. 2007 Aug;4(8):e250. | PubMed |
McAlister FA, van Diepen S, Padwal RS, Johnson JA, Majumdar SR. How evidence-based are the recommendations in evidence-based guidelines? PLoS Med. 2007 Aug;4(8):e250. | PubMed | Brouwers, Melissa. Browman, GP. Burgers J. Instrumento AGREE II. Instrumento para la evaluación de Guías de práctica clínica. 2009 [on line]. | Link |
Brouwers, Melissa. Browman, GP. Burgers J. Instrumento AGREE II. Instrumento para la evaluación de Guías de práctica clínica. 2009 [on line]. | Link | Makarski J, Brouwers MC; AGREE Enterprise. The AGREE Enterprise: a decade of advancing clinical practice guidelines. Implement Sci. 2014 Aug 15;9:103. | CrossRef | PubMed |
Makarski J, Brouwers MC; AGREE Enterprise. The AGREE Enterprise: a decade of advancing clinical practice guidelines. Implement Sci. 2014 Aug 15;9:103. | CrossRef | PubMed | Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. The Global Rating Scale complements the AGREE II in advancing the quality of practice guidelines. J Clin Epidemiol. 2012 May;65(5):526-34. | CrossRef | PubMed |
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. The Global Rating Scale complements the AGREE II in advancing the quality of practice guidelines. J Clin Epidemiol. 2012 May;65(5):526-34. | CrossRef | PubMed | Brouwers MEA. Appraisal of Guidelines for Research & Evaluation II. Agree Next Steps Consort. 2009;(May):1–56. | Link |
Brouwers MEA. Appraisal of Guidelines for Research & Evaluation II. Agree Next Steps Consort. 2009;(May):1–56. | Link | Cluzeau F, Burgers J. Appraisal of Guidelines for Research & Evaluation. Evidence-Based Medicine. London; 2001.
Cluzeau F, Burgers J. Appraisal of Guidelines for Research & Evaluation. Evidence-Based Medicine. London; 2001.  Segura L, Agustí R, Ruiz E. La hipertensión arterial en el Perú según el estudio TORNASOL II. Rev Per Cardiol. 2011;37(1):19–27.
Segura L, Agustí R, Ruiz E. La hipertensión arterial en el Perú según el estudio TORNASOL II. Rev Per Cardiol. 2011;37(1):19–27.  Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015 Jan 27;131(4):e29-322. | CrossRef | PubMed |
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015 Jan 27;131(4):e29-322. | CrossRef | PubMed | Al-Ansary LA, Tricco AC, Adi Y, Bawazeer G, Perrier L, Al-Ghonaim M, et al. A systematic review of recent clinical practice guidelines on the diagnosis, assessment and management of hypertension. PLoS One. 2013;8(1):e53744. | CrossRef | PubMed |
Al-Ansary LA, Tricco AC, Adi Y, Bawazeer G, Perrier L, Al-Ghonaim M, et al. A systematic review of recent clinical practice guidelines on the diagnosis, assessment and management of hypertension. PLoS One. 2013;8(1):e53744. | CrossRef | PubMed | Ansari S, Rashidian A. Guidelines for guidelines: are they up to the task? A comparative assessment of clinical practice guideline development handbooks. PLoS One. 2012;7(11):e49864. | CrossRef | PubMed |
Ansari S, Rashidian A. Guidelines for guidelines: are they up to the task? A comparative assessment of clinical practice guideline development handbooks. PLoS One. 2012;7(11):e49864. | CrossRef | PubMed | James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014 Feb 5;311(5):507-20. | CrossRef | PubMed |
James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014 Feb 5;311(5):507-20. | CrossRef | PubMed | Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC Practice Guidelines for the Management of Arterial Hypertension. Blood Press. 2014 Feb;23(1):3-16. | CrossRef | PubMed |
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC Practice Guidelines for the Management of Arterial Hypertension. Blood Press. 2014 Feb;23(1):3-16. | CrossRef | PubMed | Daskalopoulou SS, Rabi DM, Zarnke KB, Dasgupta K, Nerenberg K, Cloutier L, et al. The 2015 Canadian Hypertension Education Program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2015 May;31(5):549-68. | CrossRef | PubMed |
Daskalopoulou SS, Rabi DM, Zarnke KB, Dasgupta K, Nerenberg K, Cloutier L, et al. The 2015 Canadian Hypertension Education Program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2015 May;31(5):549-68. | CrossRef | PubMed | Vásquez-Kunze S, Málaga G. [New guidelines for high blood pressure and dyslipidemia: beyond the controversy, are they reliable guides?]. Rev Peru Med Exp Salud Publica. 2014;31(1):143-50. | PubMed |
Vásquez-Kunze S, Málaga G. [New guidelines for high blood pressure and dyslipidemia: beyond the controversy, are they reliable guides?]. Rev Peru Med Exp Salud Publica. 2014;31(1):143-50. | PubMed | Pak KJ, Hu T, Fee C, Wang R, Smith M, Bazzano LA. Acute hypertension: a systematic review and appraisal of guidelines. Ochsner J. 2014 Winter;14(4):655-63. | PubMed |
Pak KJ, Hu T, Fee C, Wang R, Smith M, Bazzano LA. Acute hypertension: a systematic review and appraisal of guidelines. Ochsner J. 2014 Winter;14(4):655-63. | PubMed | Go AS, Bauman MA, Coleman King SM, Fonarow GC, Lawrence W, Williams KA, et al. An effective approach to high blood pressure control: a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. J Am Coll Cardiol. 2014 Apr 1;63(12):1230-8. | CrossRef | PubMed |
Go AS, Bauman MA, Coleman King SM, Fonarow GC, Lawrence W, Williams KA, et al. An effective approach to high blood pressure control: a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. J Am Coll Cardiol. 2014 Apr 1;63(12):1230-8. | CrossRef | PubMed | Persaud N, Mamdani MM. External validity: the neglected dimension in evidence ranking. J Eval Clin Pract. 2006 Aug;12(4):450-3. | PubMed |
Persaud N, Mamdani MM. External validity: the neglected dimension in evidence ranking. J Eval Clin Pract. 2006 Aug;12(4):450-3. | PubMed | Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008 May 1;358(18):1887-98. | CrossRef | PubMed |
Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008 May 1;358(18):1887-98. | CrossRef | PubMed | Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhäger WH, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur)Trial Investigators. Lancet. 1997 Sep 13;350(9080):757-64. | PubMed |
Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhäger WH, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur)Trial Investigators. Lancet. 1997 Sep 13;350(9080):757-64. | PubMed | Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991 Jun 26;265(24):3255-64. | PubMed |
Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991 Jun 26;265(24):3255-64. | PubMed | JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res. 2008 Dec;31(12):2115-27. | CrossRef | PubMed |
JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res. 2008 Dec;31(12):2115-27. | CrossRef | PubMed | Ogihara T, Saruta T, Matsuoka H, Shimamoto K, Fujita T, Shimada K, et al. valsartan in elderly isolated systolic hypertension (VALISH) study: rationale and design. Hypertens Res. 2004 Sep;27(9):657-61. | PubMed |
Ogihara T, Saruta T, Matsuoka H, Shimamoto K, Fujita T, Shimada K, et al. valsartan in elderly isolated systolic hypertension (VALISH) study: rationale and design. Hypertens Res. 2004 Sep;27(9):657-61. | PubMed | Verdecchia P, Staessen JA, Angeli F, de Simone G, Achilli A, Ganau A, et al. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet. 2009 Aug 15;374(9689):525-33. | CrossRef | PubMed |
Verdecchia P, Staessen JA, Angeli F, de Simone G, Achilli A, Ganau A, et al. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet. 2009 Aug 15;374(9689):525-33. | CrossRef | PubMed | Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003 Dec;42(6):1206-52. | PubMed |
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003 Dec;42(6):1206-52. | PubMed | European Society of Hypertension-European Society of Cardiology Guidelines Committee. 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003 Jun;21(6):1011-53. | PubMed |
European Society of Hypertension-European Society of Cardiology Guidelines Committee. 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003 Jun;21(6):1011-53. | PubMed |