 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Palabras clave: post surgical atrial fibrillation, sleep apnea, risk factors
In this chapter, we start by discussing coronary artery bypass grafting and the most common complication after surgery – post coronary artery bypass grafting atrial fibrillation (PCAF). We then discuss the major risk factors for PCAF, and subsequently conduct an in-depth discussion of obstructive sleep apnea as a risk factor. In this endeavor, we outline how obstructive sleep apnea is diagnosed, its pathophysiological relationship to PCAF, and recent clinical studies investigating the association between obstructive sleep apnea and PCAF. We conclude with prevention and treatment strategies for PCAF, and a discussion of future research recommendations.
Post coronary artery bypass grafting atrial fibrillation (PCAF)
Coronary artery bypass grafting is one of the most common surgical procedures, with more than 50 million performed annually in the United States alone [1]. Post coronary artery bypass grafting atrial fibrillation (PCAF) remains the most common complication following coronary artery bypass grafting surgery, usually estimated to affect 20-50% of patients [2],[3],[4],[5],[6],[7],[8]. This is a supraventricular tachyarrhythmia characterized by uncoordinated atrial activation leading to damage and deterioration of atrial function. PCAF is in turn associated with a host of complications, including doubling in-hospital and 6-month mortality, tripling the risk of post-operative stroke and doubling the risk of myocardial infarction. It is also associated to increased morbidity, increased admissions to intensive care, longer intensive care unit and hospital stay, increased health system costs, and substantial burden on patients and their families [2],[3],[4],[5],[6],[7],[8],[9].
Risk factors for PCAF
Several studies have investigated which factors predict PCAF occurrence. We focused on factors that are consistently found to be significant predictors, and divided these into preoperative, intraoperative, and postoperative factors. Note that we have omitted obstructive sleep apnea in this discussion as we intend to explore it in depth in subsequent sections.
Preoperative
Advanced age is consistently reported as an independent risk factor most strongly associated with PCAF. Mathew et al. demonstrated that the odds of developing PCAF increases by 75% for every 10-year increase in age (95% confidence interval: 59% to 93%), and that anyone older than 70 years is at a high risk of developing PCAF [3]. A meta-analysis by Yin et al. showed that patients with PCAF have a 5.04-point increase (95% confidence interval: 4.16 to 5.91) in the weighted mean age difference, but the result was heterogeneous [10]. Nevertheless, every study included in the meta-analysis showed a significant increase in age in patients with PCAF [10]. The meta-analysis also demonstrated hypertension and preoperative myocardial infarction as risk factors that were significantly associated with PCAF occurrence, but the results for hypertension were highly heterogeneous.
Hernandez et al. conducted a meta-analysis to investigate obesity as a risk factor for PCAF. They demonstrated that obese patients had a 12% increased odds of PCAF than non-obese patients (95% confidence interval: 4% to 21%), with moderate heterogeneity [11]. To our knowledge, no meta-analyses were conducted for any other preoperative risk factors. Nonetheless, single studies demonstrated significant associations for many other preoperative risk factors. These include male sex [12], preoperative atrial fibrillation [3],[12], preoperative arrhythmias other than atrial fibrillation [8], left atrial enlargement [13], left atrial mechanical dysfunction [14], chronic obstructive pulmonary disease [2],[3],[15], rheumatic heart disease [15], heart failure [5],[12],[16], reduced ejection fraction [4], and the preoperative use of digoxin [2],[15].
Intraoperative
Several studies have identified intraoperative risk factors for PCAF. These include longer cross-clamp times [12],[15], not cooling the atria with a topical ice slush [2], pulmonary vein venting [2],[12], prolonged ventilation [8], and the use of cardiopulmonary bypass [8]. There is conflicting evidence as to whether cardioplegia is a risk factor for PCAF development. Some studies found it to be a significant risk factor [5], but others found a non-significant association [12]. Interestingly, Almassi et al. found that warm antegrade cardioplegia and the composition of cardioplegia were significant risk factors, but cold antegrade cardioplegia and method of cardioplegia were not significant [2].
Postoperative
Postoperative factors identified as predictors of PCAF include hypokalemia [17], hypomagnesemia [18], post-operative atrial pacing [12], withdrawal of a beta-blocker [3], and withdrawal of an angiotensin-converting enzyme inhibitor [3]. Withdrawing beta-blockers after coronary artery bypass grafting surgery could precipitate PCAF for several reasons. Firstly, the use of beta-blockers after coronary artery bypass grafting surgery is a prophylactic measure against PCAF development (see the PCAF prevention section below). Hence, withdrawing beta-blockers after coronary artery bypass grafting surgery likely increases the risk of PCAF because of a loss in their protective functions. Secondly, if patients were using beta-blockers for a long time preoperatively, withdrawing them postoperatively can induce reflex tachycardia, especially after undergoing a major physiological stressor like coronary artery bypass grafting surgery. Both of these factors together likely contribute to PCAF development.
Obstructive sleep apnea as a risk factor for PCAF
Obstructive sleep apnea is a disease recently studied as a predictor of PCAF. Investigating this relationship is important as it can facilitate the optimization of PCAF management. We will begin by examining how obstructive sleep apnea is diagnosed, followed by the putative pathophysiological relationship between obstructive sleep apnea and PCAF, and subsequently discuss the evidence for the association.
Obstructive sleep apnea has a prevalence of approximately 3-7% in North America [19]. Despite this high figure, several studies suggest that obstructive sleep apnea is grossly undiagnosed [20]. It is important to screen for and diagnose obstructive sleep apnea to avoid complications and achieve better health outcomes. The Berlin Questionnaire is a screening tool used for obstructive sleep apnea, while polysomnography is the gold standard for obstructive sleep apnea diagnosis. We will discuss both the Berlin Questionnaire and polysomnography.
The Berlin Questionnaire has been validated against polysomnography, demonstrating a sensitivity of 86%, a specificity of 77%, and a positive predictive value of 89%. It consists of 10 questions on risk factors for obstructive sleep apnea divided into three categories: 1) snoring behavior and apnea (6 points), 2) wake-time sleepiness or fatigue (3 points); and 3) the presence of obesity or hypertension (2 points). Patients mark only one response per question. If two or more categories are positive (categories 1 and 2: 2+ points; category 3: 1+ points), then patients are at a high risk of having obstructive sleep apnea. Since polysomnography is expensive and cumbersome, many centers interpret individuals with a high risk of obstructive sleep apnea as having an effective diagnosis of the disease. However, it is important to note that the Berlin Questionnaire does not give a definitive diagnosis, and this can only be done with polysomnography [21].
Polysomnography is a sleep study that is used as a diagnostic tool in sleep medicine. It records biophysiological changes during sleep, including respiratory airflow, respiratory effort, and pulse oximetry. The most commonly used indicator for obstructive sleep apnea is the apnea-hypopnea index, a measure that quantifies the number of apneic or hypopneic episodes per hour of sleep. Mild, moderate, and severe obstructive sleep apnea are classified as an apnea-hypopnea index of 5-15, 16-30, and >30, respectively [22].
Pathophysiology
The exact mechanism underlying the pathophysiology of PCAF is still under investigation. However, the pathophysiological relationship between obstructive sleep apnea and atrial fibrillation has been adequately explored. Obstructive sleep apnea is characterized by repetitive upper airway occlusion during sleep, which is thought to predispose individuals to cardiovascular disease by bringing about hypoxemia, inflammation, autonomic dysfunction, and intrathoracic pressure changes [23].
Hypoxemia
The mechanism behind which hypoxemia brings about atrial fibrillation is not well understood, but is thought to be due to continuous vascular endothelial injury via oxidative stress. As a result, patients with obstructive sleep apnea enter a state of chronic inflammation, predisposing them to hypertension and atrial fibrosis [23].
Inflammation
Patients with obstructive sleep apnea generally have both local and systemic inflammation. It is posited that local inflammation of the upper respiratory tract may facilitate airway narrowing and muscle dysfunction, thus worsening the frequency and intensity of apneic and hypopneic episodes. Systemic inflammation can potentiate cardiovascular disease due to elevated levels of proinflammatory mediators [23].
Intrathoracic pressure changes
Repetitive narrowing of the upper respiratory tract can generate large pressure gradients, which can cause atrial fibrosis and atrial enlargement – both predictors of atrial fibrillation. Animal models of obstructive sleep apnea have been demonstrated to cause left atrial distension, supporting the idea that intrathoracic pressure changes can act as a substrate for atrial fibrillation [23].
Autonomic Instability
Autonomic instability is well recognized to precipitate cardiac events and disorders, including variability in heart rate, atrial fibrillation, other arrhythmias, heart failure, myocardial infarction, stroke, and even death. This suggests that autonomic instability is a strong trigger for atrial fibrillation [23].
In addition to potentially acting as independent substrates for atrial fibrillation, the aforementioned mechanisms can cause atrial remodeling, which is a risk factor for atrial fibrillation [23].
Clinical studies
Until recently, there had been a paucity of literature investigating the association between obstructive sleep apnea and PCAF. Mooe et al. [1996] were the first group to study this association, and found obstructive sleep apnea to be an independent predictor of PCAF (multivariate adjusted odds ratio [OR], 2.8; 95% confidence interval: 1.2 to 6.8) [24]. Other studies were subsequently conducted, but these were largely underpowered. Van Oosten et al. (2014) addressed this issue and demonstrated obstructive sleep apnea to be an independent predictor of PCAF. This group used the Berlin Questionnaire to classify patients into high risk and low risk of obstructive sleep apnea, and conducted polysomnography studies for a subset of their patient sample (confirmed obstructive sleep apnea). In the low risk group, 29.7% of patients developed PCAF, whereas the proportions in the high risk, high risk + confirmed apnea, and confirmed apnea groups were consistently significantly higher – 43.3%, 45.5%, and 51.4%, respectively. This demonstrated that the risk of PCAF increases as the diagnosis of obstructive sleep apnea was more certain (i.e. a higher proportion of patients with confirmed obstructive sleep apnea developed PCAF compared to those classified as high risk by the Berlin Questionnaire). The multivariate adjusted OR for the high risk + confirmed group compared to the low risk group was 2.18 (95% confidence interval: 1.30 to 3.65). This suggested that obstructive sleep apnea is a strong predictor of PCAF [25].
Qaddoura et al. conducted a meta-analysis to synthesize the evidence for the association between obstructive sleep apnea and PCAF after elective coronary artery bypass grafting surgery. Five prospective cohort studies with 642 patients were identified, some using screening questionnaires and some using polysomnography to diagnose obstructive sleep apnea. When combining all studies, they found obstructive sleep apnea to be associated with a higher odds of developing PCAF (OR, 1.86; 95% confidence interval: 1.24 to 2.80), with moderate heterogeneity present. Three subgroup analyses were also conducted. The magnitude of the association increased for data that used polysomnography to assess obstructive sleep apnea (OR 2.34; 95% confidence interval: 1.48 to 3.70). When severe obstructive sleep apnea was included from one study (OR, 2.59; 95% confidence interval: 1.63 to 4.11), and when adjusted analyses were pooled (OR 2.38, 95% confidence interval: 1.57 to 3.62), with no detected heterogeneity in any subgroup analysis. Table 1 summarizes the results of this meta-analysis. This data suggests that the risk of PCAF increases more when obstructive sleep apnea is definitively diagnosed, and potentially with increased obstructive sleep apnea severity. It also provides confidence in the validity of the results for the unadjusted associations, as they were similar to the pooled multivariate adjusted association [26]. This is important given the aforementioned multifactorial risk for PCAF.
Table 1. Summary of results for the association between obstructive sleep apnea and PCAF. [26]
Prevention and treatment of PCAF
Several studies investigated ways to both treat and prevent PCAF occurrence. As with the section on the risk factors for PCAF, only the major preventive and treatment strategies for PCAF will be discussed.
Prevention
A large meta-analysis by Arsenault et al. demonstrated that beta-blockers have the greatest magnitude in preventing PCAF (OR, 0.33; 95% confidence interval: 0.26 to 0.43), with moderate heterogeneity [27]. Solatol had a similar efficacy in preventing PCAF (OR 0.34; 95% confidence interval: 0.26 to 0.43), with much less heterogeneity [27]. Magnesium had less efficacy, with an OR of 0.55 (95% confidence interval: 0.41 to 0.73), and moderate heterogeneity [27]. A meta-analysis of 20 randomized controlled trials (RCT) by Miller et al. showed similar values for magnesium (OR 0.54; 95% confidence interval: 0.38 to 0.75), with moderate heterogeneity [28]. Amiodarone (OR 0.43; 95% confidence interval: 0.34 to 0.54), atrial pacing (OR 0.47; 95% confidence interval: 0.36 to 0.61) and posterior pericardiotomy (OR 0.35; 95% confidence interval: 0.18 to 0.67) were all found to be effective, but had a relatively high heterogeneity [27]. A meta-analysis by Wijeysundera et al. on calcium antagonists for preventing post-cardiac surgery cardiovascular complications showed that non-dihydropyridines significantly reduced supraventricular tachycardias in general (OR 0.62; 95% confidence interval: 0.41 to 0.93) with significant heterogeneity detected [29].
Other prophylactic measures reported by individual trials include polyunsaturated fats and anti-inflammatory agents. A randomized controlled trial conducted by Calò et al. demonstrated that N-3 polyunsaturated fatty acids protected against PCAF (OR 0.32; 95% confidence interval: 0.10 to 0.98) [30]. Cheruku et al. conducted a small randomized controlled trial to investigate non-steroidal anti-inflammatory medications and found a significant benefit with ibuprofen – 28.6% of patients developed PCAF in the control group versus 9.8% of patients in the ibuprofen group (p<0.017) [31]. A different RCT by Halonen et al. demonstrated that hydrocortisone significantly reduced the incidence of PCAF (adjusted hazard ratio, 0.54; 95% confidence interval: 0.35 to 0.83) [32].
The meta-analysis by Arsenault et al. also demonstrated that prophylactic interventions decreased hospital stay, the cost of treatment, and non-significantly reduced the odds of post-operative stroke [27]. This underscores the importance for taking measures to prevent PCAF development.
Treatment
The first step in PCAF treatment, as with many disorders, is to treat underlying medical comorbidities. This includes treating electrolyte abnormalities, chronic obstructive pulmonary disease, and hypoxia [33]. Further PCAF-specific treatment is indicated for patients who remain symptomatic, are hemodynamically unstable, and develop cardiac ischemia or heart failure. Four conventional treatment strategies we will discuss: rate control, rhythm control, electrical cardioversion, and preventing thromboembolism.
Rate control
Short-acting beta-blockers are the first drug of choice, especially for patients with ischemic heart disease. However, they should be used carefully where they are relatively contraindicated. Namely, this includes patients with chronic obstructive pulmonary disease, asthma, heart failure, and atrioventricular block. One alternative is to use atrioventricular nodal blocking agents, such as nondihydropyridine calcium channel blockers. Digoxin may be used in patients with heart failure, but is less effective when adrenergic tone is elevated. Finally, amiodarone has been associated with effective control of heart rate as well as improved hemodynamic status [33].
Rhythm control
Rhythm control holds several advantages, including a decreased time to cardioversion, prolonged maintenance of sinus rhythm, and decreased length of overall hospital stay. This strategy might be used over electrical cardioversion in symptomatic patients, or when ventricular response is difficult to control. Agents include amiodarone, procainamide, ibutilide, and sotalol. Salatol’s beta-blocking action has been shown to be effective at reducing the ventricular rate, but seems to be less effective than others for inducing cardioversion of atrial fibrillation [33].
Electrical cardioversion
This treatment strategy should be urgently performed if PCAF results in hemodynamic collapse, acute heart failure, or myocardial ischemia. On the other hand, it can be used electively when drug therapy fails to resume a normal sinus rhythm. Thromboembolism is a major concern for both pharmacological and electrical cardioversion, and anticoagulation for three to four weeks before cardioversion is recommended in the general population if atrial fibrillation has lasted for more than 48 hours [33].
Preventing thromboembolism
PCAF is associated with an increased risk of perioperative stroke, and this can potentially be reduced with anticoagulation. Contrastingly, anticoagulation in the post-operative period may increase the risk of bleeding or cardiac tamponade, and clinicians should weigh the often transient and self-limited duration of PCAF against the risk of anticoagulation-induced post-operative bleeding. The increased risk of bleeding may outweigh the benefits in stroke risk reduction for some patients, especially those with advanced age, uncontrolled hypertension, and a history of bleeding [33].
PCAF often resolves spontaneously within four to six weeks. Hence, as a rule, anticoagulation is initiated for prolonged (>48 hours) and/or frequent PCAF episodes [33]. Anticoagulation is especially recommended for high-risk patients, including those with a history of stroke or transient ischemic attack and PCAF. Anticoagulation therapy for these patients should be continued for another 30 days after return to a normal sinus rhythm [34].
Future recommendations
PCAF is a serious disorder with many negative sequelae. Its risk factors should be recognized to optimally manage it, including screening for and managing obstructive sleep apnea. Several strategies have been outlined to both treat and prevent PCAF, as discussed in the previous sections. One strategy not yet been explored is the use of continuous positive airway pressure in mitigating the precipitation of PCAF and its negative consequences. Continuous positive airway pressure is used to treat obstructive sleep apnea, and its use may improve outcomes for patients with PCAF especially since obstructive sleep apnea was identified to be a strong, independent predictor of PCAF. Hence, future studies should investigate the use of continuous positive airway pressure in improving PCAF outcomes.
As well, the evidence to date suggests that severe obstructive sleep apnea may increase the magnitude of the association with PCAF. Evidently, the data is not sufficient to definitively conclude that the risk of PCAF increases with obstructive sleep apnea severity, and this provides an impetus for future studies to explore this important question.
From the editor
The authors originally submitted this article in Spanish and English. The Journal has not copyedited the English version.
Conflicts of interests
The authors completed the ICMJE conflicts of interest declaration form, translated to Spanish by Medwave, and declare not having received funding for the preparation of this report, not having any financial relationships with organizations that could have interests in the published article in the last three years, and not having other relations or activities that might influence the article´s content. Forms can be requested to the responsible author or the editorial direction of the Journal.
Funding
The authors declare that there was no funding coming from external sources.

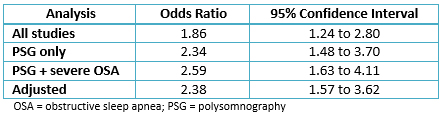 Table 1. Summary of results for the association between obstructive sleep apnea and PCAF. [26]
Table 1. Summary of results for the association between obstructive sleep apnea and PCAF. [26]
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

In this chapter, we start by discussing coronary artery bypass grafting and the most common complication after surgery – post coronary artery bypass grafting atrial fibrillation (PCAF). We then discuss the major risk factors for PCAF, and subsequently conduct an in-depth discussion of obstructive sleep apnea as a risk factor. In this endeavor, we outline how obstructive sleep apnea is diagnosed, its pathophysiological relationship to PCAF, and recent clinical studies investigating the association between obstructive sleep apnea and PCAF. We conclude with prevention and treatment strategies for PCAF, and a discussion of future research recommendations.
 Autores:
Amro Qaddoura[1], Adrian Baranchuk[1]
Autores:
Amro Qaddoura[1], Adrian Baranchuk[1]

Citación: Qaddoura A, Baranchuk A. Risk factors for post coronary artery bypass graft atrial fibrillation: role of obstructive sleep apnea. Medwave 2016;16(Suppl4).e6810 doi: 10.5867/medwave.2016.6810
Fecha de publicación: 21/12/2016
Origen: Este artículo forma parte del Suplemento 4 Especial de Cardiología cuyo editor invitado es el Dr. Alberto Morales Salinas, Cardiocentro "Ernesto Che Guevara", Villa Clara; Grupo Nacional de Cardiología, Ministerio de Salud Pública, Cuba

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 National Centre for Health Statistics. Inpatient Surgery. Centers for Disease Control and Prevention. 2014. [on line]. | Link |
National Centre for Health Statistics. Inpatient Surgery. Centers for Disease Control and Prevention. 2014. [on line]. | Link | Almassi GH, Schowalter T, Nicolosi AC, Aggarwal A, Moritz TE, Henderson WG, et al. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg. 1997 Oct;226(4):501-11; discussion 511-3. | PubMed |
Almassi GH, Schowalter T, Nicolosi AC, Aggarwal A, Moritz TE, Henderson WG, et al. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg. 1997 Oct;226(4):501-11; discussion 511-3. | PubMed | Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004 Apr 14;291(14):1720-9. | PubMed |
Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004 Apr 14;291(14):1720-9. | PubMed | Helgadottir S, Sigurdsson MI, Ingvarsdottir IL, Arnar DO, Gudbjartsson T. Atrial fibrillation following cardiac surgery: risk analysis and long-term survival. J Cardiothorac Surg. 2012 Sep 19;7:87. | CrossRef | PubMed |
Helgadottir S, Sigurdsson MI, Ingvarsdottir IL, Arnar DO, Gudbjartsson T. Atrial fibrillation following cardiac surgery: risk analysis and long-term survival. J Cardiothorac Surg. 2012 Sep 19;7:87. | CrossRef | PubMed | Shen J, Lall S, Zheng V, Buckley P, Damiano RJ Jr, Schuessler RB. The persistent problem of new-onset postoperative atrial fibrillation: a single-institution experience over two decades. J Thorac Cardiovasc Surg. 2011 Feb;141(2):559-70. | CrossRef | PubMed |
Shen J, Lall S, Zheng V, Buckley P, Damiano RJ Jr, Schuessler RB. The persistent problem of new-onset postoperative atrial fibrillation: a single-institution experience over two decades. J Thorac Cardiovasc Surg. 2011 Feb;141(2):559-70. | CrossRef | PubMed | Ahlsson A, Fengsrud E, Bodin L, Englund A. Postoperative atrial fibrillation in patients undergoing aortocoronary bypass surgery carries an eightfold risk of future atrial fibrillation and a doubled cardiovascular mortality. Eur J Cardiothorac Surg. 2010 Jun;37(6):1353-9. | CrossRef | PubMed |
Ahlsson A, Fengsrud E, Bodin L, Englund A. Postoperative atrial fibrillation in patients undergoing aortocoronary bypass surgery carries an eightfold risk of future atrial fibrillation and a doubled cardiovascular mortality. Eur J Cardiothorac Surg. 2010 Jun;37(6):1353-9. | CrossRef | PubMed | Tamis JE, Steinberg JS. Atrial fibrillation independently prolongs hospital stay after coronary artery bypass surgery. Clin Cardiol. 2000 Mar;23(3):155-9. | PubMed |
Tamis JE, Steinberg JS. Atrial fibrillation independently prolongs hospital stay after coronary artery bypass surgery. Clin Cardiol. 2000 Mar;23(3):155-9. | PubMed | Magee MJ, Herbert MA, Dewey TM, Edgerton JR, Ryan WH, Prince S, Mack MJ. Atrial fibrillation after coronary artery bypass grafting surgery: development of a predictive risk algorithm. Ann Thorac Surg. 2007 May;83(5):1707-12; discussion 1712. | PubMed |
Magee MJ, Herbert MA, Dewey TM, Edgerton JR, Ryan WH, Prince S, Mack MJ. Atrial fibrillation after coronary artery bypass grafting surgery: development of a predictive risk algorithm. Ann Thorac Surg. 2007 May;83(5):1707-12; discussion 1712. | PubMed | LaPar DJ, Speir AM, Crosby IK, Fonner E Jr, Brown M, Rich JB, e al. Postoperative atrial fibrillation significantly increases mortality, hospital readmission, and hospital costs. Ann Thorac Surg. 2014 Aug;98(2):527-33. | CrossRef | PubMed |
LaPar DJ, Speir AM, Crosby IK, Fonner E Jr, Brown M, Rich JB, e al. Postoperative atrial fibrillation significantly increases mortality, hospital readmission, and hospital costs. Ann Thorac Surg. 2014 Aug;98(2):527-33. | CrossRef | PubMed | Yin L, Wang ZN, Wang YF, Wang WT, Ji GY, Wang XW, Xu ZY. Predictors of atrial fibrillation after coronary artery bypass graft: a meta-analysis. J Geriatr Cardiol. 2009;6:162–7. | Link |
Yin L, Wang ZN, Wang YF, Wang WT, Ji GY, Wang XW, Xu ZY. Predictors of atrial fibrillation after coronary artery bypass graft: a meta-analysis. J Geriatr Cardiol. 2009;6:162–7. | Link | Hernandez AV, Kaw R, Pasupuleti V, Bina P, Ioannidis JP, Bueno H, et al. Association between obesity and postoperative atrial fibrillation in patients undergoing cardiac operations: a systematic review and meta-analysis. Ann Thorac Surg. 2013 Sep;96(3):1104-16. | CrossRef | PubMed |
Hernandez AV, Kaw R, Pasupuleti V, Bina P, Ioannidis JP, Bueno H, et al. Association between obesity and postoperative atrial fibrillation in patients undergoing cardiac operations: a systematic review and meta-analysis. Ann Thorac Surg. 2013 Sep;96(3):1104-16. | CrossRef | PubMed | Mathew JP, Parks R, Savino JS, Friedman AS, Koch C, Mangano DT, et al. Atrial fibrillation following coronary artery bypass graft surgery: predictors, outcomes, and resource utilization. MultiCenter Study of Perioperative Ischemia Research Group. JAMA. 1996 Jul 24-31;276(4):300-6. | PubMed |
Mathew JP, Parks R, Savino JS, Friedman AS, Koch C, Mangano DT, et al. Atrial fibrillation following coronary artery bypass graft surgery: predictors, outcomes, and resource utilization. MultiCenter Study of Perioperative Ischemia Research Group. JAMA. 1996 Jul 24-31;276(4):300-6. | PubMed | Osranek M, Fatema K, Qaddoura F, Al-Saileek A, Barnes ME, Bailey KR, et al. Left atrial volume predicts the risk of atrial fibrillation after cardiac surgery: a prospective study. J Am Coll Cardiol. 2006 Aug 15;48(4):779-86. | PubMed |
Osranek M, Fatema K, Qaddoura F, Al-Saileek A, Barnes ME, Bailey KR, et al. Left atrial volume predicts the risk of atrial fibrillation after cardiac surgery: a prospective study. J Am Coll Cardiol. 2006 Aug 15;48(4):779-86. | PubMed | Haffajee JA, Lee Y, Alsheikh-Ali AA, Kuvin JT, Pandian NG, Patel AR. Pre-operative left atrial mechanical function predicts risk of atrial fibrillation following cardiac surgery. JACC Cardiovasc Imaging. 2011 Aug;4(8):833-40. | CrossRef | PubMed |
Haffajee JA, Lee Y, Alsheikh-Ali AA, Kuvin JT, Pandian NG, Patel AR. Pre-operative left atrial mechanical function predicts risk of atrial fibrillation following cardiac surgery. JACC Cardiovasc Imaging. 2011 Aug;4(8):833-40. | CrossRef | PubMed | Creswell LL, Schuessler RB, Rosenbloom M, Cox JL. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993 Sep;56(3):539-49. | PubMed |
Creswell LL, Schuessler RB, Rosenbloom M, Cox JL. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993 Sep;56(3):539-49. | PubMed | Banach M, Rysz J, Drozdz JA, Okonski P, Misztal M, Barylski M, et al. Risk factors of atrial fibrillation following coronary artery bypass grafting: a preliminary report. Circ J. 2006 Apr;70(4):438-41. | PubMed |
Banach M, Rysz J, Drozdz JA, Okonski P, Misztal M, Barylski M, et al. Risk factors of atrial fibrillation following coronary artery bypass grafting: a preliminary report. Circ J. 2006 Apr;70(4):438-41. | PubMed | Wahr JA, Parks R, Boisvert D, Comunale M, Fabian J, Ramsay J, et al. Preoperative serum potassium levels and perioperative outcomes in cardiac surgery patients. Multicenter Study of Perioperative Ischemia Research Group. JAMA. 1999 Jun 16;281(23):2203-10. | PubMed |
Wahr JA, Parks R, Boisvert D, Comunale M, Fabian J, Ramsay J, et al. Preoperative serum potassium levels and perioperative outcomes in cardiac surgery patients. Multicenter Study of Perioperative Ischemia Research Group. JAMA. 1999 Jun 16;281(23):2203-10. | PubMed | England MR, Gordon G, Salem M, Chernow B. Magnesium administration and dysrhythmias after cardiac surgery. A placebo-controlled, double-blind, randomized trial. JAMA. 1992 Nov 4;268(17):2395-402. | PubMed |
England MR, Gordon G, Salem M, Chernow B. Magnesium administration and dysrhythmias after cardiac surgery. A placebo-controlled, double-blind, randomized trial. JAMA. 1992 Nov 4;268(17):2395-402. | PubMed | Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008 Feb 15;5(2):136-43. | CrossRef | PubMed |
Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008 Feb 15;5(2):136-43. | CrossRef | PubMed | Finkel KJ, Searleman AC, Tymkew H, Tanaka CY, Saager L, Safer-Zadeh E, et al. Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Med. 2009 Aug;10(7):753-8. | CrossRef | PubMed |
Finkel KJ, Searleman AC, Tymkew H, Tanaka CY, Saager L, Safer-Zadeh E, et al. Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Med. 2009 Aug;10(7):753-8. | CrossRef | PubMed | Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999 Oct 5;131(7):485-91. | PubMed |
Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999 Oct 5;131(7):485-91. | PubMed | American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999 Aug 1;22(5):667-89. | PubMed |
American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999 Aug 1;22(5):667-89. | PubMed | Drew D, Qaddoura A, Baranchuk A. The relationship between obstructive sleep apnea and atrial fibrillation in special patient populations. Expert Rev Cardiovasc Ther. 2014 Nov;12(11):1337-48. | CrossRef | PubMed |
Drew D, Qaddoura A, Baranchuk A. The relationship between obstructive sleep apnea and atrial fibrillation in special patient populations. Expert Rev Cardiovasc Ther. 2014 Nov;12(11):1337-48. | CrossRef | PubMed | Mooe T, Gullsby S, Rabben T, Eriksson P. Sleep-disordered breathing: a novel predictor of atrial fibrillation after coronary artery bypass surgery. Coron Artery Dis. 1996 Jun;7(6):475-8. | PubMed |
Mooe T, Gullsby S, Rabben T, Eriksson P. Sleep-disordered breathing: a novel predictor of atrial fibrillation after coronary artery bypass surgery. Coron Artery Dis. 1996 Jun;7(6):475-8. | PubMed | van Oosten EM, Hamilton A, Petsikas D, Payne D, Redfearn DP, Zhang S, et al. Effect of preoperative obstructive sleep apnea on the frequency of atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. 2014 Mar 15;113(6):919-23. | CrossRef | PubMed |
van Oosten EM, Hamilton A, Petsikas D, Payne D, Redfearn DP, Zhang S, et al. Effect of preoperative obstructive sleep apnea on the frequency of atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. 2014 Mar 15;113(6):919-23. | CrossRef | PubMed | Qaddoura A, Kabali C, Drew D, van Oosten EM, Michael KA, Redfearn DP, et al. Obstructive sleep apnea as a predictor of atrial fibrillation after coronary artery bypass grafting: a systematic review and meta-analysis. Can J Cardiol. 2014 Dec;30(12):1516-22. | CrossRef | PubMed |
Qaddoura A, Kabali C, Drew D, van Oosten EM, Michael KA, Redfearn DP, et al. Obstructive sleep apnea as a predictor of atrial fibrillation after coronary artery bypass grafting: a systematic review and meta-analysis. Can J Cardiol. 2014 Dec;30(12):1516-22. | CrossRef | PubMed | Arsenault KA, Yusuf AM, Crystal E, Healey JS, Morillo CA, Nair GM, et al. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst Rev. 2013 Jan 31;(1):CD003611. | CrossRef | PubMed |
Arsenault KA, Yusuf AM, Crystal E, Healey JS, Morillo CA, Nair GM, et al. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst Rev. 2013 Jan 31;(1):CD003611. | CrossRef | PubMed | Miller S, Crystal E, Garfinkle M, Lau C, Lashevsky I, Connolly SJ. Effects of magnesium on atrial fibrillation after cardiac surgery: a meta-analysis. Heart. 2005 May;91(5):618-23. | PubMed |
Miller S, Crystal E, Garfinkle M, Lau C, Lashevsky I, Connolly SJ. Effects of magnesium on atrial fibrillation after cardiac surgery: a meta-analysis. Heart. 2005 May;91(5):618-23. | PubMed | Wijeysundera DN, Beattie WS, Rao V, Karski J. Calcium antagonists reduce cardiovascular complications after cardiac surgery: a meta- analysis. J Am Coll Cardiol. 2003 May 7;41(9):1496-505. | PubMed |
Wijeysundera DN, Beattie WS, Rao V, Karski J. Calcium antagonists reduce cardiovascular complications after cardiac surgery: a meta- analysis. J Am Coll Cardiol. 2003 May 7;41(9):1496-505. | PubMed | Calò L, Bianconi L, Colivicchi F, Lamberti F, Loricchio ML, de Ruvo E, et al. N-3 Fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a randomized, controlled trial. J Am Coll Cardiol. 2005 May 17;45(10):1723-8. | PubMed |
Calò L, Bianconi L, Colivicchi F, Lamberti F, Loricchio ML, de Ruvo E, et al. N-3 Fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a randomized, controlled trial. J Am Coll Cardiol. 2005 May 17;45(10):1723-8. | PubMed | Cheruku KK, Ghani A, Ahmad F, Pappas P, Silverman PR, Zelinger A, et al. Efficacy of nonsteroidal anti-inflammatory medications for prevention of atrial fibrillation following coronary artery bypass graft surgery. Prev Cardiol. 2004 Winter;7(1):13-8. | PubMed |
Cheruku KK, Ghani A, Ahmad F, Pappas P, Silverman PR, Zelinger A, et al. Efficacy of nonsteroidal anti-inflammatory medications for prevention of atrial fibrillation following coronary artery bypass graft surgery. Prev Cardiol. 2004 Winter;7(1):13-8. | PubMed | Halonen J, Halonen P, Järvinen O, Taskinen P, Auvinen T, Tarkka M, et al. Corticosteroids for the prevention of atrial fibrillation after cardiac surgery: a randomized controlled trial. JAMA. 2007 Apr 11;297(14):1562-7. | PubMed |
Halonen J, Halonen P, Järvinen O, Taskinen P, Auvinen T, Tarkka M, et al. Corticosteroids for the prevention of atrial fibrillation after cardiac surgery: a randomized controlled trial. JAMA. 2007 Apr 11;297(14):1562-7. | PubMed | Echahidi N, Pibarot P, O'Hara G, Mathieu P. Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2008 Feb 26;51(8):793-801. | CrossRef | PubMed |
Echahidi N, Pibarot P, O'Hara G, Mathieu P. Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2008 Feb 26;51(8):793-801. | CrossRef | PubMed | Epstein AE, Alexander JC, Gutterman DD, Maisel W, Wharton JM; American College of Chest Physicians. Anticoagulation: American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest. 2005 Aug;128(2 Suppl):24S-27S. | PubMed |
Epstein AE, Alexander JC, Gutterman DD, Maisel W, Wharton JM; American College of Chest Physicians. Anticoagulation: American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest. 2005 Aug;128(2 Suppl):24S-27S. | PubMed |