 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Palabras clave: Acute mountain sickness, high altitude illness, NSAIDs, ibuprofen, acetazolamide, Epistemonikos, GRADE.
INTRODUCTION
Acute mountain sickness is a common condition occurring in healthy subjects that undergo rapid ascent without prior acclimatization, as low as 2500 meters above sea level. The classic preventive agent has been acetazolamide, although in the last decade there has been evidence favoring ibuprofen. However, it is unclear which method is more efficient.
METHODS
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis) and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified two systematic reviews that included only one primary study, which is a randomized trial. We concluded it is not possible to establish whether ibuprofen is better or worse than acetazolamide because the certainty of evidence has been evaluated as very low.
Acute mountain sickness or high altitude illness is a condition that can arise when a subject ascents to more than 2500 meters above sea level without prior acclimatization. Classically, it presents as headache plus one of the following symptoms: anorexia, nausea, insomnia, fatigue or dizziness, and can progress to more severe forms of illness such as high altitude cerebral edema or high altitude pulmonary edema [1],[2].
Acetazolamide has been the most used pharmacological prevention. It inhibits carbonic anhydrase at renal level, causing bicarbonate excretion through urine and metabolic acidosis. Its effect would offset hyperventilation and respiratory alkalosis, thus favoring the physiological response to hypoxic stimuli. However, the minimum dose (from 250 mg to 750mg per day) is still controversial.
An alternative treatment that has gained support during the last years has been ibuprofen, due to its availability and easy access [4],[5],[6]. This drug inhibits the synthesis of prostaglandin, which increases microvascular permeability at the brain as a physiopathological mechanism of acute mountain sickness. However, it is unclear which treatment is more effective.
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
|
What is the evidence. |
We found two systematic reviews [1],[2] that included one primary study [3], which is a randomized trial. |
|
What types of patients were included* |
The trial included healthy patients, between 18 to 65 years, at risk of developing acute mountain sickness; without prior acclimatization. Criteria of exclusion were use of NSAIDs or acetazolamide one to three days prior to enrollment, symptoms of acute mountain sickness or concomitant infection.[3] |
|
What types of interventions were included* |
The trial divided the participants into three groups: acetazolamide (75 mg tid), ibuprofen (600 mg tid) and placebo. All three groups had a prior three nights period of acclimatization [3]. |
|
What types of outcomes |
The trial measured multiple outcomes that were grouped in the systematic reviews as follows:
The follow-up was one day [3]. |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
The information about the effects of ibuprofen for prevention of acute mountain sickness is based on one randomized trial that included 343 subjects, from which 129 were randomized to ibuprofen, 125 to acetazolamide and 89 to placebo [3].
The summary of findings is as follows:
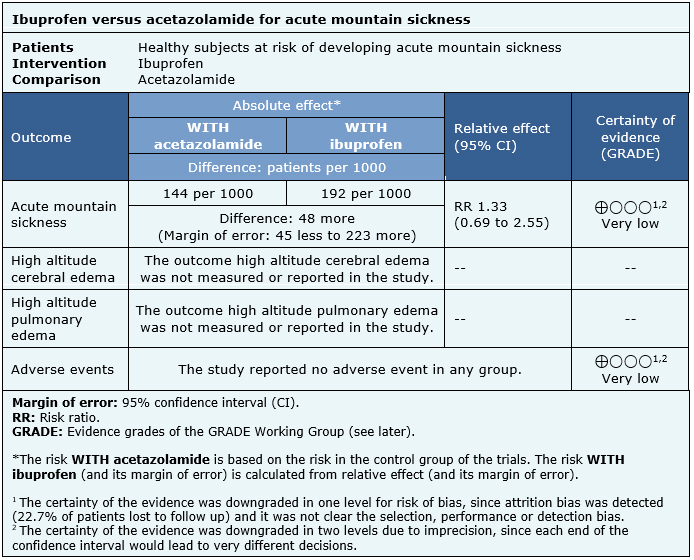
|
Follow the link to access the interactive version of this table (Interactive Summary of Findings – iSoF) |

|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.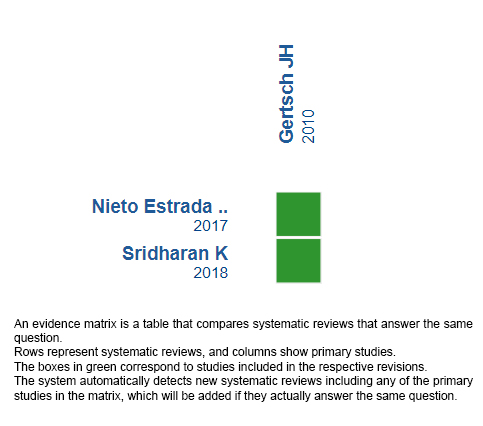
Follow the link to access the interactive version: Ibuprofen versus acetazolamide in acute mountain sickness.
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCTION
Acute mountain sickness is a common condition occurring in healthy subjects that undergo rapid ascent without prior acclimatization, as low as 2500 meters above sea level. The classic preventive agent has been acetazolamide, although in the last decade there has been evidence favoring ibuprofen. However, it is unclear which method is more efficient.
METHODS
We searched in Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis) and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified two systematic reviews that included only one primary study, which is a randomized trial. We concluded it is not possible to establish whether ibuprofen is better or worse than acetazolamide because the certainty of evidence has been evaluated as very low.
 Autores:
Maximilian Schilling[1,2], Sebastián Irarrázaval[2,3]
Autores:
Maximilian Schilling[1,2], Sebastián Irarrázaval[2,3]

Citación: Schilling M, Irarrázaval S. Ibuprofen versus acetazolamide for prevention of acute mountain sickness. Medwave 2019;20(05):e7732 doi: 10.5867/medwave.2020.05.7732
Fecha de envío: 17/10/2019
Fecha de aceptación: 21/11/2019
Fecha de publicación: 11/6/2020
Origen: Este artículo es producto del Epistemonikos Evidence Synthesis Project de la Fundación Epistemonikos, en colaboración con Medwave para su publicación.
Tipo de revisión: Con revisión por pares sin ciego por parte del equipo metodológico del Epistemonikos Evidence Synthesis Project.

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Nieto Estrada VH, Molano Franco D, Medina RD, Gonzalez Garay AG, Martí-Carvajal AJ, Arevalo-Rodriguez I. Interventions for preventing high altitude illness: Part 1. Commonly-used classes of drugs. Cochrane Database Syst Rev. 2017 Jun 27;6:CD009761. | CrossRef | PubMed | PMC |
Nieto Estrada VH, Molano Franco D, Medina RD, Gonzalez Garay AG, Martí-Carvajal AJ, Arevalo-Rodriguez I. Interventions for preventing high altitude illness: Part 1. Commonly-used classes of drugs. Cochrane Database Syst Rev. 2017 Jun 27;6:CD009761. | CrossRef | PubMed | PMC | Sridharan K, Sivaramakrishnan G. Pharmacological interventions for preventing acute mountain sickness: a network meta-analysis and trial sequential analysis of randomized clinical trials. Ann Med. 2018 Mar;50(2):147-155. | CrossRef | PubMed |
Sridharan K, Sivaramakrishnan G. Pharmacological interventions for preventing acute mountain sickness: a network meta-analysis and trial sequential analysis of randomized clinical trials. Ann Med. 2018 Mar;50(2):147-155. | CrossRef | PubMed | Gertsch JH, Lipman GS, Holck PS, Merritt A, Mulcahy A, Fisher RS, Basnyat B, Allison E, Hanzelka K, Hazan A, Meyers Z, Odegaard J, Pook B, Thompson M, Slomovic B, Wahlberg H, Wilshaw V, Weiss EA, Zafren K. Prospective, double-blind, randomized, placebo-controlled comparison of acetazolamide versus ibuprofen for prophylaxis against high altitude headache: the Headache Evaluation at Altitude Trial (HEAT). Wilderness Environ Med. 2010 Sep;21(3):236-43. | CrossRef | PubMed |
Gertsch JH, Lipman GS, Holck PS, Merritt A, Mulcahy A, Fisher RS, Basnyat B, Allison E, Hanzelka K, Hazan A, Meyers Z, Odegaard J, Pook B, Thompson M, Slomovic B, Wahlberg H, Wilshaw V, Weiss EA, Zafren K. Prospective, double-blind, randomized, placebo-controlled comparison of acetazolamide versus ibuprofen for prophylaxis against high altitude headache: the Headache Evaluation at Altitude Trial (HEAT). Wilderness Environ Med. 2010 Sep;21(3):236-43. | CrossRef | PubMed | Gertsch JH, Corbett B, Holck PS, Mulcahy A, Watts M, Stillwagon NT, Casto AM, Abramson CH, Vaughan CP, Macguire C, Farzan NN, Vo BN, Norvelle RJ, May K, Holly JE, Irons H, Stutz AM, Chapagain P, Yadav S, Pun M, Farrar J, Basnyat B. Altitude Sickness in Climbers and Efficacy of NSAIDs Trial (ASCENT): randomized, controlled trial of ibuprofen versus placebo for prevention of altitude illness. Wilderness Environ Med. 2012 Dec; 23(4):307-15. | CrossRef | PubMed |
Gertsch JH, Corbett B, Holck PS, Mulcahy A, Watts M, Stillwagon NT, Casto AM, Abramson CH, Vaughan CP, Macguire C, Farzan NN, Vo BN, Norvelle RJ, May K, Holly JE, Irons H, Stutz AM, Chapagain P, Yadav S, Pun M, Farrar J, Basnyat B. Altitude Sickness in Climbers and Efficacy of NSAIDs Trial (ASCENT): randomized, controlled trial of ibuprofen versus placebo for prevention of altitude illness. Wilderness Environ Med. 2012 Dec; 23(4):307-15. | CrossRef | PubMed | Lipman GS, Kanaan NC, Holck PS, Constance BB, Gertsch JH; PAINS Group. Ibuprofen prevents altitude illness: a randomized controlled trial for prevention of altitude illness with nonsteroidal anti-inflammatories. Ann Emerg Med. 2012 Jun;59(6):484-90. | CrossRef | PubMed |
Lipman GS, Kanaan NC, Holck PS, Constance BB, Gertsch JH; PAINS Group. Ibuprofen prevents altitude illness: a randomized controlled trial for prevention of altitude illness with nonsteroidal anti-inflammatories. Ann Emerg Med. 2012 Jun;59(6):484-90. | CrossRef | PubMed | Kanaan NC, Peterson AL, Pun M, Holck PS, Starling J, Basyal B, Freeman TF, Gehner JR, Keyes L, Levin DR, O'Leary CJ, Stuart KE, Thapa GB, Tiwari A, Velgersdyk JL, Zafren K, Basnyat B. Prophylactic Acetaminophen or Ibuprofen Results in Equivalent Acute Mountain Sickness Incidence at High Altitude: A Prospective Randomized Trial. Wilderness Environ Med. 2017 Jun;28(2):72-78. | CrossRef | PubMed |
Kanaan NC, Peterson AL, Pun M, Holck PS, Starling J, Basyal B, Freeman TF, Gehner JR, Keyes L, Levin DR, O'Leary CJ, Stuart KE, Thapa GB, Tiwari A, Velgersdyk JL, Zafren K, Basnyat B. Prophylactic Acetaminophen or Ibuprofen Results in Equivalent Acute Mountain Sickness Incidence at High Altitude: A Prospective Randomized Trial. Wilderness Environ Med. 2017 Jun;28(2):72-78. | CrossRef | PubMed | Luks AM, Auerbach PS, Freer L, Grissom CK, Keyes LE, McIntosh SE, Rodway GW, Schoene RB, Zafren K, Hackett PH. Wilderness Medical Society Practice Guidelines for the Prevention and Treatment of Acute Altitude Illness: 2019 Update. | CrossRef | PubMed |
Luks AM, Auerbach PS, Freer L, Grissom CK, Keyes LE, McIntosh SE, Rodway GW, Schoene RB, Zafren K, Hackett PH. Wilderness Medical Society Practice Guidelines for the Prevention and Treatment of Acute Altitude Illness: 2019 Update. | CrossRef | PubMed | Luks AM, McIntosh SE, Grissom CK, Auerbach PS, Rodway GW, Schoene RB, Zafren
K, Hackett PH; Wilderness Medical Society. Wilderness Medical Society practice
guidelines for the prevention and treatment of acute altitude illness: 2014 update. Wilderness Environ Med. 2014 Dec;25(4 Suppl):S4-14.
| CrossRef | PubMed |
Luks AM, McIntosh SE, Grissom CK, Auerbach PS, Rodway GW, Schoene RB, Zafren
K, Hackett PH; Wilderness Medical Society. Wilderness Medical Society practice
guidelines for the prevention and treatment of acute altitude illness: 2014 update. Wilderness Environ Med. 2014 Dec;25(4 Suppl):S4-14.
| CrossRef | PubMed |