 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
INTRODUCTION
Nausea and vomiting are common side effects in cancer patients treated with chemotherapy. Proper control of these symptoms might improve quality of life in these patients. Addition of cannabinoids to standard antiemetic treatment has been proposed in order to improve control of these symptoms.
METHODS
To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified 16 systematic reviews that include 61 primary studies. Out of these, four were randomized trials that answered our question. At present, given that the certainty of the evidence is very low, it is unclear whether the addition of cannabinoids to standard antiemetic regimes benefits patients with chemotherapy induced nausea and vomiting. Cannabinoids probably increase adverse effects substantively.
Acute and delayed nausea and vomiting are commonly associated to chemotherapy treatments. These are common side effects usually known and feared by oncological patients [1],[2]. In recent years, several new drugs have been incorporated into treatment regimes in order to prevent and manage emesis. Despite this, some patients remain unresponsive to standard management [3]. The use of cannabinoids (delta-9 tetrahydrocannabinol (THC) or its derivatives) as antiemetic has been postulated mainly based on its effects upon CB1-endocannabinoid receptors located at the central nervous system and their action over the emetic center of the brain, similar to the 5-HT3 serotonin receptors [4],[5]. However, from a clinical point of view, the role of cannabinoids upon chemotherapy-induced emesis is still controversial.
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
|
What is the evidence. |
We found 16 systematic reviews [6],[7],[8],[9],[10], This summary analyzed four trials, reported across eight references [22],[23],[24],[25],[26],[27],[28],[29], that compared the effect of cannabinoids against placebo in patients under an antiemetic regime, reporting the control of nausea and vomiting during the intervention period, which is the clinically relevant question for the authors. |
|
What types of patients were included* |
All selected trials included male and female adults. The trials also included elderly patients, up to 81 years old. Patients included in these trials had solid [22],[25], and solid or hematological tumors [23],[24]. The trials included chemotherapy regimes with a high to moderate [22], only moderate [23],[25], and not classifiable risk of emesis [24] (the latter included several chemotherapies with a high risk of emesis, but also some with low to minimum risk). Three out of four trials reported previous use of cannabinoids at any time, one of these trials included only patients with no previous exposure [24], and 2 trials included patients with or without previous exposure [22],[25]. |
|
What types of interventions were included* |
Studied cannabinoids were dronabinol (a synthetic THC derivative) with a maximum dose of 10 mg/day [22], 15 mg/day [23] or 40 mg/day [24] and nabiximol (a THC and cannabidiol extract) up to 8 sprays over a 4-hour period every 24 hours [25]. Regarding the associated antiemetic regime, two trials used corticosteroids combined with 5HT-3 antagonists [22],[23]; one trial used corticosteroids plus 5HT-3 antagonists or metoclopramide [25], and one study used prochlorperazine only [24]. |
|
What types of outcomes |
All trials evaluated the control of nausea and vomiting during the study period [22],[23],[24],[25] quantifying partial and complete responses. Three out of four of these trials [22],[24],[25] reported adverse effects. Also, some trials measured the tolerability of the intervention (measured as dropout from treatment due to adverse effects), the impact of nausea and vomiting upon quality of life, its frequency, duration and severity [22],[24],[25], percentage of patients and physicians satisfied with the intervention [25], ECOG score and quality of life [22]. Three out of four trials had a 5-day follow up [22],[23],[25] and one had a 6-day follow up [24]. |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
The information obtained about the effects of cannabinoids on nausea and vomiting induced by chemotherapy is based on four randomized trials that involved a total of 176 patients [22],[23],[24],[25]. All four trials measured control of nausea and vomiting, while three measured the occurrence of adverse events [22],[24],[25].
The summary of findings is the following:
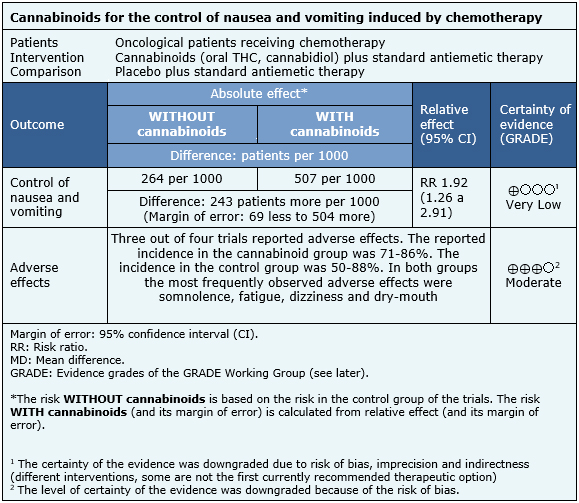
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
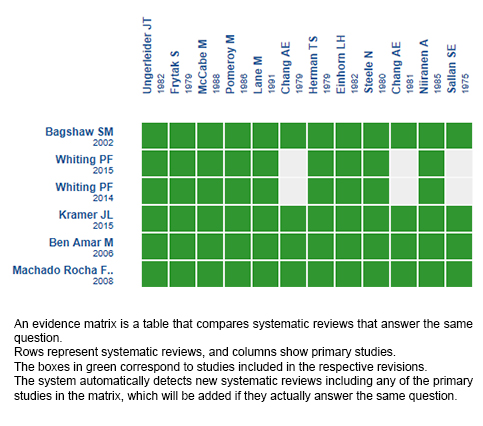
Follow the link to access the interactive version: Cannabinoids for chemotherapy induced nausea and vomiting
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCTION
Nausea and vomiting are common side effects in cancer patients treated with chemotherapy. Proper control of these symptoms might improve quality of life in these patients. Addition of cannabinoids to standard antiemetic treatment has been proposed in order to improve control of these symptoms.
METHODS
To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data of primary studies, conducted a meta-analysis and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified 16 systematic reviews that include 61 primary studies. Out of these, four were randomized trials that answered our question. At present, given that the certainty of the evidence is very low, it is unclear whether the addition of cannabinoids to standard antiemetic regimes benefits patients with chemotherapy induced nausea and vomiting. Cannabinoids probably increase adverse effects substantively.
 Autores:
Mariaignacia Morales[1,2], Oscar Corsi[2,3], José Peña[1,2,4,5]
Autores:
Mariaignacia Morales[1,2], Oscar Corsi[2,3], José Peña[1,2,4,5]

Citación: Morales M, Corsi O, Peña J. Are cannabinoids effective for the management of chemotherapy induced nausea and vomiting?. Medwave 2017 Nov-Dic;17(9):e7119 doi: 10.5867/medwave.2017.09.7119
Fecha de envío: 23/11/2017
Fecha de aceptación: 20/12/2017
Fecha de publicación: 28/12/2017
Origen: Este artículo es producto del Epistemonikos Evidence Synthesis Project de la Fundación Epistemonikos, en colaboración con Medwave para su publicación.
Tipo de revisión: Con revisión por pares sin ciego por parte del equipo metodológico del Epistemonikos Evidence Synthesis Project.

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008 Jun 5;358(23):2482-94. | CrossRef | PubMed |
Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008 Jun 5;358(23):2482-94. | CrossRef | PubMed | Schwartzberg LS. Chemotherapy-induced nausea and vomiting: clinician and patient perspectives. J Support Oncol. 2007 Feb;5(2 Suppl 1):5-12. | PubMed |
Schwartzberg LS. Chemotherapy-induced nausea and vomiting: clinician and patient perspectives. J Support Oncol. 2007 Feb;5(2 Suppl 1):5-12. | PubMed | Vanbockstael J, Coquan E, Gouerant S, Allouache D, Faveyrial A, Noal S, Delcambre C, Galais MP, Héron JF, Lefebvre AC, Sevin E, Hrab I, Polycarpe F, André M, Kaluzinski L, Gervais R, Gunzer K, Vié B, Saucier G, Lemenand N, Grellard JM, Clarisse B, Dugué AE, Joly F. How to improve the prevention of chemotherapy-induced nausea and vomiting? The French NAVI study. Support Care Cancer. 2016 Mar;24(3):1131-8. | CrossRef | PubMed |
Vanbockstael J, Coquan E, Gouerant S, Allouache D, Faveyrial A, Noal S, Delcambre C, Galais MP, Héron JF, Lefebvre AC, Sevin E, Hrab I, Polycarpe F, André M, Kaluzinski L, Gervais R, Gunzer K, Vié B, Saucier G, Lemenand N, Grellard JM, Clarisse B, Dugué AE, Joly F. How to improve the prevention of chemotherapy-induced nausea and vomiting? The French NAVI study. Support Care Cancer. 2016 Mar;24(3):1131-8. | CrossRef | PubMed | Walsh D, Nelson KA, Mahmoud FA. Established and potential therapeutic applications of cannabinoids in oncology. Support Care Cancer. 2003 Mar;11(3):137-43. | PubMed |
Walsh D, Nelson KA, Mahmoud FA. Established and potential therapeutic applications of cannabinoids in oncology. Support Care Cancer. 2003 Mar;11(3):137-43. | PubMed | Darmani NA. Delta(9)-tetrahydrocannabinol and synthetic cannabinoids prevent emesis produced by the cannabinoid CB(1) receptor antagonist/inverse agonist SR 141716A. Neuropsychopharmacology. 2001 Feb;24(2):198-203. | PubMed |
Darmani NA. Delta(9)-tetrahydrocannabinol and synthetic cannabinoids prevent emesis produced by the cannabinoid CB(1) receptor antagonist/inverse agonist SR 141716A. Neuropsychopharmacology. 2001 Feb;24(2):198-203. | PubMed | Bagshaw SM, Hagen NA. Medical efficacy of cannabinoids and marijuana: a comprehensive review of the literature. J Palliat Care. 2002 Summer;18(2):111-22. | PubMed |
Bagshaw SM, Hagen NA. Medical efficacy of cannabinoids and marijuana: a comprehensive review of the literature. J Palliat Care. 2002 Summer;18(2):111-22. | PubMed | Ben Amar M. Cannabinoids in medicine: A review of their therapeutic potential. J Ethnopharmacol. 2006 Apr 21;105(1-2):1-25. | PubMed |
Ben Amar M. Cannabinoids in medicine: A review of their therapeutic potential. J Ethnopharmacol. 2006 Apr 21;105(1-2):1-25. | PubMed | Cotter J. Efficacy of Crude Marijuana and Synthetic Delta-9-Tetrahydrocannabinol as Treatment for Chemotherapy-Induced Nausea and Vomiting: A Systematic Literature Review. Oncol Nurs Forum. 2009 May 1;36(3):345-352. | PubMed |
Cotter J. Efficacy of Crude Marijuana and Synthetic Delta-9-Tetrahydrocannabinol as Treatment for Chemotherapy-Induced Nausea and Vomiting: A Systematic Literature Review. Oncol Nurs Forum. 2009 May 1;36(3):345-352. | PubMed | Kramer JL. Medical marijuana for cancer. CA Cancer J Clin. 2015 Mar;65(2):109-22. | CrossRef | PubMed |
Kramer JL. Medical marijuana for cancer. CA Cancer J Clin. 2015 Mar;65(2):109-22. | CrossRef | PubMed | Machado Rocha FC, Stéfano SC, De Cássia Haiek R, Rosa Oliveira LM, Da Silveira DX. Therapeutic use of Cannabis sativa on chemotherapy-induced nausea and vomiting among cancer patients: systematic review and meta-analysis. Eur J Cancer Care (Engl). 2008 Sep;17(5):431-43. | CrossRef | PubMed |
Machado Rocha FC, Stéfano SC, De Cássia Haiek R, Rosa Oliveira LM, Da Silveira DX. Therapeutic use of Cannabis sativa on chemotherapy-induced nausea and vomiting among cancer patients: systematic review and meta-analysis. Eur J Cancer Care (Engl). 2008 Sep;17(5):431-43. | CrossRef | PubMed | Santana TA, Trufelli DC, Matos LL, Cruz FM, Del Giglio A. Meta-analysis of adjunctive non-NK1 receptor antagonist medications for the control of acute and delayed chemotherapy-induced nausea and vomiting. Support Care Cancer. 2015 Jan;23(1):213-22. | CrossRef | PubMed |
Santana TA, Trufelli DC, Matos LL, Cruz FM, Del Giglio A. Meta-analysis of adjunctive non-NK1 receptor antagonist medications for the control of acute and delayed chemotherapy-induced nausea and vomiting. Support Care Cancer. 2015 Jan;23(1):213-22. | CrossRef | PubMed | Smith LA, Azariah F, Lavender VT, Stoner NS, Bettiol S. Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database Syst Rev. 2015 Nov 12;(11):CD009464. | CrossRef | PubMed |
Smith LA, Azariah F, Lavender VT, Stoner NS, Bettiol S. Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database Syst Rev. 2015 Nov 12;(11):CD009464. | CrossRef | PubMed | Tramèr MR, Carroll D, Campbell FA, Reynolds DJ, Moore RA, McQuay HJ. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. BMJ. 2001 Jul 7;323(7303):16-21. | PubMed | PMC |
Tramèr MR, Carroll D, Campbell FA, Reynolds DJ, Moore RA, McQuay HJ. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. BMJ. 2001 Jul 7;323(7303):16-21. | PubMed | PMC | van den Elsen GA, Ahmed AI, Lammers M, Kramers C, Verkes RJ, van der Marck MA, Rikkert MG. Efficacy and safety of medical cannabinoids in older subjects: a systematic review. Ageing Res Rev. 2014 Mar;14:56-64. | CrossRef | PubMed |
van den Elsen GA, Ahmed AI, Lammers M, Kramers C, Verkes RJ, van der Marck MA, Rikkert MG. Efficacy and safety of medical cannabinoids in older subjects: a systematic review. Ageing Res Rev. 2014 Mar;14:56-64. | CrossRef | PubMed | Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, Keurentjes JC, Lang S, Misso K, Ryder S, Schmidlkofer S, Westwood M, Kleijnen J. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015 Jun 23-30;313(24):2456-73. Erratum in: JAMA. 2016 Apr 12;315(14):1522. JAMA. 2015 Dec 1;314(21):2308. JAMA. 2015 Aug 4;314(5):520. JAMA. 2015 Aug 25;314(8):837. | CrossRef | PubMed |
Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, Keurentjes JC, Lang S, Misso K, Ryder S, Schmidlkofer S, Westwood M, Kleijnen J. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015 Jun 23-30;313(24):2456-73. Erratum in: JAMA. 2016 Apr 12;315(14):1522. JAMA. 2015 Dec 1;314(21):2308. JAMA. 2015 Aug 4;314(5):520. JAMA. 2015 Aug 25;314(8):837. | CrossRef | PubMed | Fisher B, Johnston D, Leake P. Marijuana for medicinal purposes: an evidence-based assessment. Workers' Compensation Board – Alberta. June, 2002. | Link |
Fisher B, Johnston D, Leake P. Marijuana for medicinal purposes: an evidence-based assessment. Workers' Compensation Board – Alberta. June, 2002. | Link | Hazekamp, A , Grotenhermen, F. Review on clinical studies with cannabis and cannabinoids 2005-2009. Cannabinoids. 2010 Feb 13; 5 (special issue):1-21. | Link |
Hazekamp, A , Grotenhermen, F. Review on clinical studies with cannabis and cannabinoids 2005-2009. Cannabinoids. 2010 Feb 13; 5 (special issue):1-21. | Link | Musty R, Rossi R. Effects of Smoked Cannabis and Oral δ9-Tetrahydrocannabinol on Nausea and Emesis After Cancer Chemotherapy. Journal of Cannabis Therapeutics. 2001; 1(1): 29-42. | Link |
Musty R, Rossi R. Effects of Smoked Cannabis and Oral δ9-Tetrahydrocannabinol on Nausea and Emesis After Cancer Chemotherapy. Journal of Cannabis Therapeutics. 2001; 1(1): 29-42. | Link | Whiting P, Wolff R, Westwood M, Duffy S, Misso K, Keurentjes C, et al. Systematic Review of Cannabis for Medical Use. N.p., 2014. | Link |
Whiting P, Wolff R, Westwood M, Duffy S, Misso K, Keurentjes C, et al. Systematic Review of Cannabis for Medical Use. N.p., 2014. | Link | Kowal M, Hazekamp A, Grotenhermen F. Review on clinical studies with cannabis and cannabinoids 2010-2014. Cannabinoids. 2016. Feb; 11(special issue):1-18. | Link |
Kowal M, Hazekamp A, Grotenhermen F. Review on clinical studies with cannabis and cannabinoids 2010-2014. Cannabinoids. 2016. Feb; 11(special issue):1-18. | Link | Amato L, Davoli M, Minozzi S, Mitrova Z, Parmelli E, Saulle R. Systematic reviews on therapeutic efficacy and safety of Cannabis (including extracts and tinctures) for patients with multiple sclerosis, chronic neuropathic pain, dementia and Tourette syndrome, HIV/AIDS, and cancer receiving chemotherapy. 2016 [on line]. | Link |
Amato L, Davoli M, Minozzi S, Mitrova Z, Parmelli E, Saulle R. Systematic reviews on therapeutic efficacy and safety of Cannabis (including extracts and tinctures) for patients with multiple sclerosis, chronic neuropathic pain, dementia and Tourette syndrome, HIV/AIDS, and cancer receiving chemotherapy. 2016 [on line]. | Link | Meiri E, Jhangiani H, Vredenburgh JJ, Barbato LM, Carter FJ, Yang HM, Baranowski V. Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy-induced nausea and vomiting. Curr Med Res Opin. 2007 Mar;23(3):533-43. | PubMed |
Meiri E, Jhangiani H, Vredenburgh JJ, Barbato LM, Carter FJ, Yang HM, Baranowski V. Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy-induced nausea and vomiting. Curr Med Res Opin. 2007 Mar;23(3):533-43. | PubMed | Melhem-Bertrandt AI, MunsellMF, Fisch MJ, et al. A randomized, double-blind, placebo-controlled trial of palonosetron plus dexamethasone with or without dronabinol for the prevention of chemotherapy-induced nausea and vomiting after moderately emetogenic chemotherapy [Unpublished manuscript]. 2014:1-23.
Melhem-Bertrandt AI, MunsellMF, Fisch MJ, et al. A randomized, double-blind, placebo-controlled trial of palonosetron plus dexamethasone with or without dronabinol for the prevention of chemotherapy-induced nausea and vomiting after moderately emetogenic chemotherapy [Unpublished manuscript]. 2014:1-23.  Lane M, Smith FE, Sullivan RA, Plasse TF. Dronabinol and prochlorperazine alone and in combination as antiemetic agents for cancer chemotherapy. Am J Clin Oncol. 1990 Dec;13(6):480-4. | PubMed |
Lane M, Smith FE, Sullivan RA, Plasse TF. Dronabinol and prochlorperazine alone and in combination as antiemetic agents for cancer chemotherapy. Am J Clin Oncol. 1990 Dec;13(6):480-4. | PubMed | Duran M, Pérez E, Abanades S, Vidal X, Saura C, Majem M, Arriola E, Rabanal M, Pastor A, Farré M, Rams N, Laporte JR, Capellà D. Preliminary efficacy and safety of an oromucosal standardized cannabis extract in chemotherapy-induced nausea and vomiting. Br J Clin Pharmacol. 2010 Nov;70(5):656-63. | CrossRef | PubMed | PMC |
Duran M, Pérez E, Abanades S, Vidal X, Saura C, Majem M, Arriola E, Rabanal M, Pastor A, Farré M, Rams N, Laporte JR, Capellà D. Preliminary efficacy and safety of an oromucosal standardized cannabis extract in chemotherapy-induced nausea and vomiting. Br J Clin Pharmacol. 2010 Nov;70(5):656-63. | CrossRef | PubMed | PMC | Solvay Pharmaceuticals. Dronabinol versus standard ondansetron antiemetic therapy in preventing delayed-onset chemotherapy-induced nausea and vomiting. ClinicalTrials.gov. | Link |
Solvay Pharmaceuticals. Dronabinol versus standard ondansetron antiemetic therapy in preventing delayed-onset chemotherapy-induced nausea and vomiting. ClinicalTrials.gov. | Link | Jhangiani H, Vredenburgh JJ, Barbato L, et al. Dronabinol or ondansetron alone and combined for delayed chemotherapy-induced nausea and vomiting (CINV). Blood. 2005;106(11, part 2):477B. | Link |
Jhangiani H, Vredenburgh JJ, Barbato L, et al. Dronabinol or ondansetron alone and combined for delayed chemotherapy-induced nausea and vomiting (CINV). Blood. 2005;106(11, part 2):477B. | Link | Harden-Harrison MM, Munsell MF, Fisch MJ, et al. Dronabinol for the prevention of nausea from cyclophosphamide and/or adriamycin. Paper presented at: International MASCC/ISOO Symposium: Supportive Care in Cancer; June 28-30, 2012; New York, NY. Support Care Cancer.2012;20:S209-S210. | Link |
Harden-Harrison MM, Munsell MF, Fisch MJ, et al. Dronabinol for the prevention of nausea from cyclophosphamide and/or adriamycin. Paper presented at: International MASCC/ISOO Symposium: Supportive Care in Cancer; June 28-30, 2012; New York, NY. Support Care Cancer.2012;20:S209-S210. | Link | Lane M, Vogel CL, Ferguson J, Krasnow S, Saiers JL, Hamm J, Salva K, Wiernik PH, Holroyde CP, Hammill S, et al. Dronabinol and prochlorperazine in combination for treatment of cancer chemotherapy-induced nausea and vomiting. J Pain Symptom Manage. 1991 Aug;6(6):352-9. | PubMed |
Lane M, Vogel CL, Ferguson J, Krasnow S, Saiers JL, Hamm J, Salva K, Wiernik PH, Holroyde CP, Hammill S, et al. Dronabinol and prochlorperazine in combination for treatment of cancer chemotherapy-induced nausea and vomiting. J Pain Symptom Manage. 1991 Aug;6(6):352-9. | PubMed | Kleinman S, Weitzman SA, Cassem N, Andrews E. Double blind trial of delta-9-tetrahydrocannabinol (THC) versus placebo as an adjunct to prochlorperazine for chemotherapy induced vomiting. Current Therapeutic Research - Clinical and Experimental 1983;33(61):1014–7. | Link |
Kleinman S, Weitzman SA, Cassem N, Andrews E. Double blind trial of delta-9-tetrahydrocannabinol (THC) versus placebo as an adjunct to prochlorperazine for chemotherapy induced vomiting. Current Therapeutic Research - Clinical and Experimental 1983;33(61):1014–7. | Link | Orr LE, McKernan JF. Antiemetic effect of delta 9-tetrahydrocannabinol in chemotherapy-associated nausea and emesis as compared to placebo and compazine. J Clin Pharmacol. 1981 Aug-Sep;21(8-9 Suppl):76S-80S. | PubMed |
Orr LE, McKernan JF. Antiemetic effect of delta 9-tetrahydrocannabinol in chemotherapy-associated nausea and emesis as compared to placebo and compazine. J Clin Pharmacol. 1981 Aug-Sep;21(8-9 Suppl):76S-80S. | PubMed | Orr LE, McKernan JF, Bloome B. Antiemetic effect of tetrahydrocannabinol.Compared with placebo and prochlorperazine in chemotherapy-associated nausea and emesis. Arch Intern Med. 1980 Nov;140(11):1431-3. | PubMed |
Orr LE, McKernan JF, Bloome B. Antiemetic effect of tetrahydrocannabinol.Compared with placebo and prochlorperazine in chemotherapy-associated nausea and emesis. Arch Intern Med. 1980 Nov;140(11):1431-3. | PubMed | McCabe M, Smith FP, Goldberg D, et al. Comparative trial of oral 9 tetrahydrocannabinol and prochlorperazine for cancer chemotherapy related nausea and vomiting. Proc Am Assoc Cancer Res and Am Soc Clin Oncol. 1981; 22: 416. | Link |
McCabe M, Smith FP, Goldberg D, et al. Comparative trial of oral 9 tetrahydrocannabinol and prochlorperazine for cancer chemotherapy related nausea and vomiting. Proc Am Assoc Cancer Res and Am Soc Clin Oncol. 1981; 22: 416. | Link | McCabe M, Smith FP, Macdonald JS, Woolley PV, Goldberg D, Schein PS. Efficacy of tetrahydrocannabinol in patients refractory to standard antiemetic therapy. Invest New Drugs. 1988 Sep;6(3):243-6. | PubMed |
McCabe M, Smith FP, Macdonald JS, Woolley PV, Goldberg D, Schein PS. Efficacy of tetrahydrocannabinol in patients refractory to standard antiemetic therapy. Invest New Drugs. 1988 Sep;6(3):243-6. | PubMed | Levitt M. Nabilone vs. placebo in the treatment of chemotherapy-induced nausea and vomiting in cancer patients. Cancer Treat Rev. 1982 Dec;9 Suppl B:49-53. | PubMed |
Levitt M. Nabilone vs. placebo in the treatment of chemotherapy-induced nausea and vomiting in cancer patients. Cancer Treat Rev. 1982 Dec;9 Suppl B:49-53. | PubMed | Wada JK, Bogdon DL, Gunnell JC, Hum GJ, Gota CH, Rieth TE. Double-blind, randomized, crossover trial of nabilone vs. placebo in cancer chemotherapy. Cancer Treat Rev. 1982 Dec;9 Suppl B:39-44. | PubMed |
Wada JK, Bogdon DL, Gunnell JC, Hum GJ, Gota CH, Rieth TE. Double-blind, randomized, crossover trial of nabilone vs. placebo in cancer chemotherapy. Cancer Treat Rev. 1982 Dec;9 Suppl B:39-44. | PubMed | Johansson R, Kilkku P, Groenroos M. A double-blind, controlled trial of nabilone vs. prochlorperazine for refractory emesis induced by cancer chemotherapy. Cancer Treat Rev. 1982 Dec;9 Suppl B:25-33. | PubMed |
Johansson R, Kilkku P, Groenroos M. A double-blind, controlled trial of nabilone vs. prochlorperazine for refractory emesis induced by cancer chemotherapy. Cancer Treat Rev. 1982 Dec;9 Suppl B:25-33. | PubMed | Hutcheon AW, Palmer JB, Soukop M, Cunningham D, McArdle C, Welsh J, Stuart F, Sangster G, Kaye S, Charlton D, et al. A randomised multicentre single blind comparison of a cannabinoid anti-emetic (levonantradol) with chlorpromazine in patients receiving their first cytotoxic chemotherapy. Eur J Cancer Clin Oncol. 1983 Aug;19(8):1087-90. | PubMed |
Hutcheon AW, Palmer JB, Soukop M, Cunningham D, McArdle C, Welsh J, Stuart F, Sangster G, Kaye S, Charlton D, et al. A randomised multicentre single blind comparison of a cannabinoid anti-emetic (levonantradol) with chlorpromazine in patients receiving their first cytotoxic chemotherapy. Eur J Cancer Clin Oncol. 1983 Aug;19(8):1087-90. | PubMed | Heim ME, Queisser W, Altenburg HP. Randomized crossover study of the antiemetic activity of levonantradol and metoclopramide in cancer patients receiving chemotherapy. Cancer Chemother Pharmacol. 1984;13(2):123-5. | PubMed |
Heim ME, Queisser W, Altenburg HP. Randomized crossover study of the antiemetic activity of levonantradol and metoclopramide in cancer patients receiving chemotherapy. Cancer Chemother Pharmacol. 1984;13(2):123-5. | PubMed | Sheidler VR, Ettinger DS, Diasio RB, Enterline JP, Brown MD. Double-blind multiple-dose crossover study of the antiemetic effect of intramuscular levonantradol compared to prochlorperazine. J Clin Pharmacol. 1984 Apr;24(4):155-9. | PubMed |
Sheidler VR, Ettinger DS, Diasio RB, Enterline JP, Brown MD. Double-blind multiple-dose crossover study of the antiemetic effect of intramuscular levonantradol compared to prochlorperazine. J Clin Pharmacol. 1984 Apr;24(4):155-9. | PubMed | Niiranen A, Mattson K. A cross-over comparison of nabilone and prochlorperazine for emesis induced by cancer chemotherapy. Am J Clin Oncol. 1985 Aug;8(4):336-40. | PubMed |
Niiranen A, Mattson K. A cross-over comparison of nabilone and prochlorperazine for emesis induced by cancer chemotherapy. Am J Clin Oncol. 1985 Aug;8(4):336-40. | PubMed | Niederle N, Schütte J, Schmidt CG. Crossover comparison of the antiemetic efficacy of nabilone and alizapride in patients with nonseminomatous testicular cancer receiving cisplatin therapy. Klin Wochenschr. 1986 Apr 15;64(8):362-5. | PubMed |
Niederle N, Schütte J, Schmidt CG. Crossover comparison of the antiemetic efficacy of nabilone and alizapride in patients with nonseminomatous testicular cancer receiving cisplatin therapy. Klin Wochenschr. 1986 Apr 15;64(8):362-5. | PubMed | Chang AE, Shiling DJ, Stillman RC, Goldberg NH, Seipp CA, Barofsky I, Rosenberg SA. A prospective evaluation of delta-9-tetrahydrocannabinol as an antiemetic in patients receiving adriamycin and cytoxan chemotherapy. Cancer. 1981 Apr 1;47(7):1746-51. | PubMed |
Chang AE, Shiling DJ, Stillman RC, Goldberg NH, Seipp CA, Barofsky I, Rosenberg SA. A prospective evaluation of delta-9-tetrahydrocannabinol as an antiemetic in patients receiving adriamycin and cytoxan chemotherapy. Cancer. 1981 Apr 1;47(7):1746-51. | PubMed | Ahmedzai S, Carlyle DL, Calder IT, Moran F. Anti-emetic efficacy and toxicity of nabilone, a synthetic cannabinoid, in lung cancer chemotherapy. Br J Cancer. 1983 Nov;48(5):657-63. | PubMed | PMC |
Ahmedzai S, Carlyle DL, Calder IT, Moran F. Anti-emetic efficacy and toxicity of nabilone, a synthetic cannabinoid, in lung cancer chemotherapy. Br J Cancer. 1983 Nov;48(5):657-63. | PubMed | PMC | Sallan SE, Zinberg NE, Frei E 3rd. Antiemetic effect of delta-9-tetrahydrocannabinol in patients receiving cancer chemotherapy. N Engl J Med. 1975 Oct 16;293(16):795-7. | PubMed |
Sallan SE, Zinberg NE, Frei E 3rd. Antiemetic effect of delta-9-tetrahydrocannabinol in patients receiving cancer chemotherapy. N Engl J Med. 1975 Oct 16;293(16):795-7. | PubMed | Frytak S, Moertel CG, O'Fallon JR, Rubin J, Creagan ET, O'Connell MJ, Schutt AJ, Schwartau NW. Delta-9-tetrahydrocannabinol as an antiemetic for patients receiving cancer chemotherapy. A comparison with prochlorperazine and a placebo. Ann Intern Med. 1979 Dec;91(6):825-30. | PubMed |
Frytak S, Moertel CG, O'Fallon JR, Rubin J, Creagan ET, O'Connell MJ, Schutt AJ, Schwartau NW. Delta-9-tetrahydrocannabinol as an antiemetic for patients receiving cancer chemotherapy. A comparison with prochlorperazine and a placebo. Ann Intern Med. 1979 Dec;91(6):825-30. | PubMed | Frytak S, Moertel CG, Ofallon JR. Comparison of delta-9-tetrahydrocannabinol (THC), prochlorperazine (PCP) and placebo as anti-emetics for cancer-chemotherapy. Proc Am Assoc Cancer Res. 1979;20:391. | Link |
Frytak S, Moertel CG, Ofallon JR. Comparison of delta-9-tetrahydrocannabinol (THC), prochlorperazine (PCP) and placebo as anti-emetics for cancer-chemotherapy. Proc Am Assoc Cancer Res. 1979;20:391. | Link | Department of Social Oncology, Evaluation Unit. 1982. State of Michigan, Marihuana Therapeutic Research Project. | Link |
Department of Social Oncology, Evaluation Unit. 1982. State of Michigan, Marihuana Therapeutic Research Project. | Link | Chang AE, Shiling DJ, Stillman RC, Goldberg NH, Seipp CA, Barofsky I, Simon RM, Rosenberg SA. Delata-9-tetrahydrocannabinol as an antiemetic in cancer patients receiving high-dose methotrexate. A prospective, randomized evaluation. Ann Intern Med. 1979 Dec;91(6):819-24. | PubMed |
Chang AE, Shiling DJ, Stillman RC, Goldberg NH, Seipp CA, Barofsky I, Simon RM, Rosenberg SA. Delata-9-tetrahydrocannabinol as an antiemetic in cancer patients receiving high-dose methotrexate. A prospective, randomized evaluation. Ann Intern Med. 1979 Dec;91(6):819-24. | PubMed | Herman TS, Einhorn LH, Jones SE, Nagy C, Chester AB, Dean JC, Furnas B, Williams SD, Leigh SA, Dorr RT, Moon TE. Superiority of nabilone over prochlorperazine as an antiemetic in patients receiving cancer chemotherapy. N Engl J Med. 1979 Jun 7;300(23):1295-7. | PubMed |
Herman TS, Einhorn LH, Jones SE, Nagy C, Chester AB, Dean JC, Furnas B, Williams SD, Leigh SA, Dorr RT, Moon TE. Superiority of nabilone over prochlorperazine as an antiemetic in patients receiving cancer chemotherapy. N Engl J Med. 1979 Jun 7;300(23):1295-7. | PubMed | Niiranen A, Mattson K. Antiemetic efficacy of nabilone and dexamethasone: a randomized study of patients with lung cancer receiving chemotherapy. Am J Clin Oncol. 1987 Aug;10(4):325-9. | PubMed |
Niiranen A, Mattson K. Antiemetic efficacy of nabilone and dexamethasone: a randomized study of patients with lung cancer receiving chemotherapy. Am J Clin Oncol. 1987 Aug;10(4):325-9. | PubMed | Sallan SE, Cronin C, Zelen M, Zinberg NE. Antiemetics in patients receiving chemotherapy for cancer: a randomized comparison of delta-9-tetrahydrocannabinol and prochlorperazine. N Engl J Med. 1980 Jan 17;302(3):135-8. | PubMed |
Sallan SE, Cronin C, Zelen M, Zinberg NE. Antiemetics in patients receiving chemotherapy for cancer: a randomized comparison of delta-9-tetrahydrocannabinol and prochlorperazine. N Engl J Med. 1980 Jan 17;302(3):135-8. | PubMed | Einhorn LH, Nagy C, Furnas B, Williams SD. Nabilone: an effective antiemetic in patients receiving cancer chemotherapy. J Clin Pharmacol. 1981 Aug-Sep;21(8-9 Suppl):64S-69S. | PubMed |
Einhorn LH, Nagy C, Furnas B, Williams SD. Nabilone: an effective antiemetic in patients receiving cancer chemotherapy. J Clin Pharmacol. 1981 Aug-Sep;21(8-9 Suppl):64S-69S. | PubMed | Citron ML, Herman TS, Vreeland F, Krasnow SH, Fossieck BE Jr, Harwood S, Franklin R, Cohen MH. Antiemetic efficacy of levonantradol compared to delta-9-tetrahydrocannabinol for chemotherapy-induced nausea and vomiting. Cancer Treat Rep. 1985 Jan;69(1):109-12. | PubMed |
Citron ML, Herman TS, Vreeland F, Krasnow SH, Fossieck BE Jr, Harwood S, Franklin R, Cohen MH. Antiemetic efficacy of levonantradol compared to delta-9-tetrahydrocannabinol for chemotherapy-induced nausea and vomiting. Cancer Treat Rep. 1985 Jan;69(1):109-12. | PubMed | Cunningham D, Forrest GJ, Soukop M, Gilchrist NL, Calder IT, McArdle CS. Nabilone and prochlorperazine: a useful combination for emesis induced by cytotoxic drugs. Br Med J (Clin Res Ed). 1985 Sep 28;291(6499):864-5. | PubMed | PMC |
Cunningham D, Forrest GJ, Soukop M, Gilchrist NL, Calder IT, McArdle CS. Nabilone and prochlorperazine: a useful combination for emesis induced by cytotoxic drugs. Br Med J (Clin Res Ed). 1985 Sep 28;291(6499):864-5. | PubMed | PMC | Stambaugh JE Jr, McAdams J, Vreeland F. Dose ranging evaluation of the antiemetic efficacy and toxicity of intramuscular levonantradol in cancer subjects with chemotherapy-induced emesis. J Clin Pharmacol. 1984 Nov-Dec;24(11-12):480-5. | PubMed |
Stambaugh JE Jr, McAdams J, Vreeland F. Dose ranging evaluation of the antiemetic efficacy and toxicity of intramuscular levonantradol in cancer subjects with chemotherapy-induced emesis. J Clin Pharmacol. 1984 Nov-Dec;24(11-12):480-5. | PubMed | Crawford SM, Buckman R. Nabilone and metoclopramide in the treatment of nausea and vomiting due to cisplatinum: a double blind study. Med Oncol Tumor Pharmacother. 1986;3(1):39-42. | PubMed |
Crawford SM, Buckman R. Nabilone and metoclopramide in the treatment of nausea and vomiting due to cisplatinum: a double blind study. Med Oncol Tumor Pharmacother. 1986;3(1):39-42. | PubMed | Pomeroy M, Fennelly JJ, Towers M. Prospective randomized double-blind trial of nabilone versus domperidone in the treatment of cytotoxic-induced emesis. Cancer Chemother Pharmacol. 1986;17(3):285-8. | PubMed |
Pomeroy M, Fennelly JJ, Towers M. Prospective randomized double-blind trial of nabilone versus domperidone in the treatment of cytotoxic-induced emesis. Cancer Chemother Pharmacol. 1986;17(3):285-8. | PubMed | Gralla RJ, Tyson LB, Bordin LA, Clark RA, Kelsen DP, Kris MG, Kalman LB, Groshen S. Antiemetic therapy: a review of recent studies and a report of a random assignment trial comparing metoclopramide with delta-9-tetrahydrocannabinol. Cancer Treat Rep. 1984 Jan;68(1):163-72. | PubMed |
Gralla RJ, Tyson LB, Bordin LA, Clark RA, Kelsen DP, Kris MG, Kalman LB, Groshen S. Antiemetic therapy: a review of recent studies and a report of a random assignment trial comparing metoclopramide with delta-9-tetrahydrocannabinol. Cancer Treat Rep. 1984 Jan;68(1):163-72. | PubMed | Gralla R, Tyson L, Clark R, Bordin L, Kelsen D, Kalman L. Antiemetic trials with high dose metoclopramide: superiority over THC, and preservation of efficacy in subsequent chemotherapy courses. Proc Am Assoc Cancer Res. 1982; 1: 58. | Link |
Gralla R, Tyson L, Clark R, Bordin L, Kelsen D, Kalman L. Antiemetic trials with high dose metoclopramide: superiority over THC, and preservation of efficacy in subsequent chemotherapy courses. Proc Am Assoc Cancer Res. 1982; 1: 58. | Link | Ungerleider JT, Andrysiak T, Fairbanks L, Goodnight J, Sarna G, Jamison K. Cannabis and cancer chemotherapy: a comparison of oral delta-9-THC and prochlorperazine. Cancer. 1982 Aug 15;50(4):636-45. | PubMed |
Ungerleider JT, Andrysiak T, Fairbanks L, Goodnight J, Sarna G, Jamison K. Cannabis and cancer chemotherapy: a comparison of oral delta-9-THC and prochlorperazine. Cancer. 1982 Aug 15;50(4):636-45. | PubMed | Neidhart JA, Gagen MM, Wilson HE, Young DC. Comparative trial of the antiemetic effects of THC and haloperidol. J Clin Pharmacol. 1981 Aug-Sep;21(8-9 Suppl):38S-42S. | PubMed |
Neidhart JA, Gagen MM, Wilson HE, Young DC. Comparative trial of the antiemetic effects of THC and haloperidol. J Clin Pharmacol. 1981 Aug-Sep;21(8-9 Suppl):38S-42S. | PubMed | George M, Pejovic MH, Thuaire M, Kramar A, Wolff JP. [Randomized comparative trial of a new anti-emetic: nabilone, in cancer patients treated with cisplatin]. Biomed Pharmacother. 1983;37(1):24-7. | PubMed |
George M, Pejovic MH, Thuaire M, Kramar A, Wolff JP. [Randomized comparative trial of a new anti-emetic: nabilone, in cancer patients treated with cisplatin]. Biomed Pharmacother. 1983;37(1):24-7. | PubMed | Levitt M, Faiman C, Hawks R, Wilson A. Randomized double blind comparison of delta-9-tetrahydrocannabinol and marijuana as chemotherapy antiemetics [abstract]. Proceedings - Annual Meeting of American Society of Clinical Oncology 1984; Vol. 3:91, Abstract C-354. | Link |
Levitt M, Faiman C, Hawks R, Wilson A. Randomized double blind comparison of delta-9-tetrahydrocannabinol and marijuana as chemotherapy antiemetics [abstract]. Proceedings - Annual Meeting of American Society of Clinical Oncology 1984; Vol. 3:91, Abstract C-354. | Link | Kluin-Neleman JC, Neleman FA, Meuwissen OJ, Maes RA. delta 9-Tetrahydrocannabinol (THC) as an antiemetic in patients treated with cancerchemotherapy; a double-blind cross-over trial against placebo. Vet Hum Toxicol. 1979 Oct;21(5):338-40. | PubMed |
Kluin-Neleman JC, Neleman FA, Meuwissen OJ, Maes RA. delta 9-Tetrahydrocannabinol (THC) as an antiemetic in patients treated with cancerchemotherapy; a double-blind cross-over trial against placebo. Vet Hum Toxicol. 1979 Oct;21(5):338-40. | PubMed | Ekert H, Waters KD, Jurk IH, Mobilia J, Loughnan P. Amelioration of cancer chemotherapy-induced nausea and vomiting by delta-9-tetrahydrocannabinol. Med J Aust. 1979 Dec 15;2(12):657-9. | PubMed |
Ekert H, Waters KD, Jurk IH, Mobilia J, Loughnan P. Amelioration of cancer chemotherapy-induced nausea and vomiting by delta-9-tetrahydrocannabinol. Med J Aust. 1979 Dec 15;2(12):657-9. | PubMed | Steele N, Gralla RJ, Braun DW Jr, Young CW. Double-blind comparison of the antiemetic effects of nabilone and prochlorperazine on chemotherapy-induced emesis. Cancer Treat Rep. 1980 Feb-Mar;64(2-3):219-24. | PubMed |
Steele N, Gralla RJ, Braun DW Jr, Young CW. Double-blind comparison of the antiemetic effects of nabilone and prochlorperazine on chemotherapy-induced emesis. Cancer Treat Rep. 1980 Feb-Mar;64(2-3):219-24. | PubMed | Colls BM, Ferry DG, Gray AJ, Harvey VJ, McQueen EG. The antiemetic activity of tetrahydrocannabinol versus metoclopramide and thiethylperazine in patients undergoing cancer chemotherapy. N Z Med J. 1980 Jun 25;91(662):449-51. | PubMed |
Colls BM, Ferry DG, Gray AJ, Harvey VJ, McQueen EG. The antiemetic activity of tetrahydrocannabinol versus metoclopramide and thiethylperazine in patients undergoing cancer chemotherapy. N Z Med J. 1980 Jun 25;91(662):449-51. | PubMed | Grunberg SM, MunsellMF, Morrow PKH, et al. Randomized double-blind evaluation of dronabinol for the prevention of chemotherapy-induced nausea. Paper presented at: Annual Meeting of the American Society of Clinical Oncology (ASCO); 1-5 Jun 2012; Chicago, IL. J Clin Oncol.2012;30(15)(suppl 1):9061. | Link |
Grunberg SM, MunsellMF, Morrow PKH, et al. Randomized double-blind evaluation of dronabinol for the prevention of chemotherapy-induced nausea. Paper presented at: Annual Meeting of the American Society of Clinical Oncology (ASCO); 1-5 Jun 2012; Chicago, IL. J Clin Oncol.2012;30(15)(suppl 1):9061. | Link | Behavioral Health Sciences Division. 1984. The Lynn Pierson Therapeutic Research Program. Health and Environment Department: New Mexico. | Link |
Behavioral Health Sciences Division. 1984. The Lynn Pierson Therapeutic Research Program. Health and Environment Department: New Mexico. | Link | Kutner, MH. 1983. Evaluation of the use of both marijuana and THC in cancer patients for the relief of nausea and vomiting associated with cancer chemotherapy after failure of conventional anti-emetic therapy: Efficacy and toxicity, as prepared for the Composite State Board of Medical Examiners, Georgia Department of Health, by physicians and researchers at Emory University, Atlanta. | Link |
Kutner, MH. 1983. Evaluation of the use of both marijuana and THC in cancer patients for the relief of nausea and vomiting associated with cancer chemotherapy after failure of conventional anti-emetic therapy: Efficacy and toxicity, as prepared for the Composite State Board of Medical Examiners, Georgia Department of Health, by physicians and researchers at Emory University, Atlanta. | Link | Jones SE, Durant JR, Greco FA, Robertone A. A multi-institutional Phase III study of nabilone vs. placebo in chemotherapy-induced nausea and vomiting. Cancer Treat Rev. 1982 Dec;9 Suppl B:45-8. | PubMed |
Jones SE, Durant JR, Greco FA, Robertone A. A multi-institutional Phase III study of nabilone vs. placebo in chemotherapy-induced nausea and vomiting. Cancer Treat Rev. 1982 Dec;9 Suppl B:45-8. | PubMed | Levitt M, Wilson A, Bowman D, Kemel S, Krepart G, Marks V, Schipper H, Thomson G, Weinerman B, Weinerman R. Physiologic observations in a controlled clinical trial of the antiemetic effectiveness of 5, 10, and 15 mg of delta 9-tetrahydrocannabinol in cancer chemotherapy. Ophthalmologic implications. J Clin Pharmacol. 1981 Aug-Sep;21(8-9 Suppl):103S-109S. | PubMed |
Levitt M, Wilson A, Bowman D, Kemel S, Krepart G, Marks V, Schipper H, Thomson G, Weinerman B, Weinerman R. Physiologic observations in a controlled clinical trial of the antiemetic effectiveness of 5, 10, and 15 mg of delta 9-tetrahydrocannabinol in cancer chemotherapy. Ophthalmologic implications. J Clin Pharmacol. 1981 Aug-Sep;21(8-9 Suppl):103S-109S. | PubMed | Garb S, Beers A, Bograd M, McMahon R, Mangalik A, Ashmann A, Levine S. Two-pronged study of tetrahydrocannabinol (THC) prevention of vomiting from cancer chemotherapy. IRCS Med Sci 1980; 8: 203-204. | Link |
Garb S, Beers A, Bograd M, McMahon R, Mangalik A, Ashmann A, Levine S. Two-pronged study of tetrahydrocannabinol (THC) prevention of vomiting from cancer chemotherapy. IRCS Med Sci 1980; 8: 203-204. | Link | Behavioral Health Sciences Division. 1983. The Lynn Pierson Therapeutic Research Program. Health and Environment Department: New Mexico. | Link |
Behavioral Health Sciences Division. 1983. The Lynn Pierson Therapeutic Research Program. Health and Environment Department: New Mexico. | Link | Cunningham D, Bradley CJ, Forrest GJ, Hutcheon AW, Adams L, Sneddon M, Harding M, Kerr DJ, Soukop M, Kaye SB. A randomized trial of oral nabilone and prochlorperazine compared to intravenous metoclopramide and dexamethasone in the treatment of nausea and vomiting induced by chemotherapy regimens containing cisplatin or cisplatin analogues. Eur J Cancer Clin Oncol. 1988 Apr;24(4):685-9. | PubMed |
Cunningham D, Bradley CJ, Forrest GJ, Hutcheon AW, Adams L, Sneddon M, Harding M, Kerr DJ, Soukop M, Kaye SB. A randomized trial of oral nabilone and prochlorperazine compared to intravenous metoclopramide and dexamethasone in the treatment of nausea and vomiting induced by chemotherapy regimens containing cisplatin or cisplatin analogues. Eur J Cancer Clin Oncol. 1988 Apr;24(4):685-9. | PubMed | Broder LE, Lean NL, Hilsenbeck SG. A randomized blinded clinical trial comparing delta-9-tetrahydrocannabinol (THC) and hydroxizine (HZ) as antiemetics (AE) for cancer chemotherapy (CT). Proc Am Assoc Cancer Res. 1982;23:514. | Link |
Broder LE, Lean NL, Hilsenbeck SG. A randomized blinded clinical trial comparing delta-9-tetrahydrocannabinol (THC) and hydroxizine (HZ) as antiemetics (AE) for cancer chemotherapy (CT). Proc Am Assoc Cancer Res. 1982;23:514. | Link | Long A,Mioduszewski J, Natale R. A randomized double-blind cross-over comparison of the antiemetic activity of levonantradol and prochlorperazine. Proc Am Soc Clin Oncol. 1982;1: C-220. | Link |
Long A,Mioduszewski J, Natale R. A randomized double-blind cross-over comparison of the antiemetic activity of levonantradol and prochlorperazine. Proc Am Soc Clin Oncol. 1982;1: C-220. | Link | Board of Pharmacy, State of Tennessee. 1983. Annual Report: Evaluation of marijuana and tetrahydrocannabinol in the treatment of nausea and/or vomiting associated with cancer therapy unresponsive to conventional anti-emetic therapy: Efficacy and toxicity. | Link |
Board of Pharmacy, State of Tennessee. 1983. Annual Report: Evaluation of marijuana and tetrahydrocannabinol in the treatment of nausea and/or vomiting associated with cancer therapy unresponsive to conventional anti-emetic therapy: Efficacy and toxicity. | Link | Research Advisory Panel. 1989. Cannabis Therapeutic Research Program. Report to the California Legislature. | Link |
Research Advisory Panel. 1989. Cannabis Therapeutic Research Program. Report to the California Legislature. | Link | Cronin CM, Sallan SE, Gelber R, Lucas VS, Laszlo J. Antiemetic effect of intramuscular levonantradol in patients receiving anticancer chemotherapy. J Clin Pharmacol. 1981 Aug-Sep;21(8-9 Suppl):43S-50S. | PubMed |
Cronin CM, Sallan SE, Gelber R, Lucas VS, Laszlo J. Antiemetic effect of intramuscular levonantradol in patients receiving anticancer chemotherapy. J Clin Pharmacol. 1981 Aug-Sep;21(8-9 Suppl):43S-50S. | PubMed | Heim ME, Romer W, Queisser W. Clinical experience with levonantradol hydrochloride in the prevention of cancer chemotherapy-induced nausea and vomiting. J Clin Pharmacol. 1981 Aug-Sep;21(8-9 Suppl):86S-89S. | PubMed |
Heim ME, Romer W, Queisser W. Clinical experience with levonantradol hydrochloride in the prevention of cancer chemotherapy-induced nausea and vomiting. J Clin Pharmacol. 1981 Aug-Sep;21(8-9 Suppl):86S-89S. | PubMed | Lucas VS Jr, Laszlo J. delta 9-Tetrahydrocannabinol for refractory vomiting induced by cancer chemotherapy. JAMA. 1980 Mar 28;243(12):1241-3. | PubMed |
Lucas VS Jr, Laszlo J. delta 9-Tetrahydrocannabinol for refractory vomiting induced by cancer chemotherapy. JAMA. 1980 Mar 28;243(12):1241-3. | PubMed | Randall RC. 1990. Cancer Treatment & Marijuana Therapy. Washington DC: Galen Press, 1990. 225-34. | Link |
Randall RC. 1990. Cancer Treatment & Marijuana Therapy. Washington DC: Galen Press, 1990. 225-34. | Link | Diasio RB, Ettinger DS, Satterwhite BE. Oral levonantradol in the treatment of chemotherapy-induced emesis: preliminary observations. J Clin Pharmacol. 1981 Aug-Sep;21(8-9 Suppl):81S-85S. | PubMed |
Diasio RB, Ettinger DS, Satterwhite BE. Oral levonantradol in the treatment of chemotherapy-induced emesis: preliminary observations. J Clin Pharmacol. 1981 Aug-Sep;21(8-9 Suppl):81S-85S. | PubMed | Staquet M, Bron D, Rozencweig M, Kenis Y. Clinical studies with a THC analog (BRL-4664) in the prevention of cisplatin-induced vomiting. J Clin Pharmacol. 1981 Aug-Sep;21(8-9 Suppl):60S-63S. | PubMed |
Staquet M, Bron D, Rozencweig M, Kenis Y. Clinical studies with a THC analog (BRL-4664) in the prevention of cisplatin-induced vomiting. J Clin Pharmacol. 1981 Aug-Sep;21(8-9 Suppl):60S-63S. | PubMed | Laszlo J, Lucas VS Jr, Hanson DC, Cronin CM, Sallan SE. Levonantradol for chemotherapy-induced emesis: phase I-II oral administration. J Clin Pharmacol. 1981 Aug-Sep;21(8-9 Suppl):51S-56S. | PubMed |
Laszlo J, Lucas VS Jr, Hanson DC, Cronin CM, Sallan SE. Levonantradol for chemotherapy-induced emesis: phase I-II oral administration. J Clin Pharmacol. 1981 Aug-Sep;21(8-9 Suppl):51S-56S. | PubMed | Sweet DL, Miller NJ, Weddington W, Senay E, Sushelsky L. delta 9-Tetrahydrocannabinol as an antiemetic for patients receiving cancer chemotherapy. A pilot study. J Clin Pharmacol. 1981 Aug-Sep;21(8-9 Suppl):70S-75S. | PubMed |
Sweet DL, Miller NJ, Weddington W, Senay E, Sushelsky L. delta 9-Tetrahydrocannabinol as an antiemetic for patients receiving cancer chemotherapy. A pilot study. J Clin Pharmacol. 1981 Aug-Sep;21(8-9 Suppl):70S-75S. | PubMed | Vinciguerra V, Moore T, Brennan E. Inhalation marijuana as an antiemetic for cancer chemotherapy. N Y State J Med. 1988 Oct;88(10):525-7. | PubMed |
Vinciguerra V, Moore T, Brennan E. Inhalation marijuana as an antiemetic for cancer chemotherapy. N Y State J Med. 1988 Oct;88(10):525-7. | PubMed | Hesketh PJ, Gralla RJ, du Bois A, Tonato M. Methodology of antiemetic trials: response assessment, evaluation of new agents and definition of chemotherapy emetogenicity. Support Care Cancer. 1998 May;6(3):221-7. | PubMed |
Hesketh PJ, Gralla RJ, du Bois A, Tonato M. Methodology of antiemetic trials: response assessment, evaluation of new agents and definition of chemotherapy emetogenicity. Support Care Cancer. 1998 May;6(3):221-7. | PubMed | National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Antiemesis 2.2016 [cited Jan 17, 2017] | Link |
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Antiemesis 2.2016 [cited Jan 17, 2017] | Link | Roila F, Molassiotis A, Herrstedt J, Aapro M, Gralla RJ, Bruera E, Clark-Snow RA, Dupuis LL, Einhorn LH, Feyer P, Hesketh PJ, Jordan K, Olver I, Rapoport BL, Roscoe J, Ruhlmann CH, Walsh D, Warr D, van der Wetering M; participants of the MASCC/ESMO Consensus Conference Copenhagen 2015. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016 Sep;27(suppl 5):v119-v133. | PubMed |
Roila F, Molassiotis A, Herrstedt J, Aapro M, Gralla RJ, Bruera E, Clark-Snow RA, Dupuis LL, Einhorn LH, Feyer P, Hesketh PJ, Jordan K, Olver I, Rapoport BL, Roscoe J, Ruhlmann CH, Walsh D, Warr D, van der Wetering M; participants of the MASCC/ESMO Consensus Conference Copenhagen 2015. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016 Sep;27(suppl 5):v119-v133. | PubMed | Hesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA, Danso MA, Dennis K, Dupuis LL, Dusetzina SB, Eng C, Feyer PC, Jordan K, Noonan K, Sparacio D, Somerfield MR, Lyman GH. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017 Jul 31:JCO2017744789. | CrossRef | PubMed |
Hesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA, Danso MA, Dennis K, Dupuis LL, Dusetzina SB, Eng C, Feyer PC, Jordan K, Noonan K, Sparacio D, Somerfield MR, Lyman GH. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017 Jul 31:JCO2017744789. | CrossRef | PubMed | Grimison P. CannabisCINV: A placebo-controlled trial evaluating an oral THC/CBD cannabis extract for secondary prevention of chemotherapy-induced nausea and vomiting in patients of any known malignancy receiving chemotherapy: ACTRN12616001036404. | Link |
Grimison P. CannabisCINV: A placebo-controlled trial evaluating an oral THC/CBD cannabis extract for secondary prevention of chemotherapy-induced nausea and vomiting in patients of any known malignancy receiving chemotherapy: ACTRN12616001036404. | Link | Vitetta L. An evaluative study on the safety and efficacy of standard anti-emetics compared to standard anti-emetics plus natural cannabinoids extract for the treatment of chemotherapy induced nausea and vomiting: ACTRN12615000414516. | Link |
Vitetta L. An evaluative study on the safety and efficacy of standard anti-emetics compared to standard anti-emetics plus natural cannabinoids extract for the treatment of chemotherapy induced nausea and vomiting: ACTRN12615000414516. | Link | Fundació Institut Català de Farmacologia. Randomized, double blind, parallel groups, placebo controlled pivotal clinical trial to asses preliminary efficacy and security of a sublingual Cannabis Standardized Extract (Sativex) added to reference treatment for prevention and treatment of nausea and late vomiting induced by moderately emetogenic chemotherapy. -SATEME-08: EUCTR2004-003824-36-ES. | Link |
Fundació Institut Català de Farmacologia. Randomized, double blind, parallel groups, placebo controlled pivotal clinical trial to asses preliminary efficacy and security of a sublingual Cannabis Standardized Extract (Sativex) added to reference treatment for prevention and treatment of nausea and late vomiting induced by moderately emetogenic chemotherapy. -SATEME-08: EUCTR2004-003824-36-ES. | Link | Grunberg SM, Munsell MF, Morrow PK, Kent J, Ule UJ, Saccaro SJ, Onitilo AA, Metzner-Sadurski JK, Fisch, M. Randomized double-blind evaluation of dronabinol for the prevention of chemotherapy-induced nausea. J Clin Oncol 30, 2012 (suppl; abstr 9061). | Link |
Grunberg SM, Munsell MF, Morrow PK, Kent J, Ule UJ, Saccaro SJ, Onitilo AA, Metzner-Sadurski JK, Fisch, M. Randomized double-blind evaluation of dronabinol for the prevention of chemotherapy-induced nausea. J Clin Oncol 30, 2012 (suppl; abstr 9061). | Link | Schussel V, Miyahara L, Peixoto J, Santos A, Yoshimura E, Pachitol D, Riera R, Latorraca C. Findings and methodological quality of systematic reviews about cannabinoids for chemotherapy-related nausea and vomiting. PROSPERO 2017:CRD42017068994. | Link |
Schussel V, Miyahara L, Peixoto J, Santos A, Yoshimura E, Pachitol D, Riera R, Latorraca C. Findings and methodological quality of systematic reviews about cannabinoids for chemotherapy-related nausea and vomiting. PROSPERO 2017:CRD42017068994. | Link | Jones SE, Durant JR, Greco FA, Robertone A. A multi-institutional Phase III study of nabilone vs. placebo in chemotherapy-induced nausea and vomiting. Cancer Treat Rev. 1982 Dec;9 Suppl B:45-8. | PubMed |
Jones SE, Durant JR, Greco FA, Robertone A. A multi-institutional Phase III study of nabilone vs. placebo in chemotherapy-induced nausea and vomiting. Cancer Treat Rev. 1982 Dec;9 Suppl B:45-8. | PubMed |