 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Toxic epidermal necrolysis and Stevens-Johnson syndrome are severe adverse skin reactions to medications and infections. Steroids are described as a therapeutic alternative, but their use is still controversial. To answer this question, we searched in Epistemonikos database, which is maintained by screening multiple information sources. We identified four systematic reviews including 11 primary studies answering the question of interest. We extracted data, conducted a meta-analysis and generated a summary of findings table using the GRADE approach. We concluded it is not clear whether steroids reduce mortality or hospital stay in toxic epidermal necrolysis and Stevens-Johnson syndrome because the certainty of the evidence is very low.
Toxic epidermal necrolysis and Stevens-Johnson syndrome are severe, idiosyncratic skin reactions, mainly caused by drug exposure, or secondary to bacterial and viral infections. They are both considered part of the spectrum of the same disease characterized by generalized epidermal necrosis secondary to keratinocytes apoptosis, and differentiated according to the extent of cutaneous involvement. The main pathway leading to apoptosis is the binding of FAS, a membrane receptor present in keratinocytes, with its ligand.
The therapeutic management includes general measures of support, prevention of complications and the use of different immunosuppressive drugs such as systemic steroids. However, their effectiveness in the treatment of toxic epidermal necrolysis and Stevens-Johnson syndrome is controversial and its use is associated with significant adverse effects.
We used Epistemonikos database, which is maintained by screening multiple information sources, to identify systematic reviews and their included primary studies. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
|
What is the evidence. |
We found four systematic reviews [1],[2],[3],[4] including 11 primary studies [5],[6],[7],[8],[9],[10],[11],[12],[13], [14],[15]. None is a randomized controlled trial. |
|
What types of patients were included |
All of the studies included patients diagnosed with Stevens-Johnson syndrome (<10% of cutaneous involvement), Stevens-Johnson syndrome/toxic epidermal necrolysis overlap (10% to 30% of cutaneous involvement) and/or toxic epidermal necrolysis (>30% of cutaneous involvement). Seven studies [5],[6],[7],[8],[9],[10],[11] included patients older than 18 years, and four studies [12],[13], [14],[15] only included children. Only three studies [7],[9],[11] reported the percentage of cutaneous involvement. Six studies [6],[7],[8],[9],[10],[11] reported the etiology, with drugs being the leading cause. |
|
What types of interventions were included |
The included studies used different types of steroids, doses, route of administration, and duration. The use of oral prednisone, intravenous dexamethasone and intravenous methylprednisolone (with or without an initial bolus) was reported. Three of the studies [6],[8],[9] also incorporated intravenous immunoglobulin. |
|
What types of outcomes |
The studies measured multiple outcomes. However, those that were grouped in the identified reviews were:
|
The information about the effects of steroids on toxic epidermal necrolysis and Stevens-Johnson syndrome is based on 11 observational studies [5],[6],[7],[8],[9],[10],[11],[12],[13],[14],[15]. It was not possible to extract the information of the primary studies from the systematic reviews, in order to rebuild the meta-analysis. So, the information is presented here such as presented in the systematic reviews [1],[2],[3],[4]. The summary of findings is as follows:
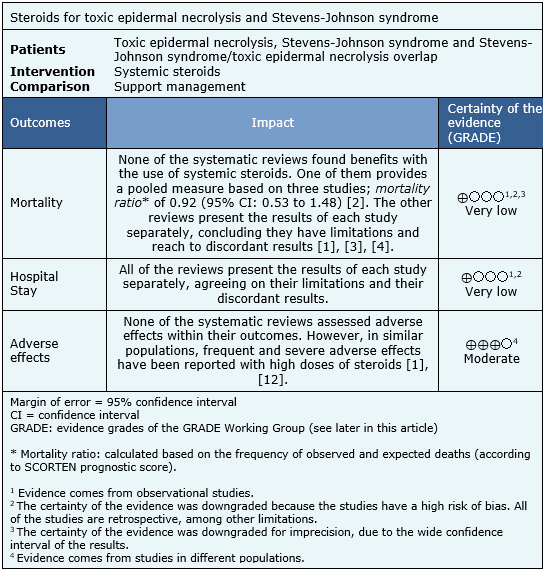

|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
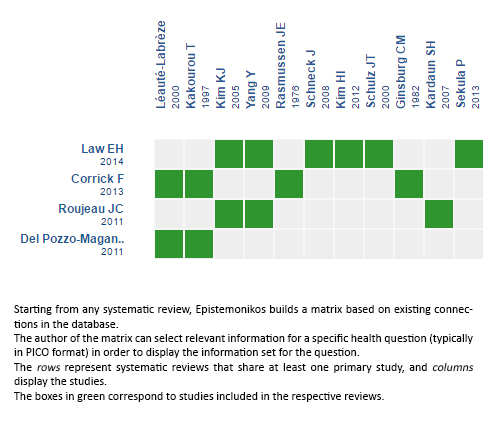
Follow the link to access the interactive version: Steroids for Stevens-Johnson syndrome and toxic epidermal necrolysis
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Toxic epidermal necrolysis and Stevens-Johnson syndrome are severe adverse skin reactions to medications and infections. Steroids are described as a therapeutic alternative, but their use is still controversial. To answer this question, we searched in Epistemonikos database, which is maintained by screening multiple information sources. We identified four systematic reviews including 11 primary studies answering the question of interest. We extracted data, conducted a meta-analysis and generated a summary of findings table using the GRADE approach. We concluded it is not clear whether steroids reduce mortality or hospital stay in toxic epidermal necrolysis and Stevens-Johnson syndrome because the certainty of the evidence is very low.
 Autores:
Rodrigo Meza[1,2], Gabriel Rada[2,3,4,5,6], Pablo Varas[2,3]
Autores:
Rodrigo Meza[1,2], Gabriel Rada[2,3,4,5,6], Pablo Varas[2,3]

Citación: Meza R, Rada , Varas P. Are steroids effective in toxic epidermal necrolysis and Stevens-Johnson syndrome?. Medwave2017;17(Suppl2):e6894 doi: 10.5867/medwave.2017.6894
Fecha de envío: 29/12/2016
Fecha de aceptación: 30/12/2016
Fecha de publicación: 5/4/2017

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Law EH, Leung M. Corticosteroids in Stevens-Johnson Syndrome/toxic epidermalnecrolysis: current evidence and implications for future research. AnnPharmacother. 2015 Mar;49(3):335-42 | CrossRef | PubMed |
Law EH, Leung M. Corticosteroids in Stevens-Johnson Syndrome/toxic epidermalnecrolysis: current evidence and implications for future research. AnnPharmacother. 2015 Mar;49(3):335-42 | CrossRef | PubMed | Roujeau JC, Bastuji-Garin S. Systematic review of treatments forStevens-Johnson syndrome and toxic epidermal necrolysis using the SCORTEN scoreas a tool for evaluating mortality. Ther Adv Drug Saf. 2011 Jun;2(3):87-94 | CrossRef | PubMed |
Roujeau JC, Bastuji-Garin S. Systematic review of treatments forStevens-Johnson syndrome and toxic epidermal necrolysis using the SCORTEN scoreas a tool for evaluating mortality. Ther Adv Drug Saf. 2011 Jun;2(3):87-94 | CrossRef | PubMed | Del Pozzo-Magana BR, Lazo-Langner A, Carleton B, Castro-Pastrana LI, Rieder MJ. A systematic review of treatment of drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in children. J PopulTherClinPharmacol. 2011;18:e121-33 | PubMed |
Del Pozzo-Magana BR, Lazo-Langner A, Carleton B, Castro-Pastrana LI, Rieder MJ. A systematic review of treatment of drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in children. J PopulTherClinPharmacol. 2011;18:e121-33 | PubMed | Corrick F, Anand G. Question 2: Would systemic steroids be useful in the management of Stevens-Johnson syndrome? Arch Dis Child. 2013 Oct;98(10):828-30 | CrossRef | PubMed |
Corrick F, Anand G. Question 2: Would systemic steroids be useful in the management of Stevens-Johnson syndrome? Arch Dis Child. 2013 Oct;98(10):828-30 | CrossRef | PubMed | Kardaun SH, Jonkman MF. Dexamethasone pulse therapy for Stevens-Johnsonsyndrome/toxic epidermal necrolysis. ActaDermVenereol. 2007;87(2):144-8 | PubMed |
Kardaun SH, Jonkman MF. Dexamethasone pulse therapy for Stevens-Johnsonsyndrome/toxic epidermal necrolysis. ActaDermVenereol. 2007;87(2):144-8 | PubMed | Kim HI, Kim SW, Park GY, Kwon EG, Kim HH, Jeong JY, et al. Causes and treatment outcomes of Stevens-Johnson syndrome and toxic epidermalnecrolysis in 82 adult patients. Korean J Intern Med. 2012 Jun;27(2):203-10 | CrossRef | PubMed |
Kim HI, Kim SW, Park GY, Kwon EG, Kim HH, Jeong JY, et al. Causes and treatment outcomes of Stevens-Johnson syndrome and toxic epidermalnecrolysis in 82 adult patients. Korean J Intern Med. 2012 Jun;27(2):203-10 | CrossRef | PubMed | Kim KJ, Lee DP, Suh HS, Lee MW, Choi JH, Moon KC, et al. Toxic epidermalnecrolysis: analysis of clinical course and SCORTEN-based comparison of mortalityrate and treatment modalities in Korean patients. ActaDermVenereol.2005;85(6):497-502 | PubMed |
Kim KJ, Lee DP, Suh HS, Lee MW, Choi JH, Moon KC, et al. Toxic epidermalnecrolysis: analysis of clinical course and SCORTEN-based comparison of mortalityrate and treatment modalities in Korean patients. ActaDermVenereol.2005;85(6):497-502 | PubMed | Schneck J, Fagot JP, Sekula P, Sassolas B, Roujeau JC, Mockenhaupt M. Effectsof treatments on the mortality of Stevens-Johnson syndrome and toxic epidermalnecrolysis: A retrospective study on patients included in the prospectiveEuroSCAR Study. J Am AcadDermatol. 2008 Jan;58(1):33-40 | PubMed |
Schneck J, Fagot JP, Sekula P, Sassolas B, Roujeau JC, Mockenhaupt M. Effectsof treatments on the mortality of Stevens-Johnson syndrome and toxic epidermalnecrolysis: A retrospective study on patients included in the prospectiveEuroSCAR Study. J Am AcadDermatol. 2008 Jan;58(1):33-40 | PubMed | Schulz JT, Sheridan RL, Ryan CM, MacKool B, Tompkins RG. A 10-year experience with toxic epidermal necrolysis. J Burn Care Rehabil. 2000 May-Jun;21(3):199-204 | PubMed |
Schulz JT, Sheridan RL, Ryan CM, MacKool B, Tompkins RG. A 10-year experience with toxic epidermal necrolysis. J Burn Care Rehabil. 2000 May-Jun;21(3):199-204 | PubMed | Sekula P, Dunant A, Mockenhaupt M, Naldi L, BouwesBavinck JN, et al. Comprehensive survival analysis of a cohort of patients with Stevens-Johnsonsyndrome and toxic epidermal necrolysis. J Invest Dermatol. 2013May;133(5):1197-204 | CrossRef | PubMed |
Sekula P, Dunant A, Mockenhaupt M, Naldi L, BouwesBavinck JN, et al. Comprehensive survival analysis of a cohort of patients with Stevens-Johnsonsyndrome and toxic epidermal necrolysis. J Invest Dermatol. 2013May;133(5):1197-204 | CrossRef | PubMed | Yang Y, Xu J, Li F, Zhu X. Combination therapy of intravenous immunoglobulinand corticosteroid in the treatment of toxic epidermal necrolysis andStevens-Johnson syndrome: a retrospective comparative study in China. Int JDermatol. 2009 Oct;48(10):1122-8 | CrossRef | PubMed |
Yang Y, Xu J, Li F, Zhu X. Combination therapy of intravenous immunoglobulinand corticosteroid in the treatment of toxic epidermal necrolysis andStevens-Johnson syndrome: a retrospective comparative study in China. Int JDermatol. 2009 Oct;48(10):1122-8 | CrossRef | PubMed | Kakourou T, Klontza D, Soteropoulou F, Kattamis C. Corticosteroid treatment of erythema multiforme major (Stevens-Johnson syndrome) in children. Eur J Pediatr. 1997 Feb;156(2):90-3 | PubMed |
Kakourou T, Klontza D, Soteropoulou F, Kattamis C. Corticosteroid treatment of erythema multiforme major (Stevens-Johnson syndrome) in children. Eur J Pediatr. 1997 Feb;156(2):90-3 | PubMed | Ginsburg CM. Stevens-Johnson syndrome in children. Pediatr Infect Dis. 1982 May-Jun;1(3):155-8 | PubMed |
Ginsburg CM. Stevens-Johnson syndrome in children. Pediatr Infect Dis. 1982 May-Jun;1(3):155-8 | PubMed | Léauté-Labrèze C, Lamireau T, Chawki D, Maleville J, Taïeb A. Diagnosis, classification, and management of erythema multiforme and Stevens-Johnson syndrome. Arch Dis Child. 2000 Oct;83(4):347-52 | PubMed |
Léauté-Labrèze C, Lamireau T, Chawki D, Maleville J, Taïeb A. Diagnosis, classification, and management of erythema multiforme and Stevens-Johnson syndrome. Arch Dis Child. 2000 Oct;83(4):347-52 | PubMed | Rasmussen JE. Erythema multiforme in children. Response to treatment with systemic corticosteroids. Br J Dermatol. 1976 Aug;95(2):181-6 | PubMed |
Rasmussen JE. Erythema multiforme in children. Response to treatment with systemic corticosteroids. Br J Dermatol. 1976 Aug;95(2):181-6 | PubMed | Endorf FW, Cancio LC, Gibran NS. Toxic epidermal necrolysis clinicalguidelines. J Burn Care Res. 2008 Sep-Oct;29(5):706-12 | CrossRef | PubMed |
Endorf FW, Cancio LC, Gibran NS. Toxic epidermal necrolysis clinicalguidelines. J Burn Care Res. 2008 Sep-Oct;29(5):706-12 | CrossRef | PubMed | Creamer D, Walsh SA, Dziewulski P, Exton LS, Lee HY, Dart JK, et al. UKguidelines for the management of Stevens-Johnson syndrome/toxicepidermal necrolysis in adults 2016 (print summary - Full guidelines available athttp://dx.doi.org/10.1016/j.bjps.2016.01.034). J PlastReconstrAesthet Surg.2016 Jun;69(6):736-41 | CrossRef | PubMed |
Creamer D, Walsh SA, Dziewulski P, Exton LS, Lee HY, Dart JK, et al. UKguidelines for the management of Stevens-Johnson syndrome/toxicepidermal necrolysis in adults 2016 (print summary - Full guidelines available athttp://dx.doi.org/10.1016/j.bjps.2016.01.034). J PlastReconstrAesthet Surg.2016 Jun;69(6):736-41 | CrossRef | PubMed |