 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Multiple beneficial effects have been proposed lately for cannabinoids in different clinical situations. Among them, it has been postulated they would control symptoms of multiple sclerosis. However, there is no consensus about their real clinical role. To answer this question, we searched in Epistemonikos database, which is maintained by screening multiple databases. We identified 25 systematic reviews including 35 studies overall, of which 26 were randomized trials. We extracted data, conducted a meta-analysis and generated a summary of findings table using the GRADE approach. We concluded cannabinoids in multiple sclerosis do not reduce spasticity or pain, and are probably associated to frequent adverse effects.
Multiple sclerosis is a demyelinating chronic disease of the central nervous system that might presents in a relapsing-remitting and/or progressive pattern. The clinical manifestations are multiple and include loss of strength and/or sensitivity in the limbs, visual loss, pain secondary to spasticity, ataxia, and bladder dysfunction. The treatment for relapses is usually based on corticosteroids. In the long term, different therapeutic alternatives are available, such as interferon beta, immunomodulatory agents, immunoglobulin, chemotherapeutic agents and sphingosine analogues. However, up to 30-40% of patients remain symptomatic.
Multiple beneficial effects have been proposed lately for cannabinoids in different clinical situations. Among them, it has been postulated tetrahydrocannabinol and cannabinidiol would control spasticity, pain and bladder dysfunction in multiple sclerosis, especially in patients with refractory symptoms. The proposed mechanisms are mediated through modulation of CB1 and CB2 receptors in the endocannabidiol system. However, the real clinical impact of cannabinoids in this condition is not clear.
We used Epistemonikos database, which is maintained by screening multiple databases, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
|
What is the evidence. |
We found 25 systematic reviews [1],[2],[3],[4],[5],[6],[7], [8],[9],[10],[11],[12],[13],[14],[15],[16],[17],[18],[19],[20], [21],[22],[23],[24],[25], including 35 primary studies reported in 57 references [26],[27],[28],[29],[30],[31],[32],[33],[34], [35],[36],[37],[38],[39],[40],[41],[42],[43],[44],[45],[46], [47],[48],[49],[50],[51],[52],[53],[54],[55],[56],[57],[58], [59],[60],[61],[62],[63],[64],[65],[66],[67],[68],[69],[70], [71],[72],[73],[74],[75],[76],[77],[78],[79],[80],[81],[82], [83],[84],[85], among them 26 randomized controlled trials [26],[28],[33],[36],[38],[39],[40],[47],[48],[49],[52],[54], [57],[59],[60],[61],[62],[63],[70],[71],[73],[74],[76],[77], [79],[80]. This table and the summary in general are based on the latter. |
|
What types of patients were included |
All of the trials included patients with multiple sclerosis, but just a few specified the subtype: six trials included patients with relapsing-remitting multiple sclerosis [39],[40],[52], [60],[73],[76],[80]; seven trials included patients with primary progressive multiple sclerosis [40],[49],[52],[65], [71],[76],[80]; and eight trials included patients with secondary progressive multiple sclerosis [28],[33],[40],[49], [52],[71],[76],[80]. The age of the included patients was reported only in some trials, with a variable range, greater than 18 years old in all the cases. The severity of the included patients was reported in only seven trials, using the EDSS score (expanded disability status scale), presenting a score greater than five in all of the trials [28],[36],[40],[49],[71],[76],[80]. In just eight trials the duration of disease was described, with a range of 4.5 to 17 years in the different trials [28],[33],[36],[49],[52],[71],[76], [80]. |
|
What types of interventions were included |
One trial administered smoked cannabis [28], nine trials used cannabis capsules [39],[40],[49],[61],[73],[74],[76],[79], [80]; two trials used dronabinol [40], [71]; and ten trials used sublingual nabiximol spray [26],[33],[36],[38],[47],[48],[52], [59],[62],[70]. Other trials used less conventional presentations. All of the trials compared against placebo. |
|
What types of outcomes |
The different systematic reviews grouped the outcomes in the following way:
|
The information about the effects of cannabinoids in multiple sclerosis is based on seven randomized trials [33],[35],[36],[43],[52],[63],[69], that included 1,985 patients. The other trials did not report any outcome of interest, or did not present the information in a way it could be incorporated in a meta-analysis. Four trials [33],[36],[63],[80] reported spasticity (1,247 patients), three trials [33],[52],[70] reported pain (327 patients) and four trials [33],[36],[52],[63] reported adverse effects (1,025 patients). The summary of findings is the following:
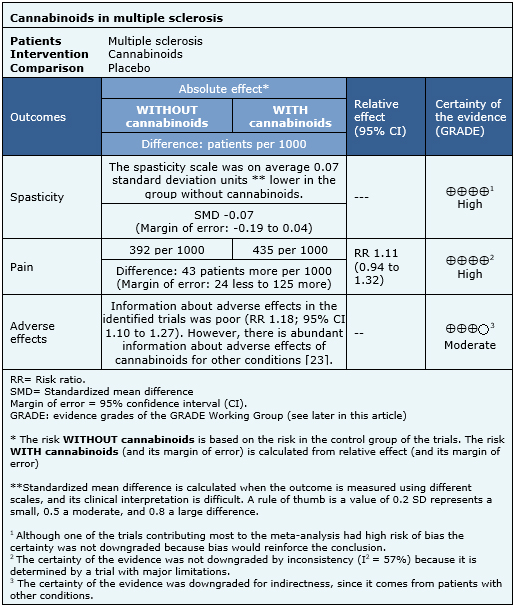

|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
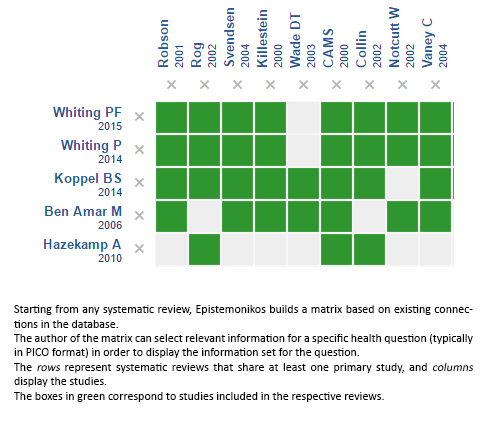
Follow the link to access the interactive version: Cannabinoids for multiple sclerosis
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Multiple beneficial effects have been proposed lately for cannabinoids in different clinical situations. Among them, it has been postulated they would control symptoms of multiple sclerosis. However, there is no consensus about their real clinical role. To answer this question, we searched in Epistemonikos database, which is maintained by screening multiple databases. We identified 25 systematic reviews including 35 studies overall, of which 26 were randomized trials. We extracted data, conducted a meta-analysis and generated a summary of findings table using the GRADE approach. We concluded cannabinoids in multiple sclerosis do not reduce spasticity or pain, and are probably associated to frequent adverse effects.
 Autores:
Rodrigo Meza[1,2], Javier Peña[1,2], Karen García[1,2], Oscar Corsi[2,4], Gabriel Rada[2,3,4,5,6]
Autores:
Rodrigo Meza[1,2], Javier Peña[1,2], Karen García[1,2], Oscar Corsi[2,4], Gabriel Rada[2,3,4,5,6]

Citación: Meza R, Peña J, García O, Corsi O, Rada G. Are cannabinoids effective in multiple sclerosis?. Medwave 2017; 17(Suppl1):e6865 doi: 10.5867/medwave.2017.6865
Fecha de envío: 27/12/2016
Fecha de aceptación: 27/12/2016
Fecha de publicación: 10/3/2017

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Bagshaw SM, Hagen NA. Medical efficacy of cannabinoids and marijuana: a comprehensive review of the literature. J Palliat Care. 2002 Summer;18(2):111-22 | PubMed |
Bagshaw SM, Hagen NA. Medical efficacy of cannabinoids and marijuana: a comprehensive review of the literature. J Palliat Care. 2002 Summer;18(2):111-22 | PubMed | Ben Amar M. Cannabinoids in medicine: A review of their therapeutic potential. J Ethnopharmacol. 2006 Apr 21;105(1-2):1-25 | PubMed |
Ben Amar M. Cannabinoids in medicine: A review of their therapeutic potential. J Ethnopharmacol. 2006 Apr 21;105(1-2):1-25 | PubMed | Burns TL, Ineck JR. Cannabinoid analgesia as a potential new therapeutic option in the treatment of chronic pain. Ann Pharmacother. 2006 Feb;40(2):251-60 | PubMed |
Burns TL, Ineck JR. Cannabinoid analgesia as a potential new therapeutic option in the treatment of chronic pain. Ann Pharmacother. 2006 Feb;40(2):251-60 | PubMed | Correa D, Moreno C. Systematic review of the literature: tratment for central pain in multiple sclerosis. Acta neurol. colomb. 2009;25(1):4 - 15. | Link |
Correa D, Moreno C. Systematic review of the literature: tratment for central pain in multiple sclerosis. Acta neurol. colomb. 2009;25(1):4 - 15. | Link | Shakespeare DT, Boggild M, Young C. Anti-spasticity agents for multiple sclerosis. Cochrane Database Syst Rev. 2003;(4):CD001332 | PubMed |
Shakespeare DT, Boggild M, Young C. Anti-spasticity agents for multiple sclerosis. Cochrane Database Syst Rev. 2003;(4):CD001332 | PubMed | Deshpande A, Mailis-Gagnon A, Zoheiry N, Lakha SF. Efficacy and adverse effects of medical marijuana for chronic noncancer pain: Systematic review of randomized controlled trials. Can Fam Physician. 2015 Aug;61(8):e372-81 | PubMed |
Deshpande A, Mailis-Gagnon A, Zoheiry N, Lakha SF. Efficacy and adverse effects of medical marijuana for chronic noncancer pain: Systematic review of randomized controlled trials. Can Fam Physician. 2015 Aug;61(8):e372-81 | PubMed | Fisher B, Johnston D, Leake P. Marijuana for medicinal purposes: an evidence-based assessment. WorkSafe BC. 2002;76. | Link |
Fisher B, Johnston D, Leake P. Marijuana for medicinal purposes: an evidence-based assessment. WorkSafe BC. 2002;76. | Link | Hazekamp A, Grotenhermen F. Review on clinical studies with cannabis and cannabinoids 2005-2009. Cannabinoids. 2010;5(special issue):1-21 | Link |
Hazekamp A, Grotenhermen F. Review on clinical studies with cannabis and cannabinoids 2005-2009. Cannabinoids. 2010;5(special issue):1-21 | Link | Iskedjian M, Bereza B, Gordon A, Piwko C, Einarson TR. Meta-analysis of cannabis based treatments for neuropathic and multiple sclerosis-related pain. Curr Med Res Opin. 2007 Jan;23(1):17-24 | PubMed |
Iskedjian M, Bereza B, Gordon A, Piwko C, Einarson TR. Meta-analysis of cannabis based treatments for neuropathic and multiple sclerosis-related pain. Curr Med Res Opin. 2007 Jan;23(1):17-24 | PubMed | Jawahar R, Oh U, Yang S, Lapane KL. A systematic review of pharmacological pain management in multiple sclerosis. Drugs. 2013 Oct;73(15):1711-22 | CrossRef | PubMed |
Jawahar R, Oh U, Yang S, Lapane KL. A systematic review of pharmacological pain management in multiple sclerosis. Drugs. 2013 Oct;73(15):1711-22 | CrossRef | PubMed | Koppel BS, Brust JC, Fife T, Bronstein J, Youssof S, Gronseth G, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014 Apr 29;82(17):1556-63 | CrossRef | PubMed |
Koppel BS, Brust JC, Fife T, Bronstein J, Youssof S, Gronseth G, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014 Apr 29;82(17):1556-63 | CrossRef | PubMed | Lakhan SE, Rowland M. Whole plant cannabis extracts in the treatment of spasticity in multiple sclerosis: a systematic review. BMC Neurol. 2009 Dec 4;9:59 | CrossRef | PubMed |
Lakhan SE, Rowland M. Whole plant cannabis extracts in the treatment of spasticity in multiple sclerosis: a systematic review. BMC Neurol. 2009 Dec 4;9:59 | CrossRef | PubMed | Lynch ME, Ware MA. Cannabinoids for the Treatment of Chronic Non-Cancer Pain: An Updated Systematic Review of Randomized Controlled Trials. J Neuroimmune Pharmacol. 2015 Jun;10(2):293-301 | CrossRef | PubMed |
Lynch ME, Ware MA. Cannabinoids for the Treatment of Chronic Non-Cancer Pain: An Updated Systematic Review of Randomized Controlled Trials. J Neuroimmune Pharmacol. 2015 Jun;10(2):293-301 | CrossRef | PubMed | Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol. 2011 Nov;72(5):735-44 | CrossRef | PubMed |
Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol. 2011 Nov;72(5):735-44 | CrossRef | PubMed | Martín-Sánchez E, Furukawa TA, Taylor J, Martin JL. Systematic review and meta-analysis of cannabis treatment for chronic pain. Pain Med. 2009 Nov;10(8):1353-68 | CrossRef | PubMed |
Martín-Sánchez E, Furukawa TA, Taylor J, Martin JL. Systematic review and meta-analysis of cannabis treatment for chronic pain. Pain Med. 2009 Nov;10(8):1353-68 | CrossRef | PubMed | Petzke F, Enax-Krumova EK, Häuser W. [Efficacy, tolerability and safety of cannabinoids for chronic neuropathic pain: A systematic review of randomized controlled studies]. Schmerz. 2016 Feb;30(1):62-88 | CrossRef | PubMed |
Petzke F, Enax-Krumova EK, Häuser W. [Efficacy, tolerability and safety of cannabinoids for chronic neuropathic pain: A systematic review of randomized controlled studies]. Schmerz. 2016 Feb;30(1):62-88 | CrossRef | PubMed | Mills RJ, Yap L, Young CA. Treatment for ataxia in multiple sclerosis. Cochrane Database Syst Rev. 2007 Jan 24;(1):CD005029 | PubMed |
Mills RJ, Yap L, Young CA. Treatment for ataxia in multiple sclerosis. Cochrane Database Syst Rev. 2007 Jan 24;(1):CD005029 | PubMed | Guerra SS. Are cannabinoids more effective than placebo in decreasing MS-related bladder dysfunction? British Journal of Neuroscience Nursing. 2012 | CrossRef |
Guerra SS. Are cannabinoids more effective than placebo in decreasing MS-related bladder dysfunction? British Journal of Neuroscience Nursing. 2012 | CrossRef | Tsang CC, Giudice MG. Nabilone for the Management of Pain. Pharmacotherapy. 2016 Mar;36(3):273-86 | CrossRef | PubMed |
Tsang CC, Giudice MG. Nabilone for the Management of Pain. Pharmacotherapy. 2016 Mar;36(3):273-86 | CrossRef | PubMed | Wade DT, Collin C, Stott C, Duncombe P. Meta-analysis of the efficacy and safety of Sativex (nabiximols), on spasticity in people with multiple sclerosis. Mult Scler. 2010 Jun;16(6):707-14 | CrossRef | PubMed |
Wade DT, Collin C, Stott C, Duncombe P. Meta-analysis of the efficacy and safety of Sativex (nabiximols), on spasticity in people with multiple sclerosis. Mult Scler. 2010 Jun;16(6):707-14 | CrossRef | PubMed | WCB Evidence Based Practice Group. Efficacy of marijuana in treating chronic non cancer pain: a short review. Richmond: WorkSafe BC. 2006 | Link |
WCB Evidence Based Practice Group. Efficacy of marijuana in treating chronic non cancer pain: a short review. Richmond: WorkSafe BC. 2006 | Link | Whiting P, Wolff R, Westwood M, Duffy S, Misso K, Keurentjes Cet al. Systematic review of cannabis for medical use. Kleijnen Systematic Reviews Ltd. 2014 | Link |
Whiting P, Wolff R, Westwood M, Duffy S, Misso K, Keurentjes Cet al. Systematic review of cannabis for medical use. Kleijnen Systematic Reviews Ltd. 2014 | Link | Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015 Jun 23-30;313(24):2456-73 | Link |
Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015 Jun 23-30;313(24):2456-73 | Link | Zhornitsky S, Potvin S. Cannabidiol in humans-the quest for therapeutic targets. Pharmaceuticals (Basel). 2012 May 21;5(5):529-52 | CrossRef | PubMed |
Zhornitsky S, Potvin S. Cannabidiol in humans-the quest for therapeutic targets. Pharmaceuticals (Basel). 2012 May 21;5(5):529-52 | CrossRef | PubMed | Andrzejewski K, Barbano R, Mink J. Cannabinoids in the treatment of movement disorders: A systematic review of case series and clinical trials. Basal Ganglia. 2016;6(3):173-181 | Link |
Andrzejewski K, Barbano R, Mink J. Cannabinoids in the treatment of movement disorders: A systematic review of case series and clinical trials. Basal Ganglia. 2016;6(3):173-181 | Link | Aragona M, Onesti E, Tomassini V, Conte A, Gupta S, Gilio F, et al. Psychopathological and cognitive effects of therapeutic cannabinoids in multiple sclerosis: a double-blind, placebo controlled, crossover study. Clin Neuropharmacol. 2009 Jan-Feb;32(1):41-7 | CrossRef | PubMed |
Aragona M, Onesti E, Tomassini V, Conte A, Gupta S, Gilio F, et al. Psychopathological and cognitive effects of therapeutic cannabinoids in multiple sclerosis: a double-blind, placebo controlled, crossover study. Clin Neuropharmacol. 2009 Jan-Feb;32(1):41-7 | CrossRef | PubMed | Brady CM, DasGupta R, Dalton C, Wiseman OJ, Berkley KJ, Fowler CJ. An open-label pilot study of cannabis-based extracts for bladder dysfunction in advanced multiple sclerosis. Mult Scler. 2004 Aug;10(4):425-33 | PubMed |
Brady CM, DasGupta R, Dalton C, Wiseman OJ, Berkley KJ, Fowler CJ. An open-label pilot study of cannabis-based extracts for bladder dysfunction in advanced multiple sclerosis. Mult Scler. 2004 Aug;10(4):425-33 | PubMed | Corey-Bloom J, Wolfson T, Gamst A, Jin S, Marcotte TD, Bentley H, et al. Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. CMAJ. 2012 Jul 10;184(10):1143-50 | CrossRef | PubMed |
Corey-Bloom J, Wolfson T, Gamst A, Jin S, Marcotte TD, Bentley H, et al. Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. CMAJ. 2012 Jul 10;184(10):1143-50 | CrossRef | PubMed | Corey-Bloom J, Wolfson TJ, Anthony GC, Bentley H, Gouaux B. Short-term effects off medicinal cannabis on spasticity in multiple sclerosis. Neurology. 2008;70(11 (suppl 1)):A86-A87. | Link |
Corey-Bloom J, Wolfson TJ, Anthony GC, Bentley H, Gouaux B. Short-term effects off medicinal cannabis on spasticity in multiple sclerosis. Neurology. 2008;70(11 (suppl 1)):A86-A87. | Link | Center for Medicinal Cannabis Research. Short-Term Effects of Medicinal Cannabis Therapy on Spasticity in Multiple Sclerosis. clinicaltrials.gov. 2001 | Link |
Center for Medicinal Cannabis Research. Short-Term Effects of Medicinal Cannabis Therapy on Spasticity in Multiple Sclerosis. clinicaltrials.gov. 2001 | Link | Centonze D, Mori F, Koch G, Buttari F, Codecà C, Rossi S, et al. Lack of effect of cannabis-based treatment on clinical and laboratory measures in multiple sclerosis. Neurol Sci. 2009 Dec;30(6):531-4 | CrossRef | PubMed |
Centonze D, Mori F, Koch G, Buttari F, Codecà C, Rossi S, et al. Lack of effect of cannabis-based treatment on clinical and laboratory measures in multiple sclerosis. Neurol Sci. 2009 Dec;30(6):531-4 | CrossRef | PubMed | Clifford DB. Tetrahydrocannabinol for tremor in multiple sclerosis. Ann Neurol. 1983 Jun;13(6):669-71 | PubMed |
Clifford DB. Tetrahydrocannabinol for tremor in multiple sclerosis. Ann Neurol. 1983 Jun;13(6):669-71 | PubMed | Collin C, Ehler E, Waberzinek G, Alsindi Z, Davies P, Powell K, et al. A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol Res. 2010 Jun;32(5):451-9 | CrossRef | PubMed |
Collin C, Ehler E, Waberzinek G, Alsindi Z, Davies P, Powell K, et al. A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol Res. 2010 Jun;32(5):451-9 | CrossRef | PubMed | Collin C, Ambler Z, Kent R, McCalla R. A randomised controlled study of Sativex® in patients with symptoms of spasticity due to multiple sclerosis. 22nd Congress of the ECTRIMS. 2006 | Link |
Collin C, Ambler Z, Kent R, McCalla R. A randomised controlled study of Sativex® in patients with symptoms of spasticity due to multiple sclerosis. 22nd Congress of the ECTRIMS. 2006 | Link | GW Pharmaceuticals Ltd. A Study to Evaluate the Efficacy of Sativex in Relieving Symptoms of Spasticity Due to Multiple Sclerosis. clinicaltrials.gov. 2005 | Link |
GW Pharmaceuticals Ltd. A Study to Evaluate the Efficacy of Sativex in Relieving Symptoms of Spasticity Due to Multiple Sclerosis. clinicaltrials.gov. 2005 | Link | Collin C, Davies P, Mutiboko IK, Ratcliffe S; Sativex Spasticity in MS Study Group. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur J Neurol. 2007 Mar;14(3):290-6. | PubMed |
Collin C, Davies P, Mutiboko IK, Ratcliffe S; Sativex Spasticity in MS Study Group. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur J Neurol. 2007 Mar;14(3):290-6. | PubMed | GW Pharmaceuticals Ltd. A Study of Sativex® for Relief of Spasticity in Subjects With Multiple Sclerosis. clinicaltrials.gov. 2002 | Link |
GW Pharmaceuticals Ltd. A Study of Sativex® for Relief of Spasticity in Subjects With Multiple Sclerosis. clinicaltrials.gov. 2002 | Link | Conte A, Bettolo CM, Onesti E, Frasca V, Iacovelli E, Gilio F, et al. Cannabinoid-induced effects on the nociceptive system: a neurophysiological study in patients with secondary progressive multiple sclerosis. Eur J Pain. 2009 May;13(5):472-7 | CrossRef | PubMed |
Conte A, Bettolo CM, Onesti E, Frasca V, Iacovelli E, Gilio F, et al. Cannabinoid-induced effects on the nociceptive system: a neurophysiological study in patients with secondary progressive multiple sclerosis. Eur J Pain. 2009 May;13(5):472-7 | CrossRef | PubMed | Fox P, Bain PG, Glickman S, Carroll C, Zajicek J. The effect of cannabis on tremor in patients with multiple sclerosis. Neurology. 2004 Apr 13;62(7):1105-9. | PubMed |
Fox P, Bain PG, Glickman S, Carroll C, Zajicek J. The effect of cannabis on tremor in patients with multiple sclerosis. Neurology. 2004 Apr 13;62(7):1105-9. | PubMed | Freeman RM, Adekanmi O, Waterfield MR, Waterfield AE, Wright D, Zajicek J. The effect of cannabis on urge incontinence in patients with multiple sclerosis: a multicentre, randomised placebo-controlled trial (CAMS-LUTS). Int Urogynecol J Pelvic Floor Dysfunct. 2006 Nov;17(6):636-41 | PubMed |
Freeman RM, Adekanmi O, Waterfield MR, Waterfield AE, Wright D, Zajicek J. The effect of cannabis on urge incontinence in patients with multiple sclerosis: a multicentre, randomised placebo-controlled trial (CAMS-LUTS). Int Urogynecol J Pelvic Floor Dysfunct. 2006 Nov;17(6):636-41 | PubMed | Katona S, Kaminski E, Sanders H, Zajicek J. Cannabinoid influence on cytokine profile in multiple sclerosis. Clin Exp Immunol. 2005 Jun;140(3):580-5 | PubMed |
Katona S, Kaminski E, Sanders H, Zajicek J. Cannabinoid influence on cytokine profile in multiple sclerosis. Clin Exp Immunol. 2005 Jun;140(3):580-5 | PubMed | Zajicek JP, Sanders HP, Wright DE, Vickery PJ, Ingram WM, Reilly SM, et al. Cannabinoids in multiple sclerosis (CAMS) study: safety and efficacy data for 12 months follow up. J Neurol Neurosurg Psychiatry. 2005 Dec;76(12):1664-9 | PubMed |
Zajicek JP, Sanders HP, Wright DE, Vickery PJ, Ingram WM, Reilly SM, et al. Cannabinoids in multiple sclerosis (CAMS) study: safety and efficacy data for 12 months follow up. J Neurol Neurosurg Psychiatry. 2005 Dec;76(12):1664-9 | PubMed | Zajicek J, Fox P, Sanders H, Wright D, Vickery J, Nunn A, et al; UK MS Research Group. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomized placebo-controlled trial. Lancet. 2003 Nov 8;362(9395):1517-26 | PubMed |
Zajicek J, Fox P, Sanders H, Wright D, Vickery J, Nunn A, et al; UK MS Research Group. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomized placebo-controlled trial. Lancet. 2003 Nov 8;362(9395):1517-26 | PubMed | Medical Research Council (MRC) (UK). A multiple randomised controlled trial of cannabinoids on spasticity in multiple sclerosis (MS). ISRCTN.com. 2000 | Link |
Medical Research Council (MRC) (UK). A multiple randomised controlled trial of cannabinoids on spasticity in multiple sclerosis (MS). ISRCTN.com. 2000 | Link | Greenberg HS, Werness SA, Pugh JE, Andrus RO, Anderson DJ, Domino EF. Short-term effects of smoking marijuana on balance in patients with multiple sclerosis and normal volunteers. Clin Pharmacol Ther. 1994 Mar;55(3):324-8. | PubMed |
Greenberg HS, Werness SA, Pugh JE, Andrus RO, Anderson DJ, Domino EF. Short-term effects of smoking marijuana on balance in patients with multiple sclerosis and normal volunteers. Clin Pharmacol Ther. 1994 Mar;55(3):324-8. | PubMed | Hamann W, di Vadi PP. Analgesic effect of the cannabinoid analogue nabilone is not mediated by opioid receptors. Lancet. 1999 Feb 13;353(9152):560 | PubMed |
Hamann W, di Vadi PP. Analgesic effect of the cannabinoid analogue nabilone is not mediated by opioid receptors. Lancet. 1999 Feb 13;353(9152):560 | PubMed | Kavia R, De Ridder D, Sarantis N, Constantinescu C. Randomised controlled trial of cannabis based medicine (CBM, Stativex®) to treat detrusor overactivity in multiple sclerosis. Neurourology and Urodynamics. 2006;25:166-166. | Link |
Kavia R, De Ridder D, Sarantis N, Constantinescu C. Randomised controlled trial of cannabis based medicine (CBM, Stativex®) to treat detrusor overactivity in multiple sclerosis. Neurourology and Urodynamics. 2006;25:166-166. | Link | Kavia RB, De Ridder D, Constantinescu CS, Stott CG, Fowler CJ. Randomized controlled trial of Sativex to treat detrusor overactivity in multiple sclerosis. Mult Scler. 2010 Nov;16(11):1349-59 | CrossRef | PubMed |
Kavia RB, De Ridder D, Constantinescu CS, Stott CG, Fowler CJ. Randomized controlled trial of Sativex to treat detrusor overactivity in multiple sclerosis. Mult Scler. 2010 Nov;16(11):1349-59 | CrossRef | PubMed | Killestein J, Hoogervorst EL, Reif M, Blauw B, Smits M, Uitdehaag BM, et al. Immunomodulatory effects of orally administered cannabinoids in multiple sclerosis. J Neuroimmunol. 2003 Apr;137(1-2):140-3. | PubMed |
Killestein J, Hoogervorst EL, Reif M, Blauw B, Smits M, Uitdehaag BM, et al. Immunomodulatory effects of orally administered cannabinoids in multiple sclerosis. J Neuroimmunol. 2003 Apr;137(1-2):140-3. | PubMed | Killestein J, Hoogervorst EL, Reif M, Kalkers NF, Van Loenen AC, Staats PG, et al. Safety, tolerability, and efficacy of orally administered cannabinoids in MS. Neurology. 2002 May 14;58(9):1404-7 | PubMed |
Killestein J, Hoogervorst EL, Reif M, Kalkers NF, Van Loenen AC, Staats PG, et al. Safety, tolerability, and efficacy of orally administered cannabinoids in MS. Neurology. 2002 May 14;58(9):1404-7 | PubMed | Killestein J, Hoogervorst ELJ, Kalkers NF, van Winsen LML, Uitdehaag BMJ, Linssen-Schuurmans CD, et al. The effects of orally administred cannabinoids in multiple sclerosis patients: a pilot study. Multiple Sclerosis. 2000;6(1 suppl 1):S27-S27 | CrossRef |
Killestein J, Hoogervorst ELJ, Kalkers NF, van Winsen LML, Uitdehaag BMJ, Linssen-Schuurmans CD, et al. The effects of orally administred cannabinoids in multiple sclerosis patients: a pilot study. Multiple Sclerosis. 2000;6(1 suppl 1):S27-S27 | CrossRef | Langford RM, Mares J, Novotna A, Vachova M, Novakova I, Notcutt W, et al. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J Neurol. 2013 Apr;260(4):984-97 | CrossRef | PubMed |
Langford RM, Mares J, Novotna A, Vachova M, Novakova I, Notcutt W, et al. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J Neurol. 2013 Apr;260(4):984-97 | CrossRef | PubMed | GW Pharmaceuticals Ltd. Sativex Versus Placebo When Added to Existing Treatment for Central Neuropathic Pain in MS. clinicaltrials.gov. 2006 | Link |
GW Pharmaceuticals Ltd. Sativex Versus Placebo When Added to Existing Treatment for Central Neuropathic Pain in MS. clinicaltrials.gov. 2006 | Link | Leocani L, Nuara A, Houdayer E, Del Carro U, Straffi L, Martinelli V, et al. Effect of THC-CBD oromucosal spray (Sativex) on measures of spasticity in multiple sclerosis: a double-blind, placebo-controlled, crossover study. Joint Americas Committee for Treatment and Research in Multiple Sclerosis ACTRIMS—European Committee for Treatment and Research in Multiple Sclerosis ECTRIMS Meeting. 2014 | Link |
Leocani L, Nuara A, Houdayer E, Del Carro U, Straffi L, Martinelli V, et al. Effect of THC-CBD oromucosal spray (Sativex) on measures of spasticity in multiple sclerosis: a double-blind, placebo-controlled, crossover study. Joint Americas Committee for Treatment and Research in Multiple Sclerosis ACTRIMS—European Committee for Treatment and Research in Multiple Sclerosis ECTRIMS Meeting. 2014 | Link | Martyn CN, Illis LS, Thom J. Nabilone in the treatment of multiple sclerosis. Lancet. 1995 Mar 4;345(8949):579 | PubMed |
Martyn CN, Illis LS, Thom J. Nabilone in the treatment of multiple sclerosis. Lancet. 1995 Mar 4;345(8949):579 | PubMed | Meinck HM, Schönle PW, Conrad B. Effect of cannabinoids on spasticity and ataxia in multiple sclerosis. J Neurol. 1989 Feb;236(2):120-2 | PubMed |
Meinck HM, Schönle PW, Conrad B. Effect of cannabinoids on spasticity and ataxia in multiple sclerosis. J Neurol. 1989 Feb;236(2):120-2 | PubMed | Notcutt W, Price M, Miller R, Newport S, Phillips C, Simmons S, et al. Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 'N of 1' studies. Anaesthesia. 2004 May;59(5):440-52 | PubMed |
Notcutt W, Price M, Miller R, Newport S, Phillips C, Simmons S, et al. Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 'N of 1' studies. Anaesthesia. 2004 May;59(5):440-52 | PubMed | GW Pharmaceuticals Ltd. A Study to Evaluate the Effects of Cannabis Based Medicine in Patients With Pain of Neurological Origin. clinicaltrials.gov. 2002 | Link |
GW Pharmaceuticals Ltd. A Study to Evaluate the Effects of Cannabis Based Medicine in Patients With Pain of Neurological Origin. clinicaltrials.gov. 2002 | Link | Notcutt W, Langford R, Davies P, Ratcliffe S, Potts R. A placebo-controlled, parallel-group, randomized withdrawal study of subjects with symptoms of spasticity due to multiple sclerosis who are receiving long-term Sativex® (nabiximols). Mult Scler. 2012 Feb;18(2):219-28 | CrossRef | PubMed |
Notcutt W, Langford R, Davies P, Ratcliffe S, Potts R. A placebo-controlled, parallel-group, randomized withdrawal study of subjects with symptoms of spasticity due to multiple sclerosis who are receiving long-term Sativex® (nabiximols). Mult Scler. 2012 Feb;18(2):219-28 | CrossRef | PubMed | Novotna A, Mares J, Ratcliffe S, Novakova I, Vachova M, Zapletalova O, et al; Sativex Spasticity Study Group. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex(®) ), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol. 2011 Sep;18(9):1122-31 | CrossRef | PubMed |
Novotna A, Mares J, Ratcliffe S, Novakova I, Vachova M, Zapletalova O, et al; Sativex Spasticity Study Group. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex(®) ), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol. 2011 Sep;18(9):1122-31 | CrossRef | PubMed | Petro DJ, Ellenberger C Jr. Treatment of human spasticity with delta 9-tetrahydrocannabinol. J Clin Pharmacol. 1981 Aug-Sep;21(8-9 Suppl):413S-416S. | PubMed |
Petro DJ, Ellenberger C Jr. Treatment of human spasticity with delta 9-tetrahydrocannabinol. J Clin Pharmacol. 1981 Aug-Sep;21(8-9 Suppl):413S-416S. | PubMed | Robson P, Wade D, Makela P, House H, Bateman C. Cannabis-based medicinal extract (Sativex) produced significant improvements in a subjective measure of spasticity which were maintained on long-term treatment with no evidence of tolerance. IACM 3rd Conference on Cannabinoids in Medicine. 2005 | Link |
Robson P, Wade D, Makela P, House H, Bateman C. Cannabis-based medicinal extract (Sativex) produced significant improvements in a subjective measure of spasticity which were maintained on long-term treatment with no evidence of tolerance. IACM 3rd Conference on Cannabinoids in Medicine. 2005 | Link | Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler. 2004 Aug;10(4):434-41 | PubMed |
Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler. 2004 Aug;10(4):434-41 | PubMed | GW Pharmaceuticals Ltd. An Investigation of Delta-9-tetrahydrocannabinol (THC) and Cannabidiol (CBD) in Multiple Sclerosis Patients. clinicaltrials.gov. 2001 | Link |
GW Pharmaceuticals Ltd. An Investigation of Delta-9-tetrahydrocannabinol (THC) and Cannabidiol (CBD) in Multiple Sclerosis Patients. clinicaltrials.gov. 2001 | Link | Rog DJ, Nurmikko TJ, Young CA. Oromucosal delta9-tetrahydrocannabinol/cannabidiol for neuropathic pain associated with multiple sclerosis: an uncontrolled, open-label, 2-year extension trial. Clin Ther. 2007 Sep;29(9):2068-79 | PubMed |
Rog DJ, Nurmikko TJ, Young CA. Oromucosal delta9-tetrahydrocannabinol/cannabidiol for neuropathic pain associated with multiple sclerosis: an uncontrolled, open-label, 2-year extension trial. Clin Ther. 2007 Sep;29(9):2068-79 | PubMed | Rog DJ, Nurmikko T, Young C, Sarantis NS. Randomized controlled trial of sativex, a cannabis based medicine (CBM), in central neuropathic pain due to multiple sclerosis, followed by an open-label extension. Neurology. 2006;66(5):A31-A31 | Link |
Rog DJ, Nurmikko T, Young C, Sarantis NS. Randomized controlled trial of sativex, a cannabis based medicine (CBM), in central neuropathic pain due to multiple sclerosis, followed by an open-label extension. Neurology. 2006;66(5):A31-A31 | Link | Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology. 2005 Sep 27;65(6):812-9 | PubMed |
Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology. 2005 Sep 27;65(6):812-9 | PubMed | Young CA, Rog DJ. Randomised controlled trial of cannabis based medicinal extracts (CBME) in central neuropathic pain due to multiple sclerosis. IV Congress of the European Federation of IASP Chapters (EFIC). 2003 | Link |
Young CA, Rog DJ. Randomised controlled trial of cannabis based medicinal extracts (CBME) in central neuropathic pain due to multiple sclerosis. IV Congress of the European Federation of IASP Chapters (EFIC). 2003 | Link | GW Pharmaceuticals Ltd. A Study of Sativex in the Treatment of Central Neuropathic Pain Due to Multiple Sclerosis. clinicaltrials.gov. 2002 | Link |
GW Pharmaceuticals Ltd. A Study of Sativex in the Treatment of Central Neuropathic Pain Due to Multiple Sclerosis. clinicaltrials.gov. 2002 | Link | Serpell MG, Notcutt W, Collin C. Sativex long-term use: an open-label trial in patients with spasticity due to multiple sclerosis. J Neurol. 2013 Jan;260(1):285-95 | CrossRef | PubMed |
Serpell MG, Notcutt W, Collin C. Sativex long-term use: an open-label trial in patients with spasticity due to multiple sclerosis. J Neurol. 2013 Jan;260(1):285-95 | CrossRef | PubMed | Svendsen KB, Jensen TS, Bach FW. [Effect of the synthetic cannabinoid dronabinol on central pain in patients with multiple sclerosis—secondary publication]. Ugeskr Laeger. 2005 Jun 20;167(25-31):2772-4 | PubMed |
Svendsen KB, Jensen TS, Bach FW. [Effect of the synthetic cannabinoid dronabinol on central pain in patients with multiple sclerosis—secondary publication]. Ugeskr Laeger. 2005 Jun 20;167(25-31):2772-4 | PubMed | Svendsen KB, Jensen TS, Bach FW. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ. 2004 Jul 31;329(7460):253 | PubMed |
Svendsen KB, Jensen TS, Bach FW. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ. 2004 Jul 31;329(7460):253 | PubMed | Turcotte D, Doupe M, Torabi M, Gomori A, Ethans K, Esfahani F, et al. Nabilone as an adjunctive to gabapentin for multiple sclerosis-induced neuropathic pain: a randomized controlled trial. Pain Med. 2015 Jan;16(1):149-59. | CrossRef | PubMed |
Turcotte D, Doupe M, Torabi M, Gomori A, Ethans K, Esfahani F, et al. Nabilone as an adjunctive to gabapentin for multiple sclerosis-induced neuropathic pain: a randomized controlled trial. Pain Med. 2015 Jan;16(1):149-59. | CrossRef | PubMed | Ungerleider JT, Andyrsiak T, Fairbanks L, Ellison GW, Myers LW. Delta-9-THC in the treatment of spasticity associated with multiple sclerosis. Adv Alcohol Subst Abuse. 1987;7(1):39-50 | PubMed |
Ungerleider JT, Andyrsiak T, Fairbanks L, Ellison GW, Myers LW. Delta-9-THC in the treatment of spasticity associated with multiple sclerosis. Adv Alcohol Subst Abuse. 1987;7(1):39-50 | PubMed | Van Amerongen G, Beumer T, Killestein J, Groeneveld GJ. Individualized dosing of a novel oral DELTA9-THC formulation improves subjective spasticity and pain in patients with progressive multiple sclerosis. Joint Americas Committee for Treatment and Research in Multiple Sclerosis ACTRIMS—European Committee for Treatment and Research in Multiple Sclerosis ECTRIMS Meeting. 2014 | Link |
Van Amerongen G, Beumer T, Killestein J, Groeneveld GJ. Individualized dosing of a novel oral DELTA9-THC formulation improves subjective spasticity and pain in patients with progressive multiple sclerosis. Joint Americas Committee for Treatment and Research in Multiple Sclerosis ACTRIMS—European Committee for Treatment and Research in Multiple Sclerosis ECTRIMS Meeting. 2014 | Link | Vaney C, Heinzel-Gutenbrunner M, Jobin P, Tschopp F, Gattlen B, Hagen U, et al. Efficacy, safety and tolerability of an orally administered cannabis extract in the treatment of spasticity in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled, crossover study. Mult Scler. 2004 Aug;10(4):417-24. | PubMed |
Vaney C, Heinzel-Gutenbrunner M, Jobin P, Tschopp F, Gattlen B, Hagen U, et al. Efficacy, safety and tolerability of an orally administered cannabis extract in the treatment of spasticity in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled, crossover study. Mult Scler. 2004 Aug;10(4):417-24. | PubMed | Wade DT, Robson P, House H, Makela P, Aram J. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin Rehabil. 2003 Feb;17(1):21-9. | PubMed |
Wade DT, Robson P, House H, Makela P, Aram J. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin Rehabil. 2003 Feb;17(1):21-9. | PubMed | Wade DT, Makela PM, House H, Bateman C, Robson P. Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis. Mult Scler. 2006 Oct;12(5):639-45 | PubMed |
Wade DT, Makela PM, House H, Bateman C, Robson P. Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis. Mult Scler. 2006 Oct;12(5):639-45 | PubMed | Wissel J, Haydn T, Müller J, Brenneis C, Berger T, Poewe W, et al. Low dose treatment with the synthetic cannabinoid Nabilone significantly reduces spasticity-related pain: a double-blind placebo-controlled cross-over trial. J Neurol. 2006 Oct;253(10):1337-41 | PubMed |
Wissel J, Haydn T, Müller J, Brenneis C, Berger T, Poewe W, et al. Low dose treatment with the synthetic cannabinoid Nabilone significantly reduces spasticity-related pain: a double-blind placebo-controlled cross-over trial. J Neurol. 2006 Oct;253(10):1337-41 | PubMed | Zajicek JP, Hobart JC, Slade A, Barnes D, Mattison PG; MUSEC Research Group. Multiple sclerosis and extract of cannabis: results of the MUSEC trial. J Neurol Neurosurg Psychiatry. 2012 Nov;83(11):1125-32 | CrossRef | PubMed |
Zajicek JP, Hobart JC, Slade A, Barnes D, Mattison PG; MUSEC Research Group. Multiple sclerosis and extract of cannabis: results of the MUSEC trial. J Neurol Neurosurg Psychiatry. 2012 Nov;83(11):1125-32 | CrossRef | PubMed | Hobart JC, Zajicek JP. Cannabis as a symptomatic treatment for MS: Clinically meaningful MUSEC to the stiffness and walking problems of people with MS. 28th Congress of the European Committee for Treatment and Research in Multiple Sclerosis. 2012:247-247 | Link |
Hobart JC, Zajicek JP. Cannabis as a symptomatic treatment for MS: Clinically meaningful MUSEC to the stiffness and walking problems of people with MS. 28th Congress of the European Committee for Treatment and Research in Multiple Sclerosis. 2012:247-247 | Link | Zajicek J, Reif M, Schnelle M. UK MUSEC Study Investigators. Cannabis extract in the treatment of muscle stiffness and other symptoms in multiple sclerosis – results of the MUSEC study. IACM 5th Conference on Cannabinoids in Medicine. 2009 | Link |
Zajicek J, Reif M, Schnelle M. UK MUSEC Study Investigators. Cannabis extract in the treatment of muscle stiffness and other symptoms in multiple sclerosis – results of the MUSEC study. IACM 5th Conference on Cannabinoids in Medicine. 2009 | Link | Zajicek, J, Reif, M, Schnelle, M. Cannabis extract in the treatment of muscle stiffness and other symptoms in multiple sclerosis—Results of the MUSEC study. 25th Congress of the European Committee for Treatment and Research in Multiple Sclerosis. 2009 | Link |
Zajicek, J, Reif, M, Schnelle, M. Cannabis extract in the treatment of muscle stiffness and other symptoms in multiple sclerosis—Results of the MUSEC study. 25th Congress of the European Committee for Treatment and Research in Multiple Sclerosis. 2009 | Link | Institut fur Klinische Forschung, Germany. Multiple Sclerosis and Extract of Cannabis (MUSEC) Study.clinicaltrials.gov. 2006 | Link |
Institut fur Klinische Forschung, Germany. Multiple Sclerosis and Extract of Cannabis (MUSEC) Study.clinicaltrials.gov. 2006 | Link | Gesellschaft fuer klinische Forschung e.V. Multiple Sclerosis and Extract of Cannabis (MUSEC): a randomised, double-blind, placebo-controlled phase III trial to determine the efficacy and safety of a standardised oral extract of cannabis sativa for the symptomatic relief of muscle stiffness and pa. EU Clinical Trial. 2006. | Link |
Gesellschaft fuer klinische Forschung e.V. Multiple Sclerosis and Extract of Cannabis (MUSEC): a randomised, double-blind, placebo-controlled phase III trial to determine the efficacy and safety of a standardised oral extract of cannabis sativa for the symptomatic relief of muscle stiffness and pa. EU Clinical Trial. 2006. | Link | FDA and Marijuana: Questions and Answers. Disponible en: http://www.fda.gov/newsevents/publichealthfocus/ucm421168.htm#notapproved). Accedido el 20 de Diciembre de 2016. | Link |
FDA and Marijuana: Questions and Answers. Disponible en: http://www.fda.gov/newsevents/publichealthfocus/ucm421168.htm#notapproved). Accedido el 20 de Diciembre de 2016. | Link | EMEA-000181-PIP02-13. Disponible en: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/pips/EMEA-000181-PIP02-13/pip_001257.jsp&mid=WC0b01ac058001d129. Accedido el 20 de Diciembre de 2016. | Link |
EMEA-000181-PIP02-13. Disponible en: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/pips/EMEA-000181-PIP02-13/pip_001257.jsp&mid=WC0b01ac058001d129. Accedido el 20 de Diciembre de 2016. | Link | Scolding N, Barnes D, Cader S, Chataway J, Chaudhuri A, Coles A, et al. Association of British Neurologists: revised (2015) guidelines for prescribing disease-modifying treatments in multiple sclerosis. Pract Neurol. 2015 Aug;15(4):273-9 | CrossRef | PubMed |
Scolding N, Barnes D, Cader S, Chataway J, Chaudhuri A, Coles A, et al. Association of British Neurologists: revised (2015) guidelines for prescribing disease-modifying treatments in multiple sclerosis. Pract Neurol. 2015 Aug;15(4):273-9 | CrossRef | PubMed | Medical Marijuana (Cannabis). Disponible en: http://www.nationalmssociety.org/Treating-MS/Complementary-Alternative-Medicines/Marijuana. Accedido el 20 de Diciembre de 2016. | Link |
Medical Marijuana (Cannabis). Disponible en: http://www.nationalmssociety.org/Treating-MS/Complementary-Alternative-Medicines/Marijuana. Accedido el 20 de Diciembre de 2016. | Link | GW Pharmaceuticals Ltd. A Study of Sativex® for Relief of Spasticity in Subjects With Multiple Sclerosis. clinicaltrials.gov. 2002
GW Pharmaceuticals Ltd. A Study of Sativex® for Relief of Spasticity in Subjects With Multiple Sclerosis. clinicaltrials.gov. 2002