 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Apart from involving skin, psoriasis can compromise the nails and adjacent structures. Even though there are multiple therapeutic alternatives, there is great interest in biological therapy, but no consensus on its role exists. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified two systematic reviews including three randomized trials. We combined the evidence using meta-analysis and generated a summary of findings table following the GRADE approach. We concluded it is not clear whether biological therapy is superior to placebo in the treatment of nail psoriasis because the certainty of the evidence is very low.
Psoriasis is a common disease that can affect skin, joints and nails. Nail involvement may be up to 50 % of patients and it is considered more difficult to treat. It corresponds to an autoimmune disorder mediated by T cells that interact with keratinocytes and other cells of the skin.
The treatment of psoriasis with biologic agents is routinely used in patients with moderate to severe psoriasis or refractory psoriatic arthritis. Good results in these situations have raised interest in evaluating them in the treatment of nail psoriasis
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information, we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
|
What is the evidence. |
We found two systematic reviews [1],[2] that include five primary studies reported in 10 references [3],[4],[5],[6],[7],[8],[9],[10],[11],[12], of which three (eight references [3],[4],[5],[6],[7],[8],[9],[10]) correspond to randomized controlled trials. This table and the overall summary are based on the latter. |
|
What types of patients were included |
All studies included adult patients, over 18 years old, of both sexes, with nail psoriasis and no other nail disease. Two studies included patients with moderate to severe psoriasis with at least 6 months from diagnosis and Psoriasis Area and Severity Index (PASI) > 12 [5],[6]. One study included patients with active psoriatic arthritis [4]. Studies of patients with pustular psoriasis of the nails, acropustulosis keratotica and acrodermatitis continua of Hallopeau were excluded. |
|
What types of interventions were included |
One study evaluated subcutaneous golimumab 50 or 100 mg at weeks 0, 4, 8, 12, 16 and 20 [4]; patients that at week 16 had less than 10% improvement had an escalation of therapy (placebo to golimumab 50 mg or golimumab 50 mg to 100 mg). It was compared with placebo at week 24 and then a crossing over was done to complete 24 more weeks. One study used subcutaneous ustekinumab 45 mg or 90 mg at weeks 0 and 4 and then every 12 weeks until week 52 [5]. Patients with improvement <50% in PASI at 28 weeks discontinued the intervention. This was compared to placebo at weeks 0 and 4, and then a crossover to ustekinumab 45 or 90 mg at week 12 was performed, administering treatment at week 16, 28, 40 and One study used intravenous infliximab 5mg/kg at week 0, 2 and 6 and every 8 weeks up to week 46 [6]: This was compared against placebo until week 24 and then crossing over to infliximab 5 mg/kg on week 24, 26 and 30 and then every 8 weeks to complete. |
|
What types of outcomes |
Nail psoriasis was measured in all studies with Nail Psoriasis Severity Index (NAPSI) in the most affected nail. Overall improvement in nail psoriasis by clinical assessment and the degree of improvement according to patient’s opinion were also assessed. Other outcomes were: adverse effects, quality of life and improvement on nail features (pain, thickness, hyperkeratosis, number of affected nails and growth). |
The information on the effects of biological therapy for nail psoriasis is based on three randomized trials involving 694 patients. All studies reported the severity of nail psoriasis and adverse effects.
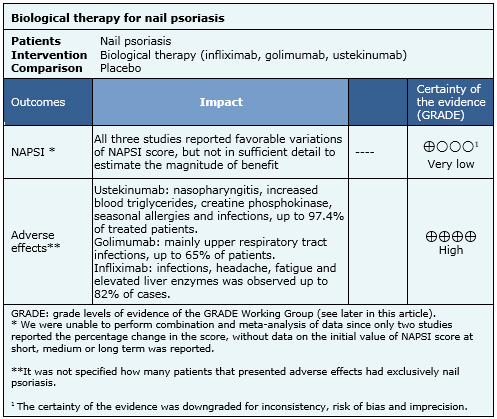

|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
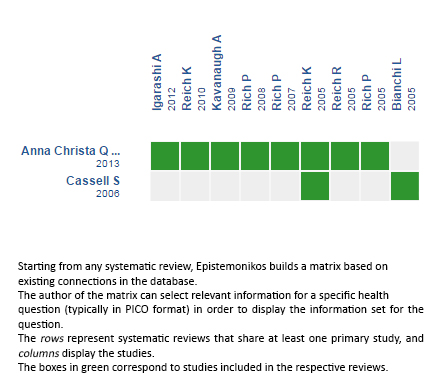
Follow the link to access the interactive version: Biologic therapy for nail psoriasis
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Apart from involving skin, psoriasis can compromise the nails and adjacent structures. Even though there are multiple therapeutic alternatives, there is great interest in biological therapy, but no consensus on its role exists. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified two systematic reviews including three randomized trials. We combined the evidence using meta-analysis and generated a summary of findings table following the GRADE approach. We concluded it is not clear whether biological therapy is superior to placebo in the treatment of nail psoriasis because the certainty of the evidence is very low.
 Autores:
Andrea Antúnez-Lay[1,2], Jorge Cabrolier[1,2], Romina Andino-Navarrete[1,2,3]
Autores:
Andrea Antúnez-Lay[1,2], Jorge Cabrolier[1,2], Romina Andino-Navarrete[1,2,3]

Citación: Antúnez-Lay A, Cabrolier J, Andino-Navarrete R. Are biologics useful for nail psoriasis?. Medwave 2016;16(Suppl 1):e6363 doi: 10.5867/medwave.2015.6363
Fecha de publicación: 11/1/2016

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 de Vries AC, Bogaards NA, Hooft L, Velema M, Pasch M, Lebwohl M, et al. Interventions for nail psoriasis. Cochrane Database Syst Rev. 2013 Jan 31;1:CD007633.
| CrossRef | PubMed |
de Vries AC, Bogaards NA, Hooft L, Velema M, Pasch M, Lebwohl M, et al. Interventions for nail psoriasis. Cochrane Database Syst Rev. 2013 Jan 31;1:CD007633.
| CrossRef | PubMed | Cassell S, Kavanaugh AF. Therapies for psoriatic nail disease. A systematic review. J Rheumatol. 2006 Jul;33(7):1452-6. | PubMed |
Cassell S, Kavanaugh AF. Therapies for psoriatic nail disease. A systematic review. J Rheumatol. 2006 Jul;33(7):1452-6. | PubMed | Rich P, Griffiths CE, Reich K, Nestle FO, Scher RK, Li S, et al. Baseline nail disease in patients with moderate to severe psoriasis and response to treatment with infliximab during 1 year. J Am Acad Dermatol. 2008 Feb;58(2):224-31. | PubMed |
Rich P, Griffiths CE, Reich K, Nestle FO, Scher RK, Li S, et al. Baseline nail disease in patients with moderate to severe psoriasis and response to treatment with infliximab during 1 year. J Am Acad Dermatol. 2008 Feb;58(2):224-31. | PubMed | Kavanaugh A, McInnes I, Mease P, Krueger GG, Gladman D, Gomez-Reino J, et al. Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: Twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum. 2009 Apr;60(4):976-86. | CrossRef | PubMed |
Kavanaugh A, McInnes I, Mease P, Krueger GG, Gladman D, Gomez-Reino J, et al. Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: Twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum. 2009 Apr;60(4):976-86. | CrossRef | PubMed | Igarashi A, Kato T, Kato M, Song M, Nakagawa H; Japanese Ustekinumab Study Group. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012 Mar;39(3):242-52. | CrossRef | PubMed |
Igarashi A, Kato T, Kato M, Song M, Nakagawa H; Japanese Ustekinumab Study Group. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012 Mar;39(3):242-52. | CrossRef | PubMed | Reich K, Nestle FO, Papp K, Ortonne JP, Evans R, Guzzo C, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005 Oct 15-21;366(9494):1367-74. | PubMed |
Reich K, Nestle FO, Papp K, Ortonne JP, Evans R, Guzzo C, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005 Oct 15-21;366(9494):1367-74. | PubMed | Reich K, Ortonne JP, Kerkmann U, Wang Y, Saurat JH, Papp K, et al. Skin and nail responses after 1 year of infliximab therapy in patients with moderate-to-severe psoriasis: a retrospective analysis of the EXPRESS Trial. Dermatology. 2010;221(2):172-8. | CrossRef | PubMed |
Reich K, Ortonne JP, Kerkmann U, Wang Y, Saurat JH, Papp K, et al. Skin and nail responses after 1 year of infliximab therapy in patients with moderate-to-severe psoriasis: a retrospective analysis of the EXPRESS Trial. Dermatology. 2010;221(2):172-8. | CrossRef | PubMed | Reich R, Nestle F, Ortonne JP, Papp K, Evans R, Guzzo C, et al. Infliximab produces a sustained and significant improvement in psoriasis over 50 weeks of continuous therapy (Abstract O-10). The 85th BAD Annual Meeting 5-8th July 2005, Glasgow, UK. British journal of dermatology. 2005;153(Suppl 1):4. | Link |
Reich R, Nestle F, Ortonne JP, Papp K, Evans R, Guzzo C, et al. Infliximab produces a sustained and significant improvement in psoriasis over 50 weeks of continuous therapy (Abstract O-10). The 85th BAD Annual Meeting 5-8th July 2005, Glasgow, UK. British journal of dermatology. 2005;153(Suppl 1):4. | Link | Rich P, Guzzo C, Li S, Reich K. Nail psoriasis improvement is maintained in patients with moderate to severe psoriasis treated with infliximab Abstract P2716. American Academy of Dermatology 65th Annual Meeting February 2-6, 2007. J Am Acad Dermatol. 2007;56(2):AB178. | CrossRef |
Rich P, Guzzo C, Li S, Reich K. Nail psoriasis improvement is maintained in patients with moderate to severe psoriasis treated with infliximab Abstract P2716. American Academy of Dermatology 65th Annual Meeting February 2-6, 2007. J Am Acad Dermatol. 2007;56(2):AB178. | CrossRef | Rich P, Scher R, Reich K, Nestle F, Papp K, Evans R, et al. Significant response of nail psoriasis to infliximab in patients with moderate to severe psoriasis disease. 14th Congress of the European Academy of Dermatology and Venereology, London,UK. Journal of the European Academy of Dermatology and Venereology 2005;19(Suppl 2):171. | Link |
Rich P, Scher R, Reich K, Nestle F, Papp K, Evans R, et al. Significant response of nail psoriasis to infliximab in patients with moderate to severe psoriasis disease. 14th Congress of the European Academy of Dermatology and Venereology, London,UK. Journal of the European Academy of Dermatology and Venereology 2005;19(Suppl 2):171. | Link | Bianchi L, Bergamin A, de Felice C, Capriotti E, Chimenti S. Remission and time of resolution of nail psoriasis during infliximab therapy. J Am Acad Dermatol. 2005 Apr;52(4):736-7. | PubMed |
Bianchi L, Bergamin A, de Felice C, Capriotti E, Chimenti S. Remission and time of resolution of nail psoriasis during infliximab therapy. J Am Acad Dermatol. 2005 Apr;52(4):736-7. | PubMed | Cassetty CT, Alexis AF, Shupack JL, Strober BE. Alefacept in the treatment of psoriatic nail disease: a small case series. J Am Acad Dermatol. 2005 Jun;52(6):1101-2. | PubMed |
Cassetty CT, Alexis AF, Shupack JL, Strober BE. Alefacept in the treatment of psoriatic nail disease: a small case series. J Am Acad Dermatol. 2005 Jun;52(6):1101-2. | PubMed |