 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Squamous cell carcinoma of the oral cavity (OSCC) is the most relevant cancer for dental surgeons. OSCC is one of the 10 most common cancers in the world, with a late clinical detection, poor prognosis, therapeutic alternatives available are highly expensive and disfiguring [1], and today it does not have specific biomarkers (BMs).
BM term refers to a broad subcategory of medical signs which can be measured accurately and reproducibly. BMs are quantifiable characteristics of biological processes [2]. Can be categorized as
i) pharmacodynamics (interaction between a drug and a target)
ii) prognostic (indicate the likely course of a disease) or
iii) predictive (patients who are likely to respond to a particular treatment) [3].
With the flourishing omics era, thousands of potential BMs have been identified and published, representing an increase of opportunities for improved diagnosis, develop more effective treatments and better cancer prognosis. Despite the auspicious omics, in 2011 it was estimated that more than 150 thousand manuscripts documenting equal or greater number of BMs, but to date less than 100 were validated in routine clinical practice [4]. Added to this, recently The Cancer Genome Atlas (TCGA) showed that a large battery of genomics and proteomics data did not improve the prognosis potential of clinical variables in patients with different types of cancer [5].
Certainly, the potential BMs must travel a difficult road to collaborate with clinical and benefit our patients. The major limitations to solve are design failures, implementation and measurement instruments and poor sensitivity and specificity in validation field [3]. To this can be added the financial difficulty to conduct long-term studies and achieve the validation, production pressure towards research teams (short time results), basic and clinical science misunderstanding (lack of integration and pathophysiology systems).
To improve the biomarkers research quality the Early Detection Research Network (EDRN) of the National Cancer Institute in the US has study design guidance tools (http://edrn.nci.nih.gov/researchers/study-design-guidance-tools). According to these publications, for example, to identify saliva BMs in patients at risk or diagnosed with oral cancer (promising research area), biological specimens can be thawed only once and then discarded.
Under recent guidelines, the following steps are necessary for a BM candidate to reach the clinic:
i) analytical validation of the BM assay,
ii) clinical validation of the BM test,
iii) demonstration of clinical value from performance of the BM test, and
iv) regulatory approval [6].
What opportunities does the OSCC to emerging BMs?. OSCC detection is eminently visual with histopathological confirmation, but current evidence shows that this is not enough, justifying the need for BMs.
Figure 1 summarizes the OSCC "natural history". Oral mucosa exposed to a set of risk factors such as snuff, alcohol, human papilloma virus, solar radiation (exposoma) and their interactions (interactome), generates aberrant keratinocyte with ecological advantages over the rest of the microenvironment. The increased chromosomal instability creates a field of susceptibility to malignancy, which can be represented by epithelial precursor lesions, which can evolve to basement membrane degradation and invasion. This natural history has windows for OSCC management and BMs origin.
Figure 1. Natural history of oral squamous cell carcinoma of the oral cavity (OSCC)
The natural history of OSCC allows the study of different phases in the malignancy progression. One opportunity is given by the etiologic field generation, influenced by risk factors and their interactions, which promotes a susceptibility state. The passage of this state to a canzerisable field gives another opportunity to study. Once the invasion was established, from oral cancer diagnosis may emerge prognostic and predictive BMs.
Some questions could or should be answered: What measurable characteristics are representative of an oral epithelium susceptible to OSCC? Is it possible to monitor the passage of a susceptibility state to canzerisable field? Is it possible to record biological processes before molecular aberrations? Are molecular aberrations permanent, despite therapy? The mouth provides an easy access to these guidelines.
For those who work with oral cancer molecular biology, studying and searching tumor markers, they are permanent questions. By naming a omics, proteomics is rethinking their experimental designs and seeking validation. It is highly likely that in the near future -following stringent criteria- will decrease the potential BMs and increase those with clinical opportunity. This is what many research groups and clinicians are expect to oral cancer.
From the editor
This article was originally submitted in Spanish and was translated into English by the author. The Journal has not copyedited this version.
Conflicts of interests
The authors have completed the conflict of interests declaration form from the ICMJE, and declare not having any conflict of interests with the matter dealt herein.

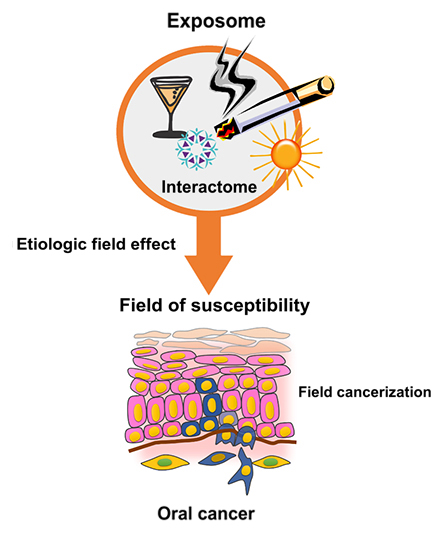 Figure 1. Natural history of oral squamous cell carcinoma of the oral cavity (OSCC)
Figure 1. Natural history of oral squamous cell carcinoma of the oral cavity (OSCC)
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

 Autor:
César Rivera[1,2]
Autor:
César Rivera[1,2]

Citación: Rivera C. Opportunities for biomarkers with potential clinical use in oral cancer. Medwave 2015 Jul;15(6):e6186 doi: 10.5867/medwave.2015.06.6186
Fecha de envío: 9/5/2015
Fecha de aceptación: 8/7/2015
Fecha de publicación: 20/7/2015
Origen: no solicitado
Tipo de revisión: con revisión por dos pares revisores externos, a doble ciego

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Rivera C, Venegas B. Histological and molecular aspects of oral squamous cell carcinoma (Review). Oncol Lett. 2014;8(1):7-11. | PubMed |
Rivera C, Venegas B. Histological and molecular aspects of oral squamous cell carcinoma (Review). Oncol Lett. 2014;8(1):7-11. | PubMed | Drucker E, Krapfenbauer K. Pitfalls and limitations in translation from biomarker discovery to clinical utility in predictive and personalised medicine. EPMA J. 2013;4(1):7. | PubMed |
Drucker E, Krapfenbauer K. Pitfalls and limitations in translation from biomarker discovery to clinical utility in predictive and personalised medicine. EPMA J. 2013;4(1):7. | PubMed | Yuan Y, Van Allen EM, Omberg L, Wagle N, Amin-Mansour A, Sokolov A, et al. Assessing the clinical utility of cancer genomic and proteomic data across tumor types. Nat Biotechnol. 2014;32(7):644-52. | PubMed |
Yuan Y, Van Allen EM, Omberg L, Wagle N, Amin-Mansour A, Sokolov A, et al. Assessing the clinical utility of cancer genomic and proteomic data across tumor types. Nat Biotechnol. 2014;32(7):644-52. | PubMed | Duffy MJ, Sturgeon CM, Soletormos G, Barak V, Molina R, Hayes DF, et al. Validation of New Cancer Biomarkers: A Position Statement from the European Group on Tumor Markers. Clin Chem. 2015 Jun;61(6):809-820. | PubMed |
Duffy MJ, Sturgeon CM, Soletormos G, Barak V, Molina R, Hayes DF, et al. Validation of New Cancer Biomarkers: A Position Statement from the European Group on Tumor Markers. Clin Chem. 2015 Jun;61(6):809-820. | PubMed |