 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Palabras clave: COVID-19, Coronavirus disease, Severe Acute Respiratory Syndrome Coronavirus 2, SARS-CoV-2 Coronavirus Infections, Systematic Review, Remdesivir, Antivirals
|
Main messages
|
COVID-19 is an infection caused by the SARS-CoV-2 coronavirus[1]. It was first identified in Wuhan, China, on 31 December 2019[2]; seven months later, more than fifteen million cases of contagion had been identified across 188 countries[3]. On 11 March 2020, the WHO characterized the COVID-19 outbreak as a pandemic[1].
While the majority of cases result in mild symptoms, some might progress to pneumonia, acute respiratory distress syndrome, and death[4],[5],[6]. The case fatality rate reported across countries, settings and age groups is highly variable, but it ranges from about 0.5% to 10%[7]. In hospitalized patients, it has been reported to be higher than 10% in some centers[8].
One of the strategies underway to identify effective interventions for COVID-19 is repurposing drugs that have been used for the treatment of other diseases. Remdesivir is among these investigational medications. It is a directly acting antiviral agent, initially developed for the treatment of Ebola virus during the 2014 outbreak in Western Africa[9]. Remdesivir displays antiviral activity against many RNA viruses, including SARS-CoV-2, in both in vitro[10] and animal studies[11].
Following the publication of the ACTT-1, a trial conducted by the National Institute of Allergy and Infectious Diseases (NIAID), the US Food and Drug Administration issued an emergency use authorization of remdesivir for the treatment of COVID-19[12].
However, the results of ACTT-1 were questioned immediately, particularly for the decision to stop it early for benefit[13]. On the other hand, the decision of the United States government to buy virtually all stocks of the drug generated an urgent need for independent, transparent information about the effects of remdesivir on COVID-19.
Using innovative and agile processes, taking advantage of technological tools, and resorting to the collective effort of several research groups, this living systematic review aims to provide a timely, rigorous and continuously updated summary of the evidence available on the effects of remdesivir in patients with COVID-19.
This manuscript complies with the 'Preferred Reporting Items for Systematic reviews and Meta-Analyses' (PRISMA) guidelines for reporting systematic reviews and meta-analyses[14] (see Appendix 1 - PRISMA Checklist).
A protocol stating the shared objectives and methodology of multiple evidence syntheses (systematic reviews and overviews of systematic reviews) to be conducted in parallel for different questions relevant to COVID-19 was published elsewhere[15]. The review was registered in PROSPERO with the number CRD42020183384, and a full protocol was made available[16].
Search strategies
Electronic searches
We used the search strategies already developed in the L·OVE (Living OVerview of Evidence) platform, a system that maps the evidence to different research questions. The full methods to maintain L·OVE are described in the website, but the process to devise the search strategies can be briefly described as:
● Identification of terms relevant to the population and intervention components of the search strategy, applying Word2vec technology[17] to the corpus of documents available in Epistemonikos Database.
● Discussion of terms with content and methods experts to identify relevant, irrelevant and missing terms.
● Creation of a sensitive boolean strategy encompassing all the relevant terms.
● Iterative analysis of articles missed by the boolean strategy, and refinement of the strategy accordingly.
All the information in the L·OVE platform comes from a repository developed and maintained by Epistemonikos Foundation through the screening of different sources relevant to COVID-19. At the time of releasing this article, this repository included more than 66 989 articles relevant to the Coronavirus disease, coming from the following databases, trial registries, preprint servers and websites relevant to COVID-19: Epistemonikos database, Pubmed, EMBASE, ICTRP Search Portal, Clinicaltrials.gov, ISRCTN registry, Chinese Clinical Trial Registry, IRCT - Iranian Registry of Clinical Trials, EU Clinical Trials Register: Clinical trials for covid-19, NIPH Clinical Trials Search (Japan) - Japan Primary Registries Network (JPRN) (JapicCTI, JMACCT CTR, jRCT, UMIN CTR), UMIN-CTR - UMIN Clinical Trials Registry, JRCT - Japan Registry of Clinical Trials, JAPIC Clinical Trials Information, Clinical Research Information Service (CRiS), Republic of Korea, ANZCTR - Australian New Zealand Clinical Trials Registry, ReBec - Brazilian Clinical Trials Registry, CTRI - Clinical Trials Registry - India, DRKS - German Clinical Trials Register, LBCTR - Lebanese Clinical Trials Registry, TCTR - Thai Clinical Trials Registry, NTR - The Netherlands National Trial Register,PACTR - Pan African Clinical Trial Registry, REPEC - Peruvian Clinical Trial Registry,SLCTR - Sri Lanka Clinical Trials Registry, medRxiv Preprints, bioRxiv Preprints, SSRN Preprints, WHO COVID-19 database.
The last version of the methods, the total number of sources screened, and a living flow diagram and report of the project is updated regularly on the website[18].
The repository is continuously updated[18], and the information is transmitted in real time to the L·OVE platform; however, it was last checked for this review the day before release on 25 August 2020. The searches covered the period from the inception date of each database, and no study design, publication status, or language restriction was applied.
The following strategy was used to retrieve from the repository the articles potentially eligible for this review: coronavir* OR coronovirus* OR betacoronavir* OR "beta-coronavirus" OR "beta-coronaviruses" OR "corona virus" OR "virus corona" OR "corono virus" OR "virus corono" OR hcov* OR covid* OR "2019-ncov" OR cv19* OR "cv-19" OR "cv 19" OR "n-cov" OR ncov* OR (wuhan* and (virus OR viruses OR viral)) OR sars* OR sari OR "severe acute respiratory syndrome" OR mers* OR "middle east respiratory syndrome" OR "middle-east respiratory syndrome" OR "2019-ncov-related" OR "cv-19-related" OR "n-cov-related" AND (remdesivir* OR "GS-5734" OR "GS 5734" OR GS5734*)
Other sources
In order to identify articles that might have been missed in the electronic searches, we proceeded as follows:
● Screened the reference lists of other systematic reviews.
● Scanned the reference lists of selected guidelines, narrative reviews, and other documents.
Eligibility criteria
We included randomized controlled trials evaluating patients infected with SARS-CoV-2 of any severity. The intervention of interest was remdesivir at any dosage, duration, timing or route of administration. The comparison of interest was a placebo (remdesivir plus standard of care versus placebo plus standard of care) or no treatment (remdesivir plus standard of care versus standard of care).
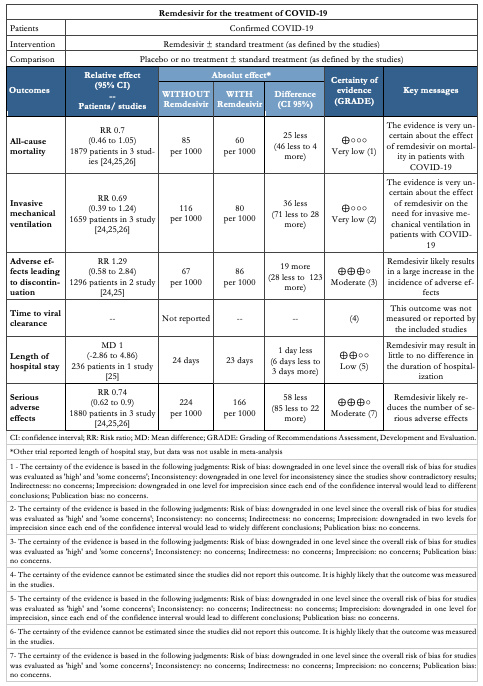
Our primary outcome of interest was all-cause mortality at longest follow-up. Secondary outcomes were invasive mechanical ventilation and adverse effects leading to discontinuation. We also extracted information on the following outcomes: time to viral clearance, length of hospital stay, and serious adverse effects.
We did not consider the outcomes as an inclusion criterion during the selection process. Any article meeting all the criteria except for the outcome criterion was preliminarily included and assessed in full text.
Selection of studies
The results of the searches in the individual sources were de-duplicated by an algorithm that compares unique identifiers (database ID, DOI, trial registry ID), and citation details (i.e. author names, journal, year of publication, volume, number, pages, article title, and article abstract). Then, the information matching the search strategy was sent in real-time to the classification tab in the L·OVE platform where at least two authors independently screened the titles and abstracts yielded against the inclusion criteria (Appendix 2). We obtained the full reports for all titles that appeared to meet the inclusion criteria or required further analysis and then decided about their inclusion.
We recorded the reasons for excluding trials in any stage of the search and outlined the study selection process in a PRISMA flow diagram that we adapted for the purpose of this project.
Extraction and management of data
Using standardized forms, two reviewers independently extracted the following data from each included trial: study design, setting, participant characteristics (including disease severity and age) and study eligibility criteria; details about the administered intervention and comparison, including dose, duration and timing (i.e. the time after diagnosis); the outcomes assessed and the time they were measured; the source of funding of the study and the conflicts of interest disclosed by the investigators; the risk of bias assessment for each individual study. We resolved disagreements by discussion, with one arbiter adjudicating unresolved disagreements.
Risk of bias assessment
The risk of bias for each randomized trial was assessed by using the 'risk of bias' tool (RoB 2.0: a revised tool to assess risk of bias in randomized trials)[19], considering the following domains of bias for each outcome result of all reported outcomes and time points: bias due to (1) the randomization process, (2) deviations from intended interventions (effects of assignment to interventions at baseline), (3) missing outcome data, (4) measurement of the outcome, and (5) selection of reported results.
Discrepancies between review authors were resolved by discussion to reach a consensus. If necessary, a third review author was consulted to achieve a decision.
Measures of treatment effect
For dichotomous outcomes, we expressed the estimate of the treatment effect of an intervention as risk ratios (RR) along with 95% confidence intervals (CI).
For continuous outcomes, we used the mean difference and standard deviation to summarize the data along with a 95% confidence interval. For continuous outcomes reported using different scales, the treatment effect was expressed as a standardized mean difference with 95% confidence interval.
Strategy for data synthesis
The results of the search and the selection of the studies is presented in the corresponding flow chart, according to recommendations of the PRISMA statement[14]. For any outcomes where it was not possible to calculate an effect estimate, a narrative synthesis is presented, describing the studies in terms of the direction and the size of effects, and any available measure of precision.
For any outcomes where data were available from more than one trial, we conducted a formal quantitative synthesis (meta-analysis) for studies clinically homogeneous using RevMan 5[20] and using the inverse variance method with the random-effects model. We assessed inconsistency by visual inspection of the forest plots and using the I² index.
Subgroup and sensitivity analysis
As few trials were found, we did not perform sensitivity or subgroup analysis.
Assessment of certainty of the evidence
The certainty of the evidence for all outcomes was judged using the Grading of Recommendations Assessment, Development and Evaluation working group methodology (GRADE Working Group)[21], across the domains of risk of bias, consistency, directness, precision and reporting bias. For the main comparisons and outcomes, we prepared a Summary of Findings (SoF) tables[22],[23].
Living evidence synthesis
An artificial intelligence algorithm deployed in the Coronavirus/COVID-19 topic of the L·OVE platform provides instant notification of articles with a high likelihood of being eligible. The authors review them, decide upon inclusion, and update the living web version of the review accordingly.
This review is part of a larger project set up to produce multiple parallel systematic reviews relevant to COVID-19[15].
Results of the search
We conducted searches using L·OVE (Living OVerview of Evidence) platform for COVID-19, a system that maps PICO questions to a repository, maintained through regular searches in 27 databases, preprint servers, trial registries and websites relevant to COVID-19. All the searches covered the period until 25 August 2020. No date or language restrictions were applied.
The search in the L·OVE platform yielded 574 records after removal of duplicates. We considered 489 as potentially eligible and obtained and evaluated their full texts. We finally included three randomized trials (11 references)[24],[25],[26].
The reasons for excluding studies at the time of full-text review were the following: not a primary study in humans (398 records); wrong study design (51 records), wrong comparison (three records) and wrong population (one record). We also identified 16 ongoing randomized trials.
The complete study selection process is summarized in the PRISMA flow chart (Figure 1) and the full list of included, excluded and ongoing trials is presented in Appendix 3.
Figure 1. PRISMA Flowchart (prepared by the authors from the study data).
Description of the included studies
The three trials identified were the Adaptive COVID-19 Treatment Trial (ACTT-1[24]), the CAP-China remdesivir 2[25] and SIMPLE 2[26]. All trials evaluated inpatient adults. ACTT-1 required for inclusion that one of the following criteria were also fulfilled: SpO2 94% on room air. Tables 1 and 2 summarize the inclusion criteria of the trials and characteristics of the intervention. More details are presented in Appendix 3. Table 1 presents the complete inclusion criteria of the trials.
Table 1. Inclusion criteria of the studies.
All trials administered the same doses of remdesivir plus standard care[24],[25],[26]. One trial included two intervention arms of remdesivir (five-day and ten-day course of remdesivir)[26]. None of the trials provides further details regarding the standard care treatment delivered. Two trials reported that the standard of care was determined by the trial site hospital[24]. The other one only reported that concomitant use of lopinavir/ritonavir, interferons, and corticoids were permitted[25].
Table 2. Characteristics of the intervention.
In total, trials included 1 896 hospitalized patients[24],[25],[26]. One trial was conducted in China[25], and the other two were multicenter trials conducted in several countries[24],[26]. All trials included patients with radiologically confirmed pneumonia[25],[26]. Baseline characteristics of participants regarding age, gender, and chronic disease were similar between studies, but the number of patients requiring supplemental oxygen or mechanical ventilation varied substantially between trials[24],[25].
Table 3. Baseline characteristics of the participants.
Risk of bias in the included studies
We judge that the overall risk of bias was "high" for all outcomes regarding the ACTT-1 trial[24]. The study was judged to raise "some concerns'' in deviations from the intended intervention domain and "high" in bias due to missing outcome data. CAP-China remdesivir 2 trial overall risk of bias was "some concern" for all outcomes, because of problems in the randomization process[25]. SIMPLE 2 overall risk of bias was some concern for all outcomes due to deviations from intended interventions[26]. Table 4 summarizes the risk of bias assessments and details of each assessment are presented in Appendix 3.
Table 4. Risk of bias in the included studies assessed by ROB-2 tool.
Efficacy of remdesivir in the treatment of patients with COVID-19
The main results are summarized in the Summary of Findings table, presented at the beginning of the manuscript.
Primary outcome
All-cause mortality
All studies reported this outcome[24],[25],[26] and the evidence is very uncertain about the effect of remdesivir on mortality (RR 0.7, 95% CI 0.46 to 1.05; very low certainty evidence).
Secondary outcomes
Invasive mechanical ventilation
All studies reported this outcome[24],[25],[26] and the evidence is very uncertain about the effect of remdesivir on the need for invasive mechanical ventilation (RR 0.69, 95% CI 0.39 to 1.24; very low certainty evidence).
Adverse effects leading to discontinuation
Two trials reported this outcome[24],[25] and remdesivir likely results in a large increase in the incidence of adverse effects (RR 1.29, 95% CI 0.58 to 2.84; moderate certainty evidence).
Time to viral clearance
This outcome was not measured or reported by the included studies.
Length of hospital stay
Two studies reported this outcome[25],[26], but only one was usable for meta analysis[25]. SIMPLE 2 trial reported that there were no significant differences between the remdesivir and standard care groups in duration of hospitalization[25]. Quantitative synthesis showed that remdesivir might result in little to no difference in the duration of hospitalization (MD 1, 95% CI -2.86 to 4.86; low certainty evidence).
Other outcomes
Serious adverse effects
All studies reported this outcome[24],[25],[26] and remdesivir likely
reduces the number of serious adverse effects (RR 0.74, 95% CI 0.62 to 0.9; moderate certainty evidence).
We conducted a systematic review and identified three randomized trials that reported data on the effect of remdesivir in patients with COVID-19[24],[25],[26]. Even though remdesivir appears to be safe, the evidence is very uncertain about the impact on the outcomes critical for decision-making in moderate and severe patients—the more relevant clinical scenario for this drug, such as mortality and need of mechanical ventilation.
It is unfortunate not knowing yet if one of the pharmaceutical interventions that have sparked more interest is effective or not. One of the limitations comes from the lack of precision of the result for the main outcomes. The early termination of the ACTT-1 trial can be seen as a missed opportunity in this regard[25]. In addition, all the trials concluded enrollment before the release of the RECOVERY trial, which showed a mortality reduction with dexamethasone[27]. It is not clear if this factor would modify the effect, if any, of remdesivir.
By now, clinicians and other decision-makers are in a difficult position. The pressure to act is high, particularly after the US Food and Drug Administration issued an emergency use authorization of remdesivir for the treatment of COVID-19[12]. We anticipate that the range of recommendations from different organizations should range between a suggestion against its use and a weak recommendation for its use in severe cases, especially in settings without resource constraints.
There are at least 46 ongoing trials that we expect will provide data in the near future. Making sense of this information is not going to be an easy task. Systematic reviews are considered the gold standard to make sense of multiple trials addressing a similar scientific question, but the traditional model for conducting reviews has several limitations, including high demand for time and resources[28] and rapid obsolescence[29]. Amid the COVID-19 crisis, researchers should make their best effort to answer the urgent needs of health decision-makers without giving up scientific accuracy. Information is being produced at a vertiginous speed[30], so alternative models are needed.
One potential solution to these shortfalls is rapid reviews, a form of knowledge synthesis that streamlines or omits specific methods of a traditional systematic review in order to move faster. Unfortunately, in many cases, this speed comes at the cost of quality[31]. Furthermore, they do not solve the issue of obsolescence. Living systematic reviews do address that issue[32]. They are continually updated by incorporating relevant new evidence as it becomes available, at a substantial effort. So, an approach combining these two models might prove more successful in providing the scientific community and other interested parties with evidence that is actionable, rapidly and efficiently produced, up to date, and of the highest quality[33].
This review is part of a larger project set up to put such an approach into practice. The project aims to produce multiple parallel living systematic reviews relevant to COVID-19 following the higher standards of quality in evidence synthesis production[15]. We believe that our methods are well suited to handle the abundance of evidence that is to come, including evidence on the role of lopinavir/ritonavir for COVID-19.
During the COVID-19 pandemic, we will maintain the search and selection of evidence for this review continuously updated, as well as an update when conclusions change or whenever there are substantial updates. Our systematic review aims to provide a high-quality, up-to-date synthesis of the evidence that is useful for clinicians and other decision-makers.
Notes
Authorship contributions
All the review authors drafted and revised the manuscript, conducted article screening and data collection, and drafted and revised the review.
The COVID-19 L·OVE Working Group was created by Epistemonikos and a number of expert teams in order to provide decision-makers with the best evidence related to COVID-19. Up-to-date information about the group and its member organizations is available here: epistemonikos.cl/working-group
Acknowledgements
The members of the COVID-19 L·OVE Working Group and Epistemonikos Foundation have made it possible to build the systems and compile the information needed by this project. Epistemonikos is a collaborative effort, based on the ongoing volunteer work of over a thousand contributors since 2012.
Competing interests
All authors declare no financial relationships with any organization that might have a real or perceived interest in this work. There are no other relationships or activities that might have influenced the submitted work.
Funding
This project was not commissioned by any organization and did not receive external funding.
Epistemonikos Foundation is providing training, support and tools at no cost for all the members of the COVID-19 L·OVE Working Group.
Ethics
As researchers will not access information that could lead to the identification of an individual participant, obtaining ethical approval was waived.
Data sharing
All data related to the project will be available. Epistemonikos Foundation will grant access to data.
PROSPERO registration number
CRD42020183384
Annexes
Annex 1.
Annex 2.
Annex 3.

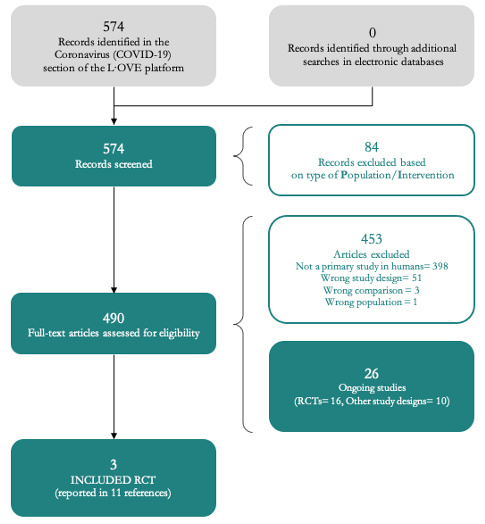 Figure 1. PRISMA Flowchart (prepared by the authors from the study data).
Figure 1. PRISMA Flowchart (prepared by the authors from the study data).

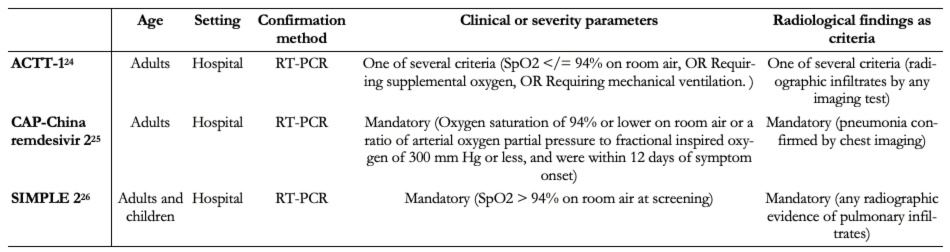 Table 1. Inclusion criteria of the studies.
Table 1. Inclusion criteria of the studies.

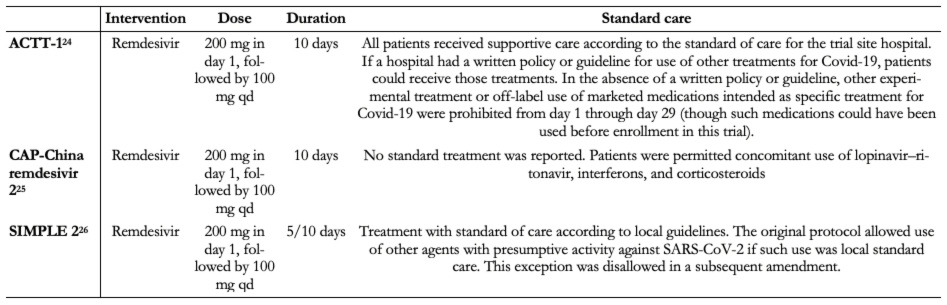 Table 2. Characteristics of the intervention.
Table 2. Characteristics of the intervention.

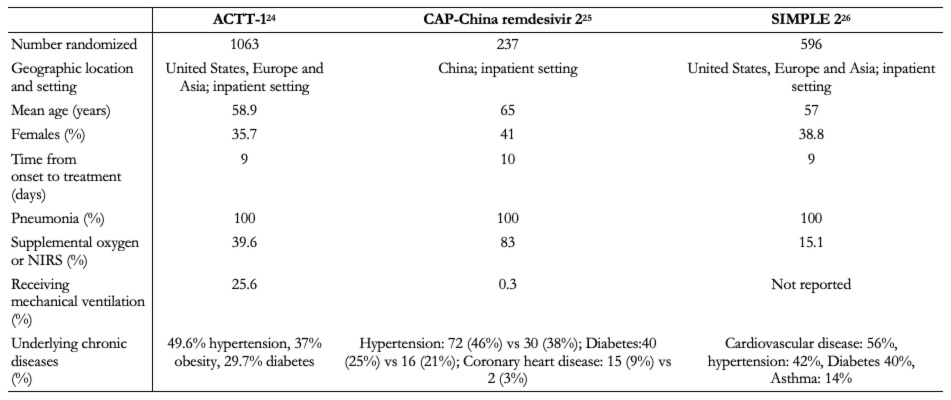 Table 3. Baseline characteristics of the participants.
Table 3. Baseline characteristics of the participants.

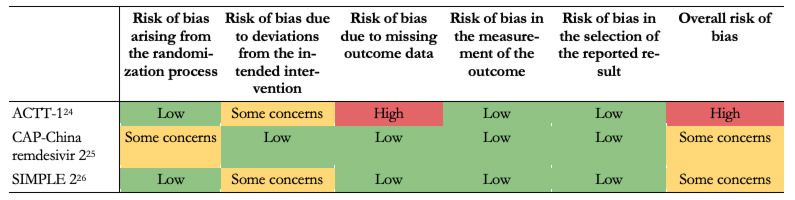 Table 4. Risk of bias in the included studies assessed by ROB-2 tool.
Table 4. Risk of bias in the included studies assessed by ROB-2 tool.

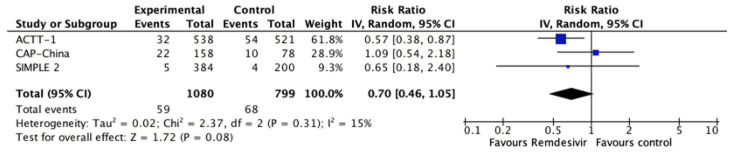 Figure 2. Relative risk for all-cause mortality for remdesivir versus standard care (prepared by the authors from the study data).
Figure 2. Relative risk for all-cause mortality for remdesivir versus standard care (prepared by the authors from the study data).

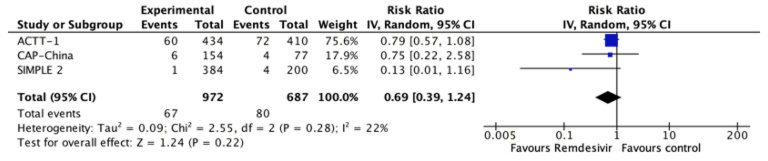 Figure 3. The relative risk for invasive mechanical ventilation for remdesivir versus standard care (prepared by the authors from the study data).
Figure 3. The relative risk for invasive mechanical ventilation for remdesivir versus standard care (prepared by the authors from the study data).

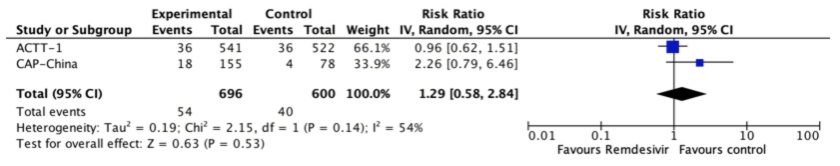 Figure 4. The relative risk for adverse effects leading to discontinuation for remdesivir versus standard care (prepared by the authors from the study data).
Figure 4. The relative risk for adverse effects leading to discontinuation for remdesivir versus standard care (prepared by the authors from the study data).

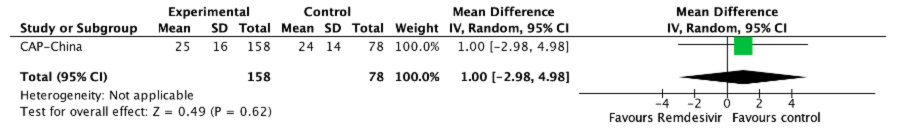 Figure 5. The relative risk for the length of hospital stay for remdesivir versus standard care (prepared by the authors from the study data).
Figure 5. The relative risk for the length of hospital stay for remdesivir versus standard care (prepared by the authors from the study data).

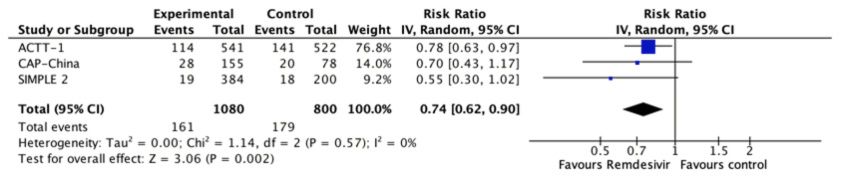 Figure 6. Relative risk for serious adverse effects for remdesivir versus standard care (prepared by the authors from the study data).
Figure 6. Relative risk for serious adverse effects for remdesivir versus standard care (prepared by the authors from the study data).
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Objetivo
Esta revisión sistemática viva tiene como objetivo entregar un resumen oportuno, riguroso y continuamente actualizado de la evidencia disponible sobre los efectos de remdesivir en pacientes con COVID-19.
Métodos
Se buscaron ensayos aleatorios que evaluaran el uso de remdesivir versus placebo o ningún tratamiento en pacientes con COVID-19. Se realizó una búsqueda en la plataforma L·OVE COVID-19 (Living OVerview of Evidence), un sistema que mantiene búsquedas regulares en bases de datos, registros de ensayos, servidores preprint y sitios web relevantes en COVID-19. Todas las búsquedas fueron realizadas hasta el 25 de agosto de 2020. No se aplicaron restricciones de fecha ni de idioma. Dos revisores evaluaron de forma independiente los artículos potencialmente elegibles, de acuerdo con criterios de selección predefinidos, y extrajeron los datos mediante un formulario estandarizado. Los resultados fueron combinados mediante un metanálisis utilizando modelos de efectos aleatorios y evaluamos la certeza de la evidencia utilizando el método GRADE. Una versión viva de esta revisión estará abiertamente disponible durante la pandemia de COVID-19.
Resultados
La búsqueda inicial arrojó 574 referencias. Finalmente, identificamos 3 ensayos aleatorios, que evaluaban el uso de remdesivir adicionado al tratamiento estándar versus tratamiento estándar. La evidencia es muy incierta acerca del efecto del remdesivir sobre la mortalidad (RR 0,7; IC del 95%: 0,46 a 1,05; certeza de la evidencia muy baja) y la necesidad de ventilación mecánica invasiva (RR 0,69; IC del 95%: 0,39 a 1,24; certeza de evidencia muy baja). Por otro lado, es probable que el uso de remdesivir produzca un aumento en la incidencia de efectos adversos en pacientes con COVID-19 (RR 1,29; IC del 95%: 0,58 a 2,84; evidencia de certeza moderada).
Conclusiones
La evidencia disponible sobre el papel del remdesivir en el tratamiento de pacientes con COVID-19 es insuficiente en relación a los desenlaces críticos para tomar decisiones, por lo que no es posible realizar un correcto balance entre los beneficios potenciales, los efectos adversos y los costos.
PROSPERO registration number
CRD42020183384
 Autores:
Francisca Verdugo-Paiva[1,2], María Paz Acuña[3,4], Iván Solá[5,6,7], Gabriel Rada[1,2,8], COVID-19 L·OVE Working Group
Autores:
Francisca Verdugo-Paiva[1,2], María Paz Acuña[3,4], Iván Solá[5,6,7], Gabriel Rada[1,2,8], COVID-19 L·OVE Working Group

Citación: Verdugo-Paiva F, Acuña MP, Solá I, Rada G. Remdesivir for the treatment of COVID-19: a living systematic review. Medwave 2020;20(11):e8080 doi: 10.5867/medwave.2020.11.8080
Fecha de envío: 28/8/2020
Fecha de aceptación: 17/11/2020
Fecha de publicación: 9/12/2020
Origen: No solicitado.
Tipo de revisión: Con revisión externa por tres pares revisores a doble ciego.

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 World Health Organization. Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020. World Health Organization; 2020 [Accessed 2020 April 12]. [Internet] | Link |
World Health Organization. Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020. World Health Organization; 2020 [Accessed 2020 April 12]. [Internet] | Link | Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020 Feb;91:264-266. | CrossRef | PubMed |
Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020 Feb;91:264-266. | CrossRef | PubMed | Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020 May;20(5):533-534. | CrossRef | PubMed |
Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020 May;20(5):533-534. | CrossRef | PubMed | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 Apr 30;382(18):1708-1720. | CrossRef | PubMed |
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 Apr 30;382(18):1708-1720. | CrossRef | PubMed | Tavakoli A, Vahdat K, Keshavarz M. Novel Coronavirus Disease 2019 (COVID-19): An Emerging Infectious Disease in the 21st Century. BPUMS. 2020;22(6):432-450. | CrossRef |
Tavakoli A, Vahdat K, Keshavarz M. Novel Coronavirus Disease 2019 (COVID-19): An Emerging Infectious Disease in the 21st Century. BPUMS. 2020;22(6):432-450. | CrossRef | Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020 Jun;92(6):577-583. | CrossRef | PubMed |
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020 Jun;92(6):577-583. | CrossRef | PubMed | Global Covid-19 Case Fatality Rates. UK: Centre for Evidence-Based Medicine [Accessed 2020 12 April]. [Internet] | Link |
Global Covid-19 Case Fatality Rates. UK: Centre for Evidence-Based Medicine [Accessed 2020 12 April]. [Internet] | Link | Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020 Mar-Apr;34:101623. | CrossRef | PubMed |
Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020 Mar-Apr;34:101623. | CrossRef | PubMed | Siegel D, Hui HC, Doerffler E, Clarke MO, Chun K, Zhang L, et al. Discovery and Synthesis of a Phosphoramidate Prodrug of a Pyrrolo[2,1-f][triazin-4-amino] Adenine C-Nucleoside (GS-5734) for the Treatment of Ebola and Emerging Viruses. J Med Chem. 2017 Mar 9;60(5):1648-1661. | CrossRef | PubMed |
Siegel D, Hui HC, Doerffler E, Clarke MO, Chun K, Zhang L, et al. Discovery and Synthesis of a Phosphoramidate Prodrug of a Pyrrolo[2,1-f][triazin-4-amino] Adenine C-Nucleoside (GS-5734) for the Treatment of Ebola and Emerging Viruses. J Med Chem. 2017 Mar 9;60(5):1648-1661. | CrossRef | PubMed | Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017 Jun 28;9(396):eaal3653. | CrossRef | PubMed |
Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017 Jun 28;9(396):eaal3653. | CrossRef | PubMed | Williamson BN, Feldmann F, Schwarz B, Meade-White K, Porter DP, Schulz J, et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature. 2020 Sep;585(7824):273-276. | CrossRef | PubMed |
Williamson BN, Feldmann F, Schwarz B, Meade-White K, Porter DP, Schulz J, et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature. 2020 Sep;585(7824):273-276. | CrossRef | PubMed | US Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment. FDA NEWS RELEASE. 2020
US Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment. FDA NEWS RELEASE. 2020  Herper M. Inside the NIH's controversial decision to stop its big remdesivir study. STAT. 2020.
Herper M. Inside the NIH's controversial decision to stop its big remdesivir study. STAT. 2020.  Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009 Oct;62(10):1006-12. | CrossRef | PubMed |
Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009 Oct;62(10):1006-12. | CrossRef | PubMed | Rada G, Verdugo-Paiva F, Ávila C, Morel-Marambio M, Bravo-Jeria R, Pesce F, et al. Evidence synthesis relevant to COVID-19: a protocol for multiple systematic reviews and overviews of systematic reviews. Medwave. 2020 Apr 1;20(3):e7868. | CrossRef | PubMed |
Rada G, Verdugo-Paiva F, Ávila C, Morel-Marambio M, Bravo-Jeria R, Pesce F, et al. Evidence synthesis relevant to COVID-19: a protocol for multiple systematic reviews and overviews of systematic reviews. Medwave. 2020 Apr 1;20(3):e7868. | CrossRef | PubMed | Verdugo F, Acuña MP, Solà I, Rada G. Remdesivir for the treatment of COVID-19: A living systematic review protocol. OSF. 2020. | CrossRef |
Verdugo F, Acuña MP, Solà I, Rada G. Remdesivir for the treatment of COVID-19: A living systematic review protocol. OSF. 2020. | CrossRef | Methods for the special L·OVE of Coronavirus infection. Santiago: Epistemonikos Foundation [Accessed 2020 3 April]. [Internet] | Link |
Methods for the special L·OVE of Coronavirus infection. Santiago: Epistemonikos Foundation [Accessed 2020 3 April]. [Internet] | Link | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019 Aug 28;366:l4898. | CrossRef | Link |
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019 Aug 28;366:l4898. | CrossRef | Link | Review Manager (RevMan) [Software]. Version 5.3.5 Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Review Manager (RevMan) [Software]. Version 5.3.5 Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.  Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008 Apr 26;336(7650):924-6. | CrossRef | PubMed |
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008 Apr 26;336(7650):924-6. | CrossRef | PubMed | Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol. 2013 Feb;66(2):158-72. | CrossRef | PubMed |
Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol. 2013 Feb;66(2):158-72. | CrossRef | PubMed | Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. Preparing summary of findings tables and evidence profiles-continuous outcomes. J Clin Epidemiol. 2013 Feb;66(2):173-83. | CrossRef | PubMed |
Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. Preparing summary of findings tables and evidence profiles-continuous outcomes. J Clin Epidemiol. 2013 Feb;66(2):173-83. | CrossRef | PubMed | Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020 Nov 5;383(19):1813-1826. | CrossRef | PubMed |
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020 Nov 5;383(19):1813-1826. | CrossRef | PubMed | Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 May 16;395(10236):1569-1578. | CrossRef | PubMed |
Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 May 16;395(10236):1569-1578. | CrossRef | PubMed | Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A, et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial. JAMA. 2020 Sep 15;324(11):1048-1057. | CrossRef | PubMed |
Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A, et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial. JAMA. 2020 Sep 15;324(11):1048-1057. | CrossRef | PubMed | Horby P, Lim WS, Emberson J, Mafham M, Bell J, Linsell L, et al. Effect of Dexamethasone in Hospitalized Patients with COVID-19: Preliminary Report. medRxiv. 2020.
Horby P, Lim WS, Emberson J, Mafham M, Bell J, Linsell L, et al. Effect of Dexamethasone in Hospitalized Patients with COVID-19: Preliminary Report. medRxiv. 2020.  Borah R, Brown AW, Capers PL, Kaiser KA. Analysis of the time and workers needed to conduct systematic reviews of medical interventions using data from the PROSPERO registry. BMJ Open. 2017 Feb 27;7(2):e012545. | CrossRef | PubMed |
Borah R, Brown AW, Capers PL, Kaiser KA. Analysis of the time and workers needed to conduct systematic reviews of medical interventions using data from the PROSPERO registry. BMJ Open. 2017 Feb 27;7(2):e012545. | CrossRef | PubMed | Shojania KG, Sampson M, Ansari MT, Ji J, Doucette S, Moher D. How quickly do systematic reviews go out of date? A survival analysis. Ann Intern Med. 2007 Aug 21;147(4):224-33. | CrossRef | PubMed |
Shojania KG, Sampson M, Ansari MT, Ji J, Doucette S, Moher D. How quickly do systematic reviews go out of date? A survival analysis. Ann Intern Med. 2007 Aug 21;147(4):224-33. | CrossRef | PubMed | Coronavirus and the risks of 'speed science'. Reuters; 2020 [Accessed 2020 12 April]. [Internet] | Link |
Coronavirus and the risks of 'speed science'. Reuters; 2020 [Accessed 2020 12 April]. [Internet] | Link | Kelly SE, Moher D, Clifford TJ. Quality of conduct and reporting in rapid reviews: an exploration of compliance with PRISMA and AMSTAR guidelines. Syst Rev. 2016 May 10;5:79. | CrossRef | PubMed |
Kelly SE, Moher D, Clifford TJ. Quality of conduct and reporting in rapid reviews: an exploration of compliance with PRISMA and AMSTAR guidelines. Syst Rev. 2016 May 10;5:79. | CrossRef | PubMed |