 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Palabras clave: epidemiology, psoriasis prescription, biological medicinal products
Introduction
Psoriasis is a chronic disease that affects the skin. One hundred twenty-five million people around the world suffer from this condition. In specific groups of patients, the joints may also be involved. To control and follow-up patients with psoriasis, psoriasis area severity and dermatological quality of life measurements were established. Both parameters are necessary for the initiation of biological therapy, as specified in the psoriasis management guide (2015) of the national committee of rheumatological, immunological, and bone metabolism diseases of the Venezuelan Institute of Social Security.
Objective
To characterize the clinical and epidemiological variables and the prescription of biological therapy in patients with psoriasis who access the high-cost dispensing program of the Venezuelan Institute Social Security (IVSS) pharmacy.
Methods
This is a descriptive, cross-sectional study.
Results
A total of 374 patient records were assessed. The male gender was more frequent, with 56.1% (p <0.001), mostly from Caracas city. In comparing age groups with sex, a difference among these was observed (p <0.05). 57.5% previously used methotrexate, 6.68% biological, 3.2% topical steroids, and 31% did not report which type of previous therapy they had received. Amongst the clinical presentations, 70% corresponded to plaque psoriasis. 79% of the patients presented moderate activity according to the Psoriasis Area and Severity Index (PASI): Eleven percent were assessed with the Dermatology Life Quality Index (DLQI); 39% of them reported an extremely important effect. The anergic range of the Mantoux test represented 70.9% of the cases, and 0.3% took the booster evaluation. Chest X-ray was reported normal in 95% of the cases. The most demanded biological medicine was etanercept, in 52% of the cases.
Conclusions
Male gender and its association with psoriasis was an important finding. The need to improve the administrative components in completing the medication request formats and strengthen clinical measurements and good medical practice was also found.
|
Main messages
|
Psoriasis is a chronic disease that affects the skin; it can also affect the joint system, and approximately 125 million people worldwide suffer from the disease[1]. The International Psoriasis Council highlights the importance of conducting epidemiological studies in every country to evaluate the disease’s impact. For adults worldwide, psoriasis has an incidence of 30.3 per 100 000 person-years, with a 0.14% prevalence, and in high-income southern Latin American countries. Its proportion ranges from 1.10%, to 0.36%, to 2.96 %[2]. Among psoriasis’ manifestations, the most common type is the vulgar or the plaque type, representing 90% of cases[3].
For mild to moderate psoriasis, front-line treatment includes topical therapies such as glucocorticosteroids, vitamin D3 analogs, and combined products. 85% of patients are inserted in this range[4]. Meanwhile, patients with the severe form[5] will require the usage of systemic therapies (methotrexate, cyclosporine, retinoids)[6] on their own or combined with biological therapies, among which are tumor necrosis factor inhibitors (TNF) -α (infliximab, adalimumab and etanercept ), as currently approved by the health authority in our country.
For the control and monitoring of patients with psoriasis, the application of psoriasis area severity index (PASI) and the application of dermatological life quality index (DLQI)[7] are established. These two parameters are necessary for the beginning of biological therapy established by the psoriasis management guide (2015) of the national committee of rheumatologic, immunologic, and bone metabolism diseases of the Venezuelan Institute of Social Security[8].
The creation of the management guide mentioned above was the result of the approved changes by the evaluation of the psoriasis program by the committee mentioned above[9], for which it was necessary to modify the norm that regulated the biological therapy dispensing on patients with psoriasis, and above all to provide timely and safe access to these as well as to offer to the physician prescriber mechanisms in order to utilize these medications.
This study characterizes psoriasis’s epidemiological profile, and the prescription of biological products on patients with psoriasis by the Venezuelan Institute of Social Security approved medical personnel.
Study design and locations
A descriptive, cross-sectional study was performed on 374 psoriasis patients over 18 years of age and registered in the high-cost IVSS pharmacy dispensing program. A review of the digitized files was carried out from October 2015 to May 2016. The variables to be studied, such as age, sex, origin, nationality, definitive clinical form, PASI index, DLQI index, complementary studies, previous and requested medications, were analyzed and consigned in a determined format. It was established, as exclusion criteria, the records that include insufficient clinical, socio-demographic history data in order to gather information.
Data analysis
With this information obtained, the database was assembled in Microsoft Excel 2010, ordered to allow for processing, analysis, and subsequent preparation of tables and graphics to present and discuss the results. The mean and standard deviation of continuous variables were calculated; regarding the nominal variables, their frequencies and percentages were calculated, a test of comparison of proportions and crossed tables was carried out from the chi2 statistic, and a statistically significant difference was considered when p <0.05, considering a 95% of confidence. The data were analyzed with the SPSS 20.0 version.
Ethics committee review
This current study was approved by the Bioethics IVSS Central Hospital Committee “Dr. Miguel Pérez Carreño” in compliance with the provisions of the Helsinki Declaration. Given that the study’s unit is not patients but records, the confidentiality commitment was made through the informed consent presented as an access requirement to the high-cost program following the Declaration of Taiwan.
Social-demographic characteristics and usage of topical and systemic medicines
The sample was represented by 374 histories that met the inclusion criteria, where the average age of the patients was 48 ± 18 years. The males presented as more prevalent with 56.1% (n = 210) compared to the females at 43.9% (n = 164), a difference that turned out to be statistically significant according to the comparison proportions test (Chi2 = 10.75; p <0.001). Regarding the requests for federal entity evaluations, 83% of the states analyzed constitutes the Bolivarian Republic of Venezuela, in which the highest percentage of cases came from Caracas city (the capital district) with 24.3% (n = 91), followed by Apure with 10.7% (n = 40), and then Monagas with 10.2% (n = 38). (Table 1).
Concerning the therapies used to control the disease, the type of therapy (topical or systemic) patients received to change from initiation to biological therapy was determined, finding that 57.5% (n = 215) of the patients had used methotrexate, followed by biological therapy with 6.6% (n = 25). In comparison, 31% (n = 116) of the cases did not report receiving any previous therapy. (Table 1).
Age distribution in patients with psoriasis
When analyzing psoriasis manifestations comparing age groups with gender, a statistically significant difference between the categories was observed (Chi2 = 12.26; p <0.05). In the male gender, the prevalence was between 39 and 48 years of age (n = 61), followed by the group ranging from 29 to 38 years (n = 45). Meanwhile, for females, the prevalence was found in the range between 29 and 38 years (n = 42) and then the group that ranges from 18 to 28 years (n = 37). Referring to the total number of psoriasis cases, the first group ranged between 39 and 48 years (n = 88), and the second ranged between 29 and 38 years (n = 87). The lowest proportion of patients in the analyzed groups ranged over 69 years (Graphic 1).
Distribution of therapies and time of usage in patients with psoriasis
Of the time of use of methotrexate, the most frequent group was less than 12 months (n = 73), and with 42 cases, the range was greater than 49 months. In this last interval, three cases of cyclosporine were presented. Topical steroid therapy was distributed among five cases in the less than 12-month group and four in the greater than 49-month range.
Likewise, it was observed that 6.6% (n = 25) of patients had had prior biological therapy, where the most consumed drug was etanercept with 52% (n = 13). Adalimumab with 32% (n = 8) was second, and the third on the line was infliximab with 16% (n = 4). The most frequent usage was the range of greater than 49 months, where etanercept is present in seven cases, four cases correspond to adalimumab, and all four infliximab cases are inside this category. The second group is represented by the interval of 25 to 36 months, and in this range, only etanercept is present with four cases, followed by the range of 13 to 24 months, with adalimumab presenting two cases. (Graphic 2).
Clinical characteristics of patients with psoriasis
Within the clinical form, plaque psoriasis was predominant with 70% (n = 262), followed by psoriatic arthritis with 17.6% (n = 66), and guttate psoriasis with 4.8% (n = 18). Regarding compliance with the application of clinimetry to evaluate severity (PASI) and quality of life (DLQI), it was observed that the PASI activity scale had been filled out by 77.5% (n = 290) of patients, where the group that represented moderated activity represented 79.3% (n = 230). Regarding the DLQI quality of life scale, this was applied in 11% (n = 41), being this the category with the highest prevalence with an extremely important effect on 39% (n = 16), and in second place the moderated effect group was located with 34% (n = 14). (Table 2).
Specific complementary studies for the initiation of biological therapy
The erythrocyte sedimentation rate was performed in 91.2% (n = 341) of patients with a value of 16.8 ± 6.9 millimeters. 88% (n = 329) had aspartate aminotransferase with 34.2 ± 17.4, 87% (n = 325), alanine aminotransferase with a value of 48.5 ± 34.5, alkaline phosphatase was performed in 40% (n = 149) of the cases with 161 ± 104.3, 64% reported glycemia with 103 ± 37.6, and C-reactive protein was performed on the 78.1% (n = 292), where 69.2% (n = 202) was <6 mg / L.
The mantoux test report in patients was distributed in the following way: anergy with 70.9% (n = 265), followed by the group greater than ten millimeters with 10.4% (n = 39). The booster effect wasn't registered in 99.6% (n = 264) of the patients with an anergy mantoux test report (n = 265). The serology for the human immunodeficiency virus in 93.3% (n = 349) of the cases was negative, and for the hepatitis B virus it was negative in 90.4%, (n = 338) and 0.8% (n = 3) resulted positive. The given evaluation by the radiologist regarding the thorax radiography reported normal in 95.5% (n = 357), 1.6% (n = 6) represented pulmonary infiltrates, and cardiomegaly in 1.1% (n = 4). (Table 3).
The most required biological drug for the evaluation to change and initiate this therapy was etanercept in 52% (n = 196) followed by adalimumab in 47% (n = 176) and infliximab in a 1% ( n = 2) of the total.
When comparing males and females with psoriasis, males were more representative. This finding corresponds with the study carried out at the “Hospital Universitario” in Caracas by Marrón et al.[10], where the male sex had a higher proportion corresponding to 54%. It must be noted that while worldwide there is disagreement regarding the predominance of one sex over another, in Venezuela, the male gender is prevalent, for which we also cite another study carried out by Vivas et al.[11] where males were predominant. It could be considered that this variable would evidence the existence of a higher risk of developing psoriasis in our population.
There is no record of national studies, which precludes a comparison of the disease distributed by federal entities. The largest number of applications was filled by the capital district, which may be associated with this administrative entity’s demographics. It is the headquarters of three of the five postgraduate dermatology programs in the country and has a greater number of professionals in this medical specialty. However, the second place is occupied by the state of Apure and the third by Monagas, according to the 2017 census. In terms of population size, the first Venezuelan state occupies the 19th and second the 13th.
The study by Parisi R et al.[2] showed that there is an increase up to the group of 39 years of age in psoriasis incidence, then the incidence decreases at 40 to 49 years before increasing again with a second peak around 50 to 59 years or 60 to 69 years; finally, a decline occurs at the end of life. This differs from the results obtained, where the two largest presentation groups were between 39 and 48 years old and 29 and 38 years old. Additionally, concerning the average age, the lowest presentation occurred in patients older than 59 years. This final result may be associated with the existing differences in the population pyramid of developed countries and their differences with Venezuela when referring to percentages of the population over 59 years of age.
Before initiating biological drugs, the most widely used systemic therapy was methotrexate, which constitutes the most prescribed medication. This drug has shown its usefulness in patients with psoriasis, as indicated by the METOP study[12] where methotrexate had a favorable risk-benefit profile. As for recommendations, the authors consider the importance of evaluating the most appropriate administration route, the dosage according to the patient’s requirements, and trying to reach the maximum doses of the drug. This examination on the type of administration route (oral vs. subcutaneous) and maximum doses to be achieved is a topic that must be discussed in the institutional management guide.
In mild forms of Psoriasis, management is given by topical treatments, unlike moderate to severe forms, which use systemic therapy as a method of control once topical therapy has not fulfilled the expected effect[13].
In the formats of prescription in the Venezuelan Institute of Social Security high-cost treatment, the different management strategies such as the combination of medications and the rotation of these were not registered, as alternatives that increase their effectiveness that could improve tolerance and decrease the risk of toxicity associated with a single agent. This is evidenced in the work of Mrowietz et al.[6], who stipulated the validity of systemic monotherapy therapy when the disease was controlled, and combination therapy was recommended in patients where monotherapy failed. The finding of low reported use of topical steroids as the main or adjuvant treatment in patients with psoriasis is a fact that is not correlated with the management of this pathology, and it is not comparable to the Korean national cohort results where the use of topical therapy ranged between 88.47% and 86.92% among their patients[14].
In the study carried out by Castro et al.[15], the following findings were found related to the distribution of clinical forms: vulgar psoriasis represented 88.9%, followed by gout psoriasis, and no psoriatic arthritis cases, which matches our clinical profile of the disease where the most frequent type was vulgaris or plaque (70%). This was in contrast to psoriatic arthritis, which, in our case, represented the second presentation. It could be related to the significant presence of Spanish descendants in the country and may be consistent with the results of the study by Romero et al.[16], which demonstrated a high prevalence of psoriatic arthritis in the Spanish population with 0.58%.
Biological therapy has become an important component of care to treat moderate to severe psoriasis. Its use without complying with some requirements can generate adverse events, highlighting infections[17]; therefore, one of the recommendations is the Mantoux test’s performance and the booster effect in case of anergy. It was discovered that the most frequent Mantoux test report was the range of anergy; however, only one patient underwent the booster effect, as opposed to what is established in the institutional management guide. It must be noted that the percentage of anergy (70.9%) and the number of positive cases (23%) for the Mantoux test, coincide with the results obtained in the Spanish study by Ribera et al.[18], where the anergy group was 77%, and the positive group had 23%. The non-practice of the booster could induce the presentation of adverse reactions that compromise the patient’s safety.
For its monitoring, psoriasis requires the utilization of clinimetry (PASI, DLQI); these constitute an important factor for the change or initiation of biologics in patients without response, severe cases, and adverse event to other therapies. However, a low report of the DLQI scale could be evidenced, and the PASI, although it had a higher report, did not reach 100%, which must be a reason to reinforce and to encourage the importance of its use in daily practice, as well as to evaluate the level of medical adherence to management guidelines.
Also disturbing is a percentage of 31% of patients that did not report receiving any therapy. This type of problem was addressed in the study made by Van de Kerkhof et al.[19], which evaluated the doctors’ perspectives in the psoriasis treatment and psoriatic arthritis. The dermatologists classified 20.3 and 25.7% of their patients with severe psoriasis and severe psoriatic joint disease, respectively, of whom 74.9% were receiving topical therapy, 19.5% conventional oral therapy, and 19.6% biological. Unmet treatment needs and insufficient in the management of their patients were also recognized.
The most prescribed biological medicine was etanercept, a similar result to the one obtained by Carretero et al.[20]. Regarding the time of use of biological medicines (drug survival), it can be observed that approximately 60% maintained it for more than 49 months; these results are not similar to the Rappersberger K et al.[21] study, which obtained an estimated median survival of all biologic agents together (etanercept, adalimumab, ustekinumab) of 36 months. Due to the socio-economic problems that Venezuela is currently facing, none of these drugs are dispensed, which represents a great challenge in clinical practice.
Regarding the study’s limitations, the prescriber’s data may obviate relevant information, and it can be challenging to verify the information recorded. Because the population study comes from different care centers in the country, the prescription strategies for systemic and biological treatments may vary according to each care unit’s criteria. They may also be affected by access to specific therapies (phototherapy) that limit patients’ management.
The existence of a significant association (p < 0.05) of sex and age groups regarding the presence of psoriasis was observed. Therefore, it is necessary to carry out studies that expand the analysis of this finding in our population. The results also show problems in filling out the requests to start the biological therapies dispensed by the Venezuelan Institute of Social Security, which generates patients who, if they require their use, see their use limited. The need to improve the clinimetry report, particularly the DLQI, as well as the performance of the booster effect is observed.
The improvement of the Venezuelan Institute of Social Security auditing systems is required to continue observing the established therapeutic behaviors and their results in safety and efficacy.
Roles and contributions of authorship
JR: conceptualization, design methodology, direction and administration of the project, research, data management, manuscript preparation (original draft development), writing (revisions and editions), allocation of resources for research. PT: formal analysis, methodology, data presentation, manuscript preparation (original draft development), writing (revisions and editions). LL: formal analysis, methodology, manuscript preparation (original draft development), writing (revisions and editions).
Declaration of conflicts of interest
The authors have completed the ICMJE conflict of interest declaration form and declare that they have no conflicts of interest with the subject of this study.
Funding
This work has been funded by the Biomedical Center for Research in Internal Medicine – CEBIMI.
Statement of ethical aspects
This study was reviewed and approved by the Bioethics Committee of the IVSS Central Hospital of the “Dr. Miguel Pérez Carreño.”
From the editors
The original version of this manuscript was submitted in Spanish. This English version was submitted by the authors and has been copyedited by the Journal.

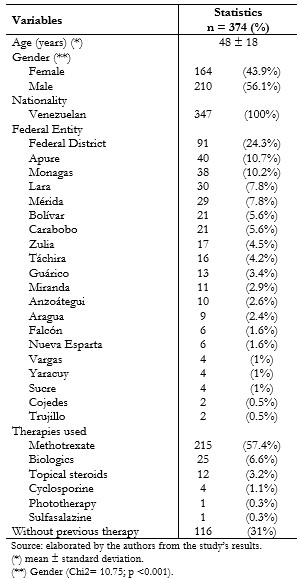 Table 1. Social-demographic characteristics of patients with psoriasis. Referred to IVSS high-cost pharmacies, from October 2015 to May 2016.
Table 1. Social-demographic characteristics of patients with psoriasis. Referred to IVSS high-cost pharmacies, from October 2015 to May 2016.

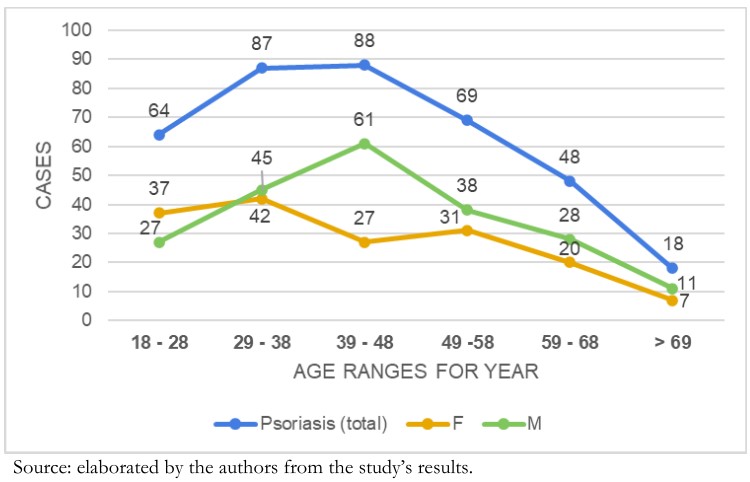 Figure 1. Age distribution in patients with psoriasis referred to IVSS high-cost pharmacies from October 2015 to May 2016.
Figure 1. Age distribution in patients with psoriasis referred to IVSS high-cost pharmacies from October 2015 to May 2016.

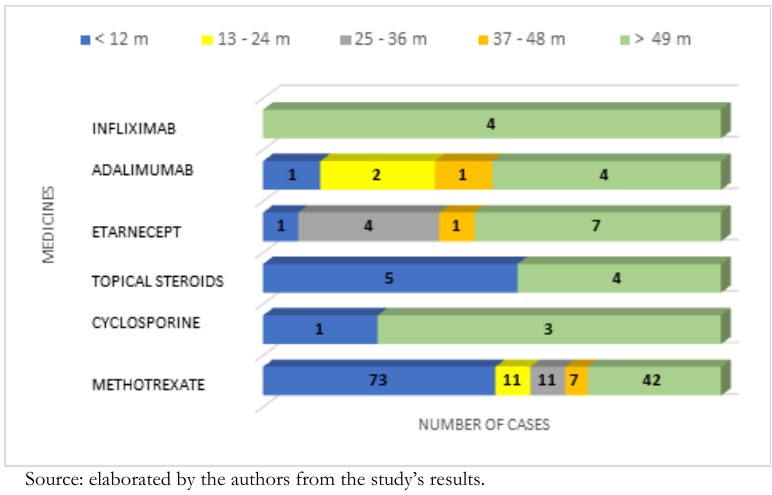 Figure 2. Distribution of therapies and time of usage on patients with psoriasis referred to IVSS high-cost pharmacies, October 2015 to May 2016.
Figure 2. Distribution of therapies and time of usage on patients with psoriasis referred to IVSS high-cost pharmacies, October 2015 to May 2016.

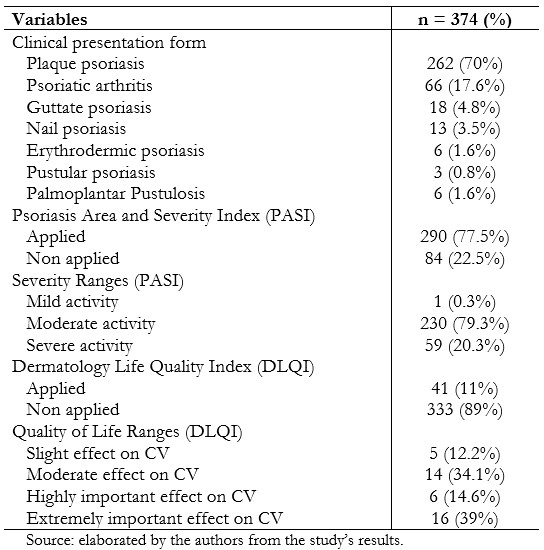 Table 2. Clinical and clinimetry characteristics in patients with psoriasis referred to IVSS high-cost pharmacies, October 2015 to May 2016.
Table 2. Clinical and clinimetry characteristics in patients with psoriasis referred to IVSS high-cost pharmacies, October 2015 to May 2016.

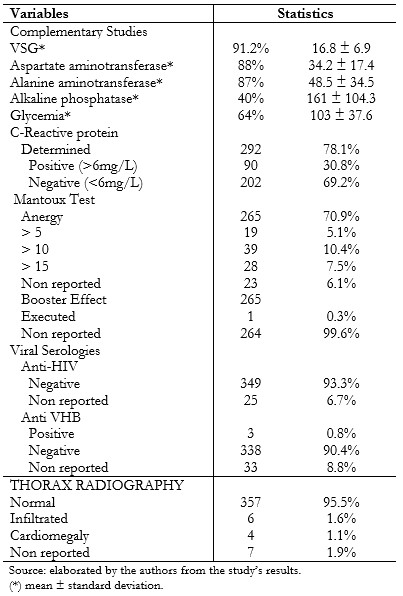 Table 3. Complementary studies and thorax X-ray prior to initiation of biological therapy in patients with psoriasis referred to IVSS high-cost pharmacies, October 2015 to May 2016.
Table 3. Complementary studies and thorax X-ray prior to initiation of biological therapy in patients with psoriasis referred to IVSS high-cost pharmacies, October 2015 to May 2016.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Introduction
Psoriasis is a chronic disease that affects the skin. One hundred twenty-five million people around the world suffer from this condition. In specific groups of patients, the joints may also be involved. To control and follow-up patients with psoriasis, psoriasis area severity and dermatological quality of life measurements were established. Both parameters are necessary for the initiation of biological therapy, as specified in the psoriasis management guide (2015) of the national committee of rheumatological, immunological, and bone metabolism diseases of the Venezuelan Institute of Social Security.
Objective
To characterize the clinical and epidemiological variables and the prescription of biological therapy in patients with psoriasis who access the high-cost dispensing program of the Venezuelan Institute Social Security (IVSS) pharmacy.
Methods
This is a descriptive, cross-sectional study.
Results
A total of 374 patient records were assessed. The male gender was more frequent, with 56.1% (p <0.001), mostly from Caracas city. In comparing age groups with sex, a difference among these was observed (p <0.05). 57.5% previously used methotrexate, 6.68% biological, 3.2% topical steroids, and 31% did not report which type of previous therapy they had received. Amongst the clinical presentations, 70% corresponded to plaque psoriasis. 79% of the patients presented moderate activity according to the Psoriasis Area and Severity Index (PASI): Eleven percent were assessed with the Dermatology Life Quality Index (DLQI); 39% of them reported an extremely important effect. The anergic range of the Mantoux test represented 70.9% of the cases, and 0.3% took the booster evaluation. Chest X-ray was reported normal in 95% of the cases. The most demanded biological medicine was etanercept, in 52% of the cases.
Conclusions
Male gender and its association with psoriasis was an important finding. The need to improve the administrative components in completing the medication request formats and strengthen clinical measurements and good medical practice was also found.
 Autores:
Jairo Rojano Rada[1], Paulette Terán Pereira[1], Liliana López Grassa[2]
Autores:
Jairo Rojano Rada[1], Paulette Terán Pereira[1], Liliana López Grassa[2]

Citación: Rojano Rada J, Terán Pereira P, López Grassa L. Clinical and epidemiological characterization of patients with psoriasis and the prescription of biological therapy in Venezuela: a cross-sectional study. Medwave 2020;20(10):e8064 doi: 10.5867/medwave.2020.10.8064
Fecha de envío: 20/4/2020
Fecha de aceptación: 27/10/2020
Fecha de publicación: 23/11/2020
Origen: No solicitado
Tipo de revisión: Con revisión por pares externa, por cuatro árbitros a doble ciego

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Griffiths CEM, van der Walt JM, Ashcroft DM, Flohr C, Naldi L, Nijsten T, et al. The global state of psoriasis disease epidemiology: a workshop report. Br J Dermatol. 2017 Jul;177(1):e4-e7. | CrossRef | PubMed |
Griffiths CEM, van der Walt JM, Ashcroft DM, Flohr C, Naldi L, Nijsten T, et al. The global state of psoriasis disease epidemiology: a workshop report. Br J Dermatol. 2017 Jul;177(1):e4-e7. | CrossRef | PubMed | Parisi R, Iskandar IYK, Kontopantelis E, Augustin M, Griffiths CEM, Ashcroft DM, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020 May 28;369:m1590. | CrossRef | PubMed |
Parisi R, Iskandar IYK, Kontopantelis E, Augustin M, Griffiths CEM, Ashcroft DM, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020 May 28;369:m1590. | CrossRef | PubMed | Rendon A, Schäkel K. Psoriasis Pathogenesis and Treatment. Int J Mol Sci. 2019 Mar 23;20(6):1475. | CrossRef | PubMed |
Rendon A, Schäkel K. Psoriasis Pathogenesis and Treatment. Int J Mol Sci. 2019 Mar 23;20(6):1475. | CrossRef | PubMed | Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can Fam Physician. 2017 Apr;63(4):278-285. | PubMed |
Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can Fam Physician. 2017 Apr;63(4):278-285. | PubMed | Mrowietz U, de Jong EM, Kragballe K, Langley R, Nast A, Puig L, et al. A consensus report on appropriate treatment optimization and transitioning in the management of moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2014 Apr;28(4):438-53. | CrossRef | PubMed |
Mrowietz U, de Jong EM, Kragballe K, Langley R, Nast A, Puig L, et al. A consensus report on appropriate treatment optimization and transitioning in the management of moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2014 Apr;28(4):438-53. | CrossRef | PubMed | Páez E, M LMA, Cabello I, Crespo L, Chassaigne J, Chirinos ME, et al. CONSENSO VENEZOLANO DE PSORIASIS. Dermatol Venez. 2015;53(2). [On line]. | Link |
Páez E, M LMA, Cabello I, Crespo L, Chassaigne J, Chirinos ME, et al. CONSENSO VENEZOLANO DE PSORIASIS. Dermatol Venez. 2015;53(2). [On line]. | Link | González L, Rojano J, Rodríguez G, Pereira M, Alam B. Guía de manejo de psoriasis de Venezuela. Comité nacional de enfermedades reumáticas, inmunológicas, dermatológicas y metabolismo óseo del Instituto Venezolano de los Seguros Sociales. IVSS: Caracas; 2015:1-57.
González L, Rojano J, Rodríguez G, Pereira M, Alam B. Guía de manejo de psoriasis de Venezuela. Comité nacional de enfermedades reumáticas, inmunológicas, dermatológicas y metabolismo óseo del Instituto Venezolano de los Seguros Sociales. IVSS: Caracas; 2015:1-57.  González L, Rodríguez G, Rojano J. Informe sobre uso de biológicos en pacientes con artritis reumatoide en Venezuela. Caracas: Comité nacional de enfermedades reumáticas, inmunológicas, dermatológicas y metabolismo óseo del Instituto Venezolano de los Seguros Sociales. IVSS: Caracas; 2015:1-19.
González L, Rodríguez G, Rojano J. Informe sobre uso de biológicos en pacientes con artritis reumatoide en Venezuela. Caracas: Comité nacional de enfermedades reumáticas, inmunológicas, dermatológicas y metabolismo óseo del Instituto Venezolano de los Seguros Sociales. IVSS: Caracas; 2015:1-19.  Marrón M, Flores A, Pinedo S, García R, Ruíz Á, Ferreiro MC. Estudio clínico epidemiológico de pacientes con psoriasis del Hospital Universitario de Caracas. Período 2010 – 2014. Dermatol Venez. 2016;54(2). [On line]. | Link |
Marrón M, Flores A, Pinedo S, García R, Ruíz Á, Ferreiro MC. Estudio clínico epidemiológico de pacientes con psoriasis del Hospital Universitario de Caracas. Período 2010 – 2014. Dermatol Venez. 2016;54(2). [On line]. | Link | Vivas S, Núñez, Z, González, L, Acosta, D, Puerta L, Ochoa F. Psoriasis: Perfil Clínico Epidemiológico de la consulta. Servicio de Dermatología, ciudad hospitalaria "Dr. Enrique Tejera". 2002-2012. Comunidad y salud. 2014;12(1). [On line]. | Link |
Vivas S, Núñez, Z, González, L, Acosta, D, Puerta L, Ochoa F. Psoriasis: Perfil Clínico Epidemiológico de la consulta. Servicio de Dermatología, ciudad hospitalaria "Dr. Enrique Tejera". 2002-2012. Comunidad y salud. 2014;12(1). [On line]. | Link | Warren RB, Mrowietz U, von Kiedrowski R, Niesmann J, Wilsmann-Theis D, Ghoreschi K, et al. An intensified dosing schedule of subcutaneous methotrexate in patients with moderate to severe plaque-type psoriasis (METOP): a 52 week, multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017 Feb 4;389(10068):528-537. | CrossRef | PubMed |
Warren RB, Mrowietz U, von Kiedrowski R, Niesmann J, Wilsmann-Theis D, Ghoreschi K, et al. An intensified dosing schedule of subcutaneous methotrexate in patients with moderate to severe plaque-type psoriasis (METOP): a 52 week, multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017 Feb 4;389(10068):528-537. | CrossRef | PubMed | Kimball AB, Gladman D, Gelfand JM, Gordon K, Horn EJ, Korman NJ, et al. National Psoriasis Foundation clinical consensus on psoriasis comorbidities and recommendations for screening. J Am Acad Dermatol. 2008 Jun;58(6):1031-42. | CrossRef | PubMed |
Kimball AB, Gladman D, Gelfand JM, Gordon K, Horn EJ, Korman NJ, et al. National Psoriasis Foundation clinical consensus on psoriasis comorbidities and recommendations for screening. J Am Acad Dermatol. 2008 Jun;58(6):1031-42. | CrossRef | PubMed | Han JH, Lee JH, Han KD, Seo HM, Bang CH, Park YM, et al. Epidemiology and Medication Trends in Patients with Psoriasis: A Nationwide Population-based Cohort Study from Korea. Acta Derm Venereol. 2018 Apr 16;98(4):396-400. | CrossRef | PubMed |
Han JH, Lee JH, Han KD, Seo HM, Bang CH, Park YM, et al. Epidemiology and Medication Trends in Patients with Psoriasis: A Nationwide Population-based Cohort Study from Korea. Acta Derm Venereol. 2018 Apr 16;98(4):396-400. | CrossRef | PubMed | Castro J, Casadiego E, Medina D. Descripción de los pacientes con diagnóstico de psoriasis en un centro dermatológico de referencia de Bogotá, Colombia. Dermatol Rev Mex. 2017;61(4):283-291. [On line]. | Link |
Castro J, Casadiego E, Medina D. Descripción de los pacientes con diagnóstico de psoriasis en un centro dermatológico de referencia de Bogotá, Colombia. Dermatol Rev Mex. 2017;61(4):283-291. [On line]. | Link | Romero Pérez A, Queiro R, Seoane-Mato D, Graell E, Chamizo E, Chaves Chaparro L, et al. Higher prevalence of psoriatic arthritis in the adult population in Spain? A population-based cross-sectional study. PLoS One. 2020 Jun 17;15(6):e0234556. | CrossRef | PubMed |
Romero Pérez A, Queiro R, Seoane-Mato D, Graell E, Chamizo E, Chaves Chaparro L, et al. Higher prevalence of psoriatic arthritis in the adult population in Spain? A population-based cross-sectional study. PLoS One. 2020 Jun 17;15(6):e0234556. | CrossRef | PubMed | Rivera R, García-Doval I, Carretero G, Daudén E, Sánchez-Carazo J, Ferrándiz C, et al. BIOBADADERM: registro español de acontecimientos adversos de terapias biológicas en Dermatología. Primer informe [BIOBADADERM, the Spanish Registry of Adverse Events Associated with Biologic Drugs in Dermatology: first report]. Actas Dermosifiliogr. 2011 Mar;102(2):132-41. | CrossRef | PubMed |
Rivera R, García-Doval I, Carretero G, Daudén E, Sánchez-Carazo J, Ferrándiz C, et al. BIOBADADERM: registro español de acontecimientos adversos de terapias biológicas en Dermatología. Primer informe [BIOBADADERM, the Spanish Registry of Adverse Events Associated with Biologic Drugs in Dermatology: first report]. Actas Dermosifiliogr. 2011 Mar;102(2):132-41. | CrossRef | PubMed | Ribera M, Zulaica A, Pujol C, Alonso ML, Rodriguez IM, Garcia-Calvo C, et al. Estimation of the prevalence of latent tuberculosis infection in patients with moderate to severe plaque psoriasis in Spain: The Latent study. Actas Dermosifiliogr. 2015 Dec;106(10):823-9. | CrossRef | PubMed |
Ribera M, Zulaica A, Pujol C, Alonso ML, Rodriguez IM, Garcia-Calvo C, et al. Estimation of the prevalence of latent tuberculosis infection in patients with moderate to severe plaque psoriasis in Spain: The Latent study. Actas Dermosifiliogr. 2015 Dec;106(10):823-9. | CrossRef | PubMed | van de Kerkhof PC, Reich K, Kavanaugh A, Bachelez H, Barker J, Girolomoni G, et al. Physician perspectives in the management of psoriasis and psoriatic arthritis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis survey. J Eur Acad Dermatol Venereol. 2015 Oct;29(10):2002-10. | CrossRef | PubMed |
van de Kerkhof PC, Reich K, Kavanaugh A, Bachelez H, Barker J, Girolomoni G, et al. Physician perspectives in the management of psoriasis and psoriatic arthritis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis survey. J Eur Acad Dermatol Venereol. 2015 Oct;29(10):2002-10. | CrossRef | PubMed | Carretero Hernández G, Ferrándiz C, Rivera Díaz R, Daudén Tello E, de la Cueva-Dobao P, Gómez-García FJ, et al. Description of Patients Treated with Biologic Drugs as First-Line Systemic Therapy in the BIOBADADERM Registry Between 2008 and 2016. Actas Dermosifiliogr. 2018 Sep;109(7):617-623. | CrossRef | PubMed |
Carretero Hernández G, Ferrándiz C, Rivera Díaz R, Daudén Tello E, de la Cueva-Dobao P, Gómez-García FJ, et al. Description of Patients Treated with Biologic Drugs as First-Line Systemic Therapy in the BIOBADADERM Registry Between 2008 and 2016. Actas Dermosifiliogr. 2018 Sep;109(7):617-623. | CrossRef | PubMed |