 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Palabras clave: sleep wake disorders, postural balance, older adults
Objetive
To determine the relationship between sleep quality and postural balance in older adults living in the community.
Methods
An analytical cross-sectional study was used. We recruited 53 subjects who fulfilled the inclusion criteria. The Pittsburgh Sleep Quality Index (PSQI) was administered, and a static balance test was performed through a computerized posturography to evaluate the postural balance.
Results
Fifty-three subjects 60 to 80 years old (M = 70.13; standard deviation = 6.06) were evaluated; 69.8% were women and 30.2% were men. The correlation test found a statistically significant and positive relationship, but moderate (p = 0.002; r = 0.417) between total displacement and sleep quality in the Romberg open eyes test. The same happened with the total displacement in the Romberg close eyes test (p = 0.002, r = 0.445). Displacements were always greater in the anteroposterior plane, where a statistically significant and positive correlation with sleep quality was also found in all posturological tests. The correlation was moderate in all cases.
Conclusion
There is a relationship between the quality of sleep measured and postural balance. However, this relationship is low to moderate, and the size of the effect must be considered before accepting the hypothesis.
|
Key ideas
|
The change in world demographics has increased life expectancy, thus an interesting growth in the adult population. This increase results in new social and political challenges that cater to an emerging older population of adults over the need of the under 15 years old[1]. As we age, a series of changes associated with the same process occurs, both morphologically and functionally[2]. While some changes may be related to the natural depletion of energy reserves and respond to the phenotype of aging, others may respond to associated pathological factors and aging syndromes[3].
Changes in sleep architecture accompany normal aging. There is a significant decrease in the ability to initiate and maintain sleep. It is also common to see an increased proportion of time in stages N1 and N2 sleep (lighter stages of sleep), a decreased proportion of time in stage N3 sleep (deeper stage of sleep) and in rapid eye movement, also more time to achieve the onset of sleep and increase in the number of transitions from one stage to another[4],[5]. It is possible to emphasize that a difficulty in the uninterrupted maintenance of sleep, the quantitative reduction of the phases of a deep sleep, the decrease of the threshold of excitation due to noise, increase the number of naps during the day and disturbances of sleep[6]. Excessive daytime sleepiness can affect between 10 to 33% of people and has been associated in older adults with an increased incidence of functional impairment, increased risk of falls, cognitive deficits, decreased quality of life and mortality[7]. In this area, it has been described that age is an important factor; however, the consumption of drugs, inactivity during the day, consumption of stimulating foods, and smoking[8]. Generally, the association between inadequate sleep quality and the state of health of older people is underestimated by health teams and tends to be associated with a normal aging process without considering the additional burden of disease that this entails and the increase in expenditure at the health level[4].
Most reports of sleep disorders in older people are associated with cognitive impairment[9],[10],[11], but another of the consequences that sleep-related problems have on the health of the elderly is the deterioration of physical function and postural control, commonly associated with an increased risk of falls. Falls are a serious public health problem, with a higher incidence among women than among men. Several fall risk factors have been described, which are often classified as extrinsic (such as effects of medications or risks in the home) or intrinsic (such as functional and health status). While extrinsic factors are modifiable, only some of the intrinsic factors are susceptible to modification. One of the main intrinsic risk factors for the fall is the balance deficit, which is the ability to maintain the position of the body on its support base, whether stationary or moving[12]. Although some factors are potentially modifiable, in current evidence, only exercise, multidisciplinary interventions, and drug withdrawal have been shown to reduce the falls risk[13],[14].
Because sleep duration is a modifiable factor, information regarding the quality of sleep on factors related to falls and subsequent efforts to maintain adequate duration would have major clinical implications for the prevention or maintenance of postural balance and consequent reduction of the incidence of falls[4],[13],[14].
The findings of an association between sleep problems and loss of postural balance are controversial, mainly because of the measurement methods, the psychometric characteristics of these methods, and the subjectivity that acquire part of them. But the first approximations show that specific sleep problems, such as extremely short (less than five hours) and extremely long (more than nine hours) duration, daytime sleepiness and naps during the day, seem to be significantly related to the loss of postural balance in the elderly, and therefore, to the prevalence of a fall event[15]. A U-shaped relationship has been established between the duration of sleep and the incidence of falls in older persons, where groups with seven hours of sleep showed the lowest incidence of falls[16],[17].
The brain uses all available sensory signals from the vestibular, visual, and proprioceptive inputs, which are also integrated by the central nervous system to execute appropriate motor responses to maintain balance. In this way, the deterioration of the balance related to age does not seem to behave as a standardized or isolated phenomenon, but on the contrary, it seems to be extremely variable from one subject to another[18]. Also, minor new or acute deficiencies may disproportionately affect your ability to cope with balance scenarios, as each sensory modality may already be partially impaired. Current knowledge focuses on the determination of anomalies in the balance tests and how these are related to an increased risk of falls, and to the profiling of balance disorder of selective deficiencies that can guide a specific treatment[19].
The objective of this study was to determine the relationship between postural balance (expressed as the body pressure center) and sleep quality in older persons in the community consulting in a private rehabilitation center.
Participants
For this analytical, cross-sectional study, 84 subjects were initially contacted, of which 53 accepted to participate. The participants were recruited from a private rehabilitation center. The data collection took place from August 2017 to November 2017. This study was approved by the Research Ethics Committee of the Universidad de las Américas of Chile. All participants provided written informed consent to participate in this study, which was conducted by good clinical practice and all applicable laws and regulations. First, they answered the anamnesis form, then the Pittsburgh Sleep Quality Index (PSQI) and, finally, they performed the computerized posturography test in their static equilibrium protocol in which the body pressure center was evaluated.
Procedure
The Pittsburgh Sleep Quality Index is a self-administered questionnaire of 24 questions; It has 19 self-assessment questions, and five questions addressed to the roommate or bedmate, being only the first 19, those used to obtain the overall score. The questionnaire was created by Buysse (1989)[20] to provide a general qualification of sleep quality. The Pittsburgh Sleep Quality Index investigates sleep schedules, sleep-related events, such as difficulties in starting to sleep (awakenings, nightmares, snoring, respiratory disorders), sleep quality, the intake of sleep medications and the existence of sleepiness during the day. The 19 questions are grouped into seven components that are rated on a scale of 0 to 3. The sum of the components results in a general score, where a lower score indicates a higher overall quality of sleep. In the original study by Buysse et al. (1989), the Pittsburgh Sleep Quality Index showed a high coefficient of internal homogeneity (Cronbach's α= 0.83) and moderate to high correlation coefficients between the components and the general score (Pearson r = 0.46 to 0.85). The authors established a score of less than five as a cut-off point, distinguishing subjects with poor sleep from those who sleep well, with high sensitivity and specificity (89.6 and 86.5%, respectively)[21],[22]. For the present study, only the self-administered version of 19 questions of the version validated in Spanish[23].
The computerized static posturography technique analyzes the postural control of the subject in stable standing and destabilizing conditions. For this purpose, it uses a dynamometric platform that analyzes postural oscillations by recording the vertical projection of the force of gravity. To perform these tests a method similar to the Norré Sensory Interaction Test was followed, which includes Romberg with open eyes (ROA) both on a rigid surface and on a foam rubber surface and with closed eyes (Romberg close eyes). In the Romberg with open eyes test, the participant stood on the platform with bare feet at an angle of 20°, arms extended and glued to the body. Maintaining this position, he had to face forward towards a point located on a monitor of the posturographer's own[24],[25],[26]. Additionally, the variables of body composition such as weight, length, percentage of fat, percentage of lean mass and weight of the bones were obtained by electrical bioimpedance and some sociodemographic variables with the object to characterize the sample.
The eligibility criteria included were a person 60 years of age or older who were self-sufficient in the instrumental activities of daily life, with scores higher than 43 points in Part A of the Elderly Functionality Examination of the Ministry of Health of Chile. Participants were excluded if they showed difficulties in comprehension or execution that interfered with the posturography test; subjects with acute skeletal muscle injuries that prevented or impeded the walking; subjects with labyrinth diseases; and those who consumed central nervous system depressants and labyrinth depressants (labyrinth functions), but only those who are not indicated for the regulation of sleep activity.
Data analysis
The normal distribution assumption was evaluated using the Kolmogorov-Smirnov test, as this assumption was not fulfilled for any of the variables, the inferential analysis was performed using the non-parametric Spearman Correlation test statistic with a significance level of α < 0.05. All analyses were performed in the IBM SPSS statistical program.
Ethics
This study was approved by the institutional review board of the Universidad de Las Américas, dated June 7, 2018.
A sample of 53 subjects between 60 and 80 years of age was recruited with an average of 70.13 ± 6.06; of these, 69.8% were female, and 30.2% were male. Table 1 presents some characteristics of the sample and shows that 83% of the sample referred to poor sleep quality (score < 5 points), with an average of 9.91 ± 4.26 points in the Pittsburgh Sleep Quality Index.
Table 2 presents the mean of the scores for the Total Displacement Index, the Anterior-Posterior Displacement Index, and the mediolateral displacement index in the respective tests (Romberg open eyes and Romberg close eyes). It is observed that the test with the highest total displacement was the Romberg close eyes, followed by Romberg open eyes and that in all cases the lowest index was that of the anteroposterior stability index over the mediolateral stability index.
Then the same displacement descriptors are presented for the group cataloged as "with good sleep quality" (Table 3) and "with poor sleep quality" (Table 4).
Sleep quality and total displacement
A statistically significant and positive relationship (p = 0.002) between total displacement and sleep quality in the Romberg open eyes test was found; this correlation is moderate (r = 0.417). The same occurred with total displacement in the Romberg close eyes test (p = 0.002; r = 0.445) (Figure 1-2).
Figure 2. Correlation between sleep quality (PSQI) and TSI, RCE test.
Quality of sleep and anteroposterior displacement
When evaluating movements in the anteroposterior plane, a statistically significant and positive correlation with sleep quality was also found in all posturographic tests. This correlation was moderate in all cases (p = 0.002, r = 0.417 and p = 0.003, r = 0.445; Romberg with open eyes and Romberg close eyes, respectively) (Figure 3-4).
Figure 3. Correlation between sleep quality (PSQI) and APSI, ROE test
Figure 4. Correlation between sleep quality (PSQI) and APSI, RCE test.
Sleep quality and mediolateral displacement
Finally, in evaluating the relationship of sleep quality to mediolateral displacement, no statistically significant correlation was found in any of the tests. In addition, the statistically significant values are supplemented with low effect size values (p = 0.085, r = 0.239 and p = 0.152, r = 0.217, Romberg with open eyes and Romberg close eyes, respectively) (Figure 5-6).
Figure 5. Correlation between sleep quality (PSQI) and the MLSI, ROE test.
Figure 6. Correlation between sleep quality (PSQI) and the MLSI, RCE test.
The findings of this study show a statistically significant and positive relationship of moderate character between sleep quality and postural balance; wherein all the tests the greatest displacement was found in the anterolateral plane, both in older people consigned with good and poor sleep quality (Table 2). However, in the group of older adults with poor sleep quality, the mean number of trips was higher in all planes.
Postural stability is an important function of the human body, and the results obtained in this study are based on the premise that postural control depends on the coordination of the central nervous system with visual, proprioceptive and vestibular information to control the effector organs (skeletal muscles of the lower limb, gait, and trunk)[27]. In this sense, any alteration in these integrative processes will negatively influence the postural balance. In a healthy individual, the central nervous system uses small amplitude postural oscillations to keep the body close to vertical alignment; but in older people it has been found that there is an increase in the amplitude of the body pressure center and a greater frequency of this signal in the sagittal plane, a premise consistent with the findings of this study[28],[29].
It is necessary to point out that the human body in a bipedal position manages to regulate the postural balance using the intervention of the hip and ankle muscles, while the knee usually remains in a blocking position[30]. This transforms into a suspension strategy of the knee that contributes to diminishing the center of mass to stabilize the posture during the change of the back-pressure center in the elderly, being a fully functional articulation in the vertical postural control[31]. The anteroposterior displacement, which is the one with the highest rates of displacement, is mainly regulated by the muscles of the ankle; while the displacement of the mediolateral plane is essentially based on the execution of the abductor and adductor muscles of the hip; and it is believed that the components of postural control in the frontal plane acquire importance in complex postural situations and facilitate the initiation of a lateral step to restore postural balance[32]. The literature mentions that age-related muscle atrophy increases the sensitivity of the ankle muscles to sleep loss and therefore increases the mobilization of the mediolateral muscles during quiet posture in the face of sleep deprivation. Because the hip muscles are closer than the ankle muscles to the center of the mass, older people subject to sleep loss recruit the mediolateral muscles to exert a more direct effect on the center of mass, counteracting the loss of postural balance and generating a lower inertia effect[17].
Something to take into consideration when observing the results is that the majority of the studied sample consumed more than three drugs, so they were in a polypharmacy situation. In this sense, polypharmacy has been considered as a significant risk factor for falls among the elderly[33],[34]. The risk of falls depends on the type of medication ingested, varying with drugs of the central nervous system, including antipsychotics, antiparkinsonians, and narcotic analgesics, considered to be the most strongly associated with falls[35]. On the other hand, cardiovascular drugs, especially antihypertensives, which are the most frequent drugs, still have an inconsistent relationship[34],[36]. The study by Sevilla et al. (2012) documented that 36% of the elderly have more than three chronic diseases, which means that they use a high number of drugs for long periods, including within a category of polypharmacy[37]. Regarding this, a linear negative relationship between the number of medications used and the score of the equilibrium measures has been reported[38]. Pugh et al. (2007) were the first to define a polypharmacy cut-off value, of five medicinal molecules, for adverse outcomes on functional mobility, as assessed by a static and dynamic balance test[39]. Leipzig (1999), had already documented the influence of drug use and risk of falls, even reported that polypharmacy tripled this event[40]. Some drugs have been described as risk factors for falls, among which include psychoactive drugs (such as benzodiazepines, antidepressants, and neuroleptics), as well as antiarrhythmics, digitalis, peripheral vasodilators, and insulin. Among the most prominent are antidepressant drugs that can generate adverse events such as daytime drowsiness, abnormal gait, dizziness, among others[41]. On the other hand, metformin has not been directly related to the risk of falls; however, if the neuropathy secondary to vitamin B12 deficiency and insulin dependent subjects[42]. Further research is needed to determine the effects of medication review and drug withdrawal on postural balance, as well as the comorbidities for which these drugs are prescribed.
Other factors to consider are the anthropometric variables and their incidence in the postural domain since when the Romberg close eyes test was performed; it was where the greatest displacements of the body pressure center were registered. By annulling visual information, the displacement of body pressure center be increased, either by mass distribution, height, and inertial properties of body segments, which increases the difference between the distribution of the center of mass and the center of body pressure[43]. The study by Alonso et al. (2015) suggests that a lower lean body mass and a higher waist-hip ratio may be risk factors for postural imbalance[44]. Based on the literature, anthropometric variables can be considered as another component within the affectation of sleep characteristics. Previous studies have observed that higher body mass index is associated with longer sleep duration in the elderly and that individuals classified as obese experience greater excessive daytime sleepiness[45].
On the other hand, the body mass index and the fat mass do not seem to influence the equilibrium measurements calculated in a force platform during the elderly. The men showed poorer postural control than the women for all the variables of the posture center. These results have implications for the assessment of balance and the readjustment of balance in older people related to fall prevention programs, including people with different body mass index and fat mass characteristics[46].
Piovezan et al. (2015) recorded that age-related sleep impairment acts on neuroendocrine factors associated with the development of sarcopenia. Sleep disorders such as decreased duration and impaired sleep quality favor proteolysis, modify body composition and increase the risk of insulin resistance, all factors previously associated with sarcopenia. This occurs through various pathways of protein synthesis and degradation mediated by growth hormone, insulin growth factor type 1, testosterone, cortisol, and insulin itself[47].
One of the limitations is that the results are mainly contrasted with studies carried out in non-Latin American countries, where the language, culture, and way of interpreting the health-disease process are not shared in the same way as in the geographical area of the present study. In several studies, the use of the questionnaire does not correspond with older persons to evaluate age groups, so we had limited literature to compare the results. The original version is designed for patients with psychiatric disorders; later, it was validated for different groups, and it has been culturally adapted[48]. Another limitation is that sleep questions typically used in epidemiologic studies do not closely correspond with objective measures of sleep, such as assessed using polysomnography and actigraphy[49]. These findings have implications in our study that used an auto administrated form. However, the application is based on the advantages of self-reports, which include its ease of use, convenience, low expenditure, a reflection of the natural environment, lack of relative discretion and recording of the experience of sleep perceived by the person. The objective measure alone is not enough to reflect the quality of the variable the daily functioning perceived by the person.
Functioning has multiple levels, is closely related, and must be considered when it comes to welfare. In academics, while some argue that well-being is in the subjective perception of life or the psychological functioning of a person, others argue that well-being is found in objective conditions and the broader environment[50]. Therefore, regardless of the nature of the variables, none should be dismissed.
The present investigation also indicates the need to validate the screening tools both linguistically and to the different transcultural realities, so that researchers and clinicians would have greater points of contrast. In the interpretation of the findings of the present study, it is important to consider that the variables of comorbidity of the sample were not expressly detailed, and a neater future collection is suggested.
There is a correlation between the quality of sleep measured with the Pittsburgh Sleep Quality Index and the postural balance in older people analyzed with computerized posturography. However, this relationship is low to moderate; the effect size is a factor that must be considered to accept the hypothesis easily. Future studies should address the dependence of each of the variables. Also, they could equate more objective measures for each variable. However, we emphasize that the variables that are considered more subjective should not be ignored since they can provide the daily functioning perceived and declared by the person.
Contributor Roles
LFH: Conceptualization, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing.
JAA: Conceptualization, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing.
MBM: Conceptualization, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing.
KCL: Conceptualization, Investigation, Data curation, Formal analysis, Methodology, Validation, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Competing interests
The authors have completed the declaration of conflicts of interest of the ICMJE form and declare not having received financing for the completion of the report; the authors have not financial relationships with organizations that might have interests in the published article.
Funding statement
The authors declare that there were no external sources of funding.
Statement on the multiple affiliations of the authors
From the editors
The manuscript was submitted in English. This article has been lightly edited by the editorial office.

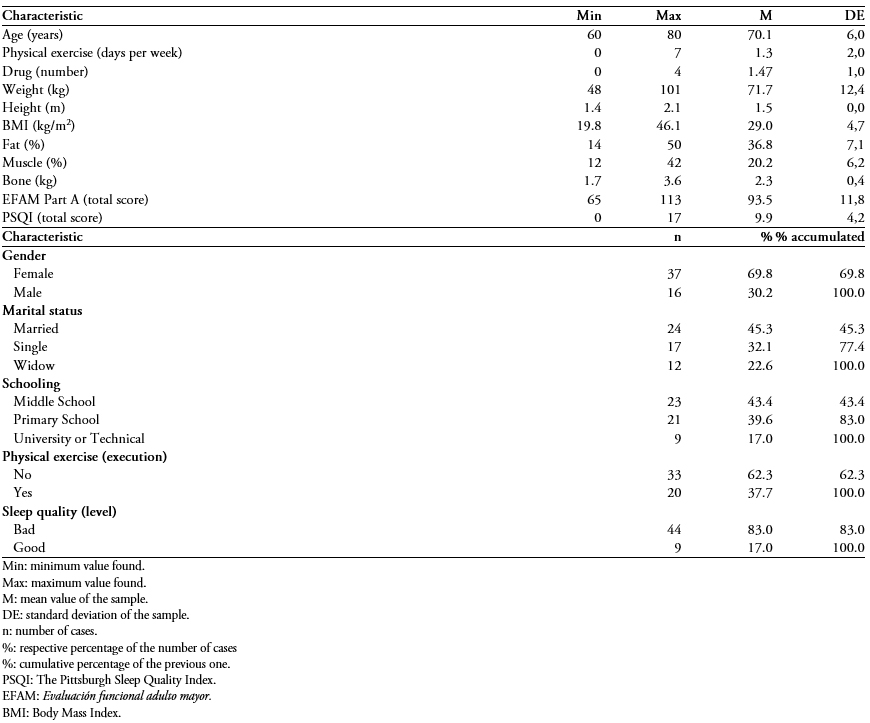 Table 1. Descriptive characteristics of the sample of 53 older people attending a private rehabilitation center.
Table 1. Descriptive characteristics of the sample of 53 older people attending a private rehabilitation center.

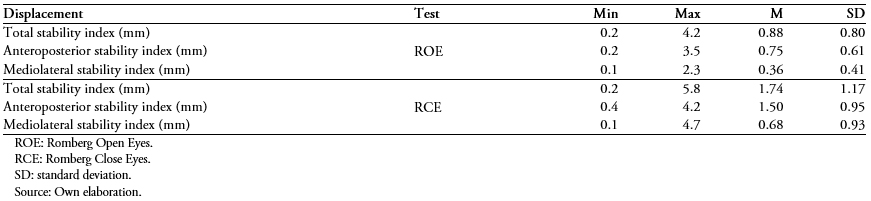 Table 2. Static posturograph stability test for 53 older people attending a private rehabilitation center.
Table 2. Static posturograph stability test for 53 older people attending a private rehabilitation center.

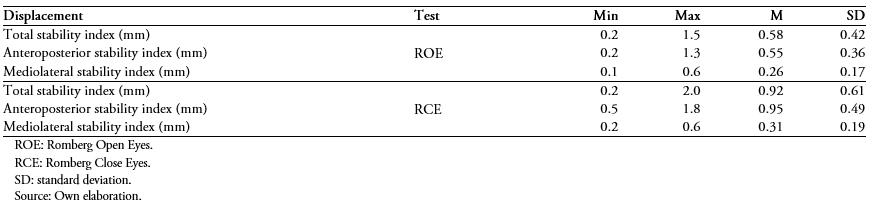 Table 3. Static posturograph stability test for older adults classified in the "good sleep quality" category (n = 9).
Table 3. Static posturograph stability test for older adults classified in the "good sleep quality" category (n = 9).

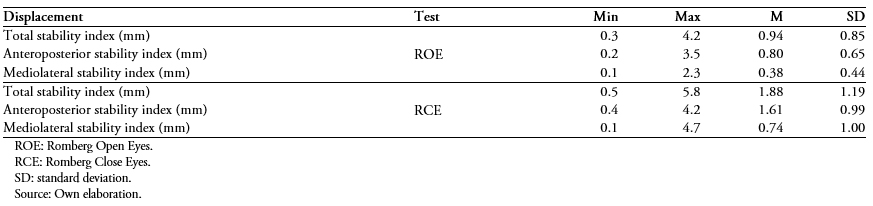 Table 4. Static posturograph stability test for older adults classified in the "poor sleep quality" category (n = 44).
Table 4. Static posturograph stability test for older adults classified in the "poor sleep quality" category (n = 44).

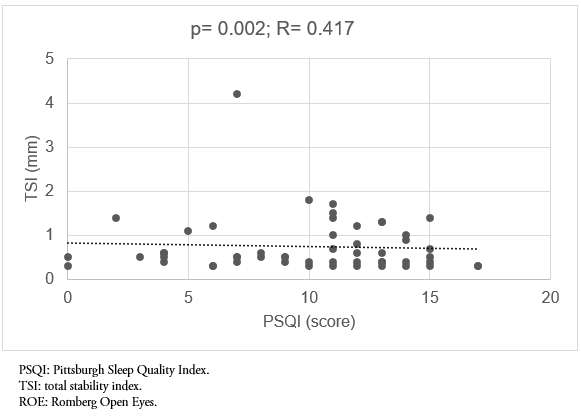 Figure 1. Correlación entre la calidad del sueño (PSQI) y el índice de estabilidad total, prueba de ROA.
Figure 1. Correlación entre la calidad del sueño (PSQI) y el índice de estabilidad total, prueba de ROA.

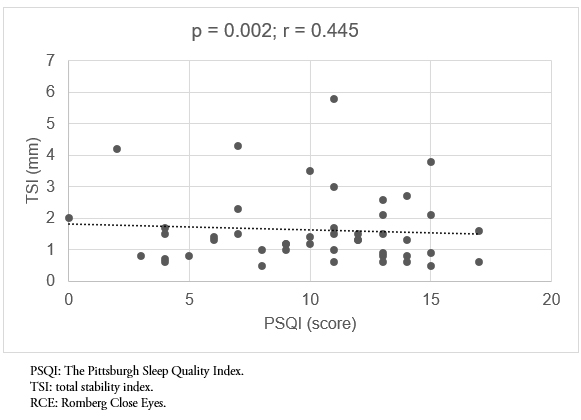 Figure 2. Correlation between sleep quality (PSQI) and TSI, RCE test.
Figure 2. Correlation between sleep quality (PSQI) and TSI, RCE test.

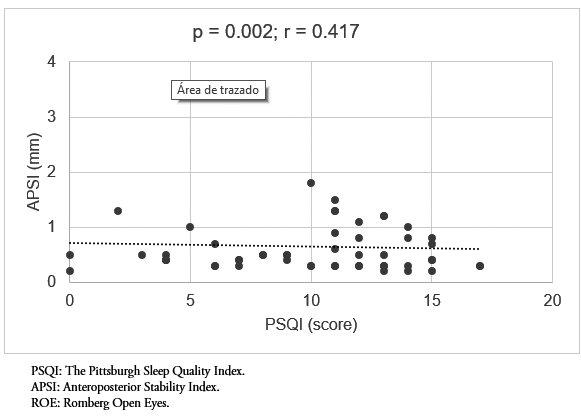 Figure 3. Correlation between sleep quality (PSQI) and APSI, ROE test
Figure 3. Correlation between sleep quality (PSQI) and APSI, ROE test

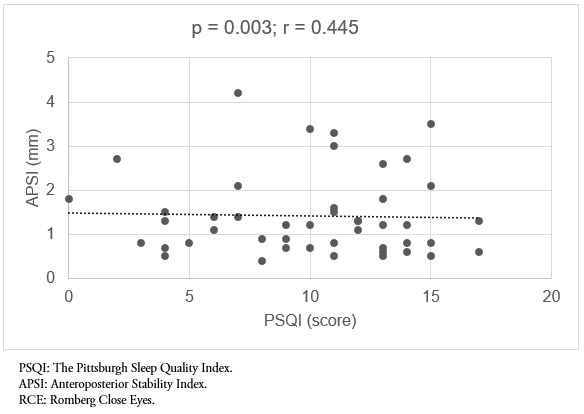 Figure 4. Correlation between sleep quality (PSQI) and APSI, RCE test.
Figure 4. Correlation between sleep quality (PSQI) and APSI, RCE test.

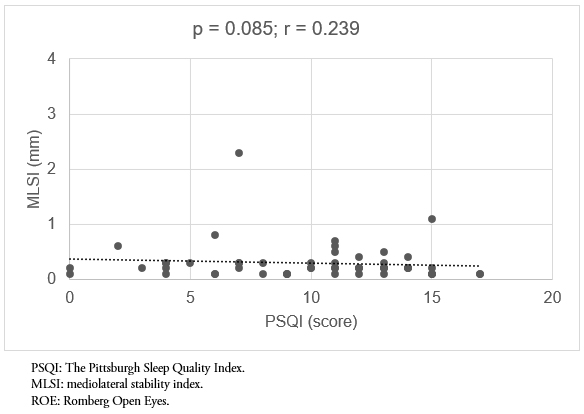 Figure 5. Correlation between sleep quality (PSQI) and the MLSI, ROE test.
Figure 5. Correlation between sleep quality (PSQI) and the MLSI, ROE test.

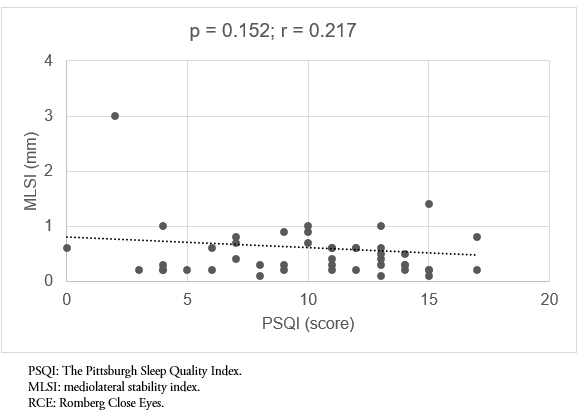 Figure 6. Correlation between sleep quality (PSQI) and the MLSI, RCE test.
Figure 6. Correlation between sleep quality (PSQI) and the MLSI, RCE test.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Objetive
To determine the relationship between sleep quality and postural balance in older adults living in the community.
Methods
An analytical cross-sectional study was used. We recruited 53 subjects who fulfilled the inclusion criteria. The Pittsburgh Sleep Quality Index (PSQI) was administered, and a static balance test was performed through a computerized posturography to evaluate the postural balance.
Results
Fifty-three subjects 60 to 80 years old (M = 70.13; standard deviation = 6.06) were evaluated; 69.8% were women and 30.2% were men. The correlation test found a statistically significant and positive relationship, but moderate (p = 0.002; r = 0.417) between total displacement and sleep quality in the Romberg open eyes test. The same happened with the total displacement in the Romberg close eyes test (p = 0.002, r = 0.445). Displacements were always greater in the anteroposterior plane, where a statistically significant and positive correlation with sleep quality was also found in all posturological tests. The correlation was moderate in all cases.
Conclusion
There is a relationship between the quality of sleep measured and postural balance. However, this relationship is low to moderate, and the size of the effect must be considered before accepting the hypothesis.
 Autores:
Lincoyán Fernández-Huerta [1,2,3], Jorge Aravena-Arriagada[2], Marcos Bernales-Montero[2], Karen Córdova-León[3]
Autores:
Lincoyán Fernández-Huerta [1,2,3], Jorge Aravena-Arriagada[2], Marcos Bernales-Montero[2], Karen Córdova-León[3]

Citación: Fernández-Huerta L, Aravena-Arriagada J, Bernales-Montero M, Córdova-León K. Relationship between sleep quality and postural balance in community-dwelling older persons: A cross-sectional study. Medwave 2019; 19(5):e7651 doi: 10.5867/medwave.2019.05.7652
Fecha de envío: 4/2/2019
Fecha de aceptación: 25/4/2019
Fecha de publicación: 13/6/2019
Origen: no solicitado
Tipo de revisión: con revisión por cuatro pares revisores externos, a doble ciego

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Götmark F, Cafaro P, O'Sullivan J. Aging Human Populations: Good for Us, Good for the Earth. Trends Ecol Evol. 2018 Nov;33(11):851-862. | CrossRef | PubMed |
Götmark F, Cafaro P, O'Sullivan J. Aging Human Populations: Good for Us, Good for the Earth. Trends Ecol Evol. 2018 Nov;33(11):851-862. | CrossRef | PubMed | Normal Physiological Changes in the Aging Adult. Adult-Gerontology Practice Guidelines. Springer Publishing Company. Jan;2019 | CrossRef |
Normal Physiological Changes in the Aging Adult. Adult-Gerontology Practice Guidelines. Springer Publishing Company. Jan;2019 | CrossRef | Medina-Chávez JH, Fuentes-Alexandro SA, Gil-Palafox IB, Adame-Galván L, Solís-Lam F, Sánchez-Herrera LY, et al. Clinical practice guideline. Diagnosis and treatment of insomnia in the elderly. Rev Med Inst Mex Seguro Soc. 2014; 52(1):108-119. | Link |
Medina-Chávez JH, Fuentes-Alexandro SA, Gil-Palafox IB, Adame-Galván L, Solís-Lam F, Sánchez-Herrera LY, et al. Clinical practice guideline. Diagnosis and treatment of insomnia in the elderly. Rev Med Inst Mex Seguro Soc. 2014; 52(1):108-119. | Link | Durán AS, Mattar AP, Bravo BN, Moreno BC, Reyes GS. [Association of quality of life perception with sleeping patterns in Chilean older people]. Rev Med Chil. 2014 Nov;142(11):1371-6. | CrossRef | PubMed |
Durán AS, Mattar AP, Bravo BN, Moreno BC, Reyes GS. [Association of quality of life perception with sleeping patterns in Chilean older people]. Rev Med Chil. 2014 Nov;142(11):1371-6. | CrossRef | PubMed | Durán Agüero S & Vásquez Leiva A. (2015). Caracterización antropométrica, calidad y estilos de vida del anciano chileno octogenario. Nutrición Hospitalaria. 2015;31(6):2554-2560. | CrossRef |
Durán Agüero S & Vásquez Leiva A. (2015). Caracterización antropométrica, calidad y estilos de vida del anciano chileno octogenario. Nutrición Hospitalaria. 2015;31(6):2554-2560. | CrossRef | Rodriguez JC, Dzierzewski JM, Alessi CA. Sleep problems in the elderly. Med Clin North Am. 2015 Mar;99(2):431-9. | CrossRef | PubMed |
Rodriguez JC, Dzierzewski JM, Alessi CA. Sleep problems in the elderly. Med Clin North Am. 2015 Mar;99(2):431-9. | CrossRef | PubMed | Ancoli-Israel S, Poceta JS, Stepnowsky C, Martin J, Gehrman P. Identification and treatment of sleep problems in the elderly. Sleep Med Rev.1997;1:3-17. | CrossRef |
Ancoli-Israel S, Poceta JS, Stepnowsky C, Martin J, Gehrman P. Identification and treatment of sleep problems in the elderly. Sleep Med Rev.1997;1:3-17. | CrossRef | Gooneratne NS, Richards KC, Joffe M, Lam RW, Pack F, Staley B, et al. Sleep disordered breathing with excessive daytime sleepiness is a risk factor for mortality in older adults. Sleep. 2011 Apr 1;34(4):435-42. | PubMed |
Gooneratne NS, Richards KC, Joffe M, Lam RW, Pack F, Staley B, et al. Sleep disordered breathing with excessive daytime sleepiness is a risk factor for mortality in older adults. Sleep. 2011 Apr 1;34(4):435-42. | PubMed | Brandão GS, Camelier FWR, Sampaio AAC, Brandão GS, Silva AS, Gomes GSBF, et al. Association of sleep quality with excessive daytime somnolence and quality of life of elderlies of community. Multidiscip Respir Med. 2018 Mar 15;13:8. | CrossRef | PubMed |
Brandão GS, Camelier FWR, Sampaio AAC, Brandão GS, Silva AS, Gomes GSBF, et al. Association of sleep quality with excessive daytime somnolence and quality of life of elderlies of community. Multidiscip Respir Med. 2018 Mar 15;13:8. | CrossRef | PubMed | Gooneratne NS, Weaver TE, Cater JR, Pack FM, Arner HM, Greenberg AS, et al. Functional outcomes of excessive daytime sleepiness in older adults. J Am Geriatr Soc. 2003 May;51(5):642-9. | PubMed |
Gooneratne NS, Weaver TE, Cater JR, Pack FM, Arner HM, Greenberg AS, et al. Functional outcomes of excessive daytime sleepiness in older adults. J Am Geriatr Soc. 2003 May;51(5):642-9. | PubMed | Blatter K, Opwis K, Münch M, Wirz-Justice A, Cajochen C. Sleep loss-related decrements in planning performance in healthy elderly depend on task difficulty. J Sleep Res. 2005 Dec;14(4):409-17. | CrossRef | PubMed |
Blatter K, Opwis K, Münch M, Wirz-Justice A, Cajochen C. Sleep loss-related decrements in planning performance in healthy elderly depend on task difficulty. J Sleep Res. 2005 Dec;14(4):409-17. | CrossRef | PubMed | Huang AR, Mallet L, Rochefort CM, Eguale T, Buckeridge DL, Tamblyn R. Medication-related falls in the elderly: causative factors and preventive strategies. Drugs Aging. 2012 May 1;29(5):359-76. | CrossRef | PubMed |
Huang AR, Mallet L, Rochefort CM, Eguale T, Buckeridge DL, Tamblyn R. Medication-related falls in the elderly: causative factors and preventive strategies. Drugs Aging. 2012 May 1;29(5):359-76. | CrossRef | PubMed | Hita-Contreras F, Zagalaz-Anula N, Martínez-Amat A, Cruz-Díaz D, Sánchez-Montesinos I, Aibar-Almazán A, et al. Sleep quality and its association with postural stability and fear of falling among Spanish postmenopausal women. Menopause. 2018 Jan;25(1):62-69. | CrossRef | PubMed |
Hita-Contreras F, Zagalaz-Anula N, Martínez-Amat A, Cruz-Díaz D, Sánchez-Montesinos I, Aibar-Almazán A, et al. Sleep quality and its association with postural stability and fear of falling among Spanish postmenopausal women. Menopause. 2018 Jan;25(1):62-69. | CrossRef | PubMed | Tinetti ME, Kumar C. The patient who falls: "It's always a trade-off". JAMA. 2010 Jan 20;303(3):258-66. | CrossRef | PubMed |
Tinetti ME, Kumar C. The patient who falls: "It's always a trade-off". JAMA. 2010 Jan 20;303(3):258-66. | CrossRef | PubMed | Vieira ER, Palmer RC, Chaves PH. Prevention of falls in older people living in the community. BMJ. 2016 Apr 28;353:i1419. | CrossRef | PubMed |
Vieira ER, Palmer RC, Chaves PH. Prevention of falls in older people living in the community. BMJ. 2016 Apr 28;353:i1419. | CrossRef | PubMed | Cadore EL, Rodríguez-Mañas L, Sinclair A, Izquierdo M. Effects of different exercise interventions on risk of falls, gait ability, and balance in physically frail older adults: a systematic review. Rejuvenation Res. 2013 Apr;16(2):105-14. | CrossRef | PubMed |
Cadore EL, Rodríguez-Mañas L, Sinclair A, Izquierdo M. Effects of different exercise interventions on risk of falls, gait ability, and balance in physically frail older adults: a systematic review. Rejuvenation Res. 2013 Apr;16(2):105-14. | CrossRef | PubMed | Kim SY, Kim SG, Sim S, Park B, Choi HG. Excessive sleep and lack of sleep are associated with slips and falls in the adult Korean population: a population-based cross-sectional study. Medicine. 95(4):e2397.
| CrossRef |
Kim SY, Kim SG, Sim S, Park B, Choi HG. Excessive sleep and lack of sleep are associated with slips and falls in the adult Korean population: a population-based cross-sectional study. Medicine. 95(4):e2397.
| CrossRef | Robillard R, Prince F, Filipini D, Carrier J. Aging worsens the effects of sleep deprivation on postural control. PLoS One. 2011;6(12):e28731. | CrossRef | PubMed |
Robillard R, Prince F, Filipini D, Carrier J. Aging worsens the effects of sleep deprivation on postural control. PLoS One. 2011;6(12):e28731. | CrossRef | PubMed | Fernández L, Breinbauer HA, Delano PH. Vertigo and Dizziness in the Elderly. Front Neurol. 2015 Jun 26;6:144. | CrossRef | PubMed |
Fernández L, Breinbauer HA, Delano PH. Vertigo and Dizziness in the Elderly. Front Neurol. 2015 Jun 26;6:144. | CrossRef | PubMed | Davalos-Bichara M, Agrawal Y. Normative results of healthy older adults on standard clinical vestibular tests. Otol Neurotol. 2014 Feb;35(2):297-300. | CrossRef | PubMed |
Davalos-Bichara M, Agrawal Y. Normative results of healthy older adults on standard clinical vestibular tests. Otol Neurotol. 2014 Feb;35(2):297-300. | CrossRef | PubMed | Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989 May;28(2):193-213. | CrossRef | PubMed |
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989 May;28(2):193-213. | CrossRef | PubMed | Blumen S, Bayona H, Givoli S, Pecker G, Fine S. Estudio de validación de una prueba de integridad multimétodo en una muestra peruana. Revista de Psicología. 2017;35(1):347-370. | CrossRef |
Blumen S, Bayona H, Givoli S, Pecker G, Fine S. Estudio de validación de una prueba de integridad multimétodo en una muestra peruana. Revista de Psicología. 2017;35(1):347-370. | CrossRef | Royuela A, Macías JA. Propiedades clinimétricas de la versión castellana del Cuestionario de Pittsburgh. Vigilia-Sueño. 1997;9:81-94. | Link |
Royuela A, Macías JA. Propiedades clinimétricas de la versión castellana del Cuestionario de Pittsburgh. Vigilia-Sueño. 1997;9:81-94. | Link | Peydro de Moya MF, Baydal Bertomeu JM, Vivas Broseta MJ. Evaluación y rehabilitación del equilibrio mediante posturografía. Rehabilitación. 2005; 39(6): 315-323. | CrossRef |
Peydro de Moya MF, Baydal Bertomeu JM, Vivas Broseta MJ. Evaluación y rehabilitación del equilibrio mediante posturografía. Rehabilitación. 2005; 39(6): 315-323. | CrossRef | Soto-Varela A, Faraldo-García A, Rossi-Izquierdo M, Lirola-Delgado A, Vaamonde-Sánchez-Andrade I, del-Río-Valeiras M, et al. Can we predict the risk of falls in elderly patients with instability? Auris Nasus Larynx. 2015 Feb;42(1):8-14. | CrossRef | PubMed |
Soto-Varela A, Faraldo-García A, Rossi-Izquierdo M, Lirola-Delgado A, Vaamonde-Sánchez-Andrade I, del-Río-Valeiras M, et al. Can we predict the risk of falls in elderly patients with instability? Auris Nasus Larynx. 2015 Feb;42(1):8-14. | CrossRef | PubMed | Nardone A, Tarantola J, Giordano A, Schieppati M. Fatigue effects on body balance. Electroencephalogr Clin Neurophysiol. 1997 Aug;105(4):309-20. | CrossRef | PubMed |
Nardone A, Tarantola J, Giordano A, Schieppati M. Fatigue effects on body balance. Electroencephalogr Clin Neurophysiol. 1997 Aug;105(4):309-20. | CrossRef | PubMed | Winter DA, Patla AE, Ishac M, Gage WH. Motor mechanisms of balance during quiet standing. J Electromyogr Kinesiol. 2003 Feb;13(1):49-56. | CrossRef | PubMed |
Winter DA, Patla AE, Ishac M, Gage WH. Motor mechanisms of balance during quiet standing. J Electromyogr Kinesiol. 2003 Feb;13(1):49-56. | CrossRef | PubMed | Kolomiiets B, Popov A. Analysis of Stabilograms of Healthy Human Using Time and Frequency Characteristics. 2018 IEEE 38th International Conference on Electronics and Nanotechnology (ELNANO). IEEE; 2018 Apr. [on line]. | CrossRef |
Kolomiiets B, Popov A. Analysis of Stabilograms of Healthy Human Using Time and Frequency Characteristics. 2018 IEEE 38th International Conference on Electronics and Nanotechnology (ELNANO). IEEE; 2018 Apr. [on line]. | CrossRef | Winter DA. Human balance and posture control during standing and walking. Gait and Posture. 1995;3(4):193-214. | CrossRef |
Winter DA. Human balance and posture control during standing and walking. Gait and Posture. 1995;3(4):193-214. | CrossRef | Kasahara S, Saito H, Anjiki T, Osanai H. The effect of aging on vertical postural control during the forward and backward shift of the center of pressure. Gait Posture. 2015 Oct;42(4):448-54. | CrossRef | PubMed |
Kasahara S, Saito H, Anjiki T, Osanai H. The effect of aging on vertical postural control during the forward and backward shift of the center of pressure. Gait Posture. 2015 Oct;42(4):448-54. | CrossRef | PubMed | Horak FB, Nashner LM, Diener HC. Postural strategies associated with somatosensory and vestibular loss. Exp Brain Res. 1990;82(1):167-77. | CrossRef | PubMed |
Horak FB, Nashner LM, Diener HC. Postural strategies associated with somatosensory and vestibular loss. Exp Brain Res. 1990;82(1):167-77. | CrossRef | PubMed | Wu TY, Chie WC, Yang RS, Liu JP, Kuo KL, Wong WK, Liaw CK. Factors associated with falls among community-dwelling older people in Taiwan. Ann Acad Med Singapore. 2013 Jul;42(7):320-7. | PubMed |
Wu TY, Chie WC, Yang RS, Liu JP, Kuo KL, Wong WK, Liaw CK. Factors associated with falls among community-dwelling older people in Taiwan. Ann Acad Med Singapore. 2013 Jul;42(7):320-7. | PubMed | Zia A, Kamaruzzaman SB, Tan MP. Polypharmacy and falls in older people: Balancing evidence-based medicine against falls risk. Postgrad Med. 2015 Apr;127(3):330-7. | CrossRef | PubMed |
Zia A, Kamaruzzaman SB, Tan MP. Polypharmacy and falls in older people: Balancing evidence-based medicine against falls risk. Postgrad Med. 2015 Apr;127(3):330-7. | CrossRef | PubMed | Shuto H, Imakyure O, Matsumoto J, Egawa T, Jiang Y, Hirakawa M, et al. Medication use as a risk factor for inpatient falls in an acute care hospital: a case-crossover study. Br J Clin Pharmacol. 2010 May;69(5):535-42. | CrossRef | PubMed |
Shuto H, Imakyure O, Matsumoto J, Egawa T, Jiang Y, Hirakawa M, et al. Medication use as a risk factor for inpatient falls in an acute care hospital: a case-crossover study. Br J Clin Pharmacol. 2010 May;69(5):535-42. | CrossRef | PubMed | Callisaya ML, Sharman JE, Close J, Lord SR, Srikanth VK. Greater daily defined dose of antihypertensive medication increases the risk of falls in older people--a population-based study. J Am Geriatr Soc. 2014 Aug;62(8):1527-33. | CrossRef | PubMed |
Callisaya ML, Sharman JE, Close J, Lord SR, Srikanth VK. Greater daily defined dose of antihypertensive medication increases the risk of falls in older people--a population-based study. J Am Geriatr Soc. 2014 Aug;62(8):1527-33. | CrossRef | PubMed | Sevilla-Sánchez D, Espaulella-Panicot J, de Andrés-Lazaro AM, Torres-Allezpuz R, Soldevila-Llagostera M, Codina-Jane C. Medicación potencialmente inapropiada al ingreso en una unidad de media estancia según los criterios STOPP & START. Revista Española de Geriatría y Gerontología. Elsevier BV; 2012 Jul;47(4):155-7. | CrossRef |
Sevilla-Sánchez D, Espaulella-Panicot J, de Andrés-Lazaro AM, Torres-Allezpuz R, Soldevila-Llagostera M, Codina-Jane C. Medicación potencialmente inapropiada al ingreso en una unidad de media estancia según los criterios STOPP & START. Revista Española de Geriatría y Gerontología. Elsevier BV; 2012 Jul;47(4):155-7. | CrossRef | Pugh MJ, Palmer RF, Parchman ML, Mortensen E, Markides K, Espino DV. Association of suboptimal prescribing and change in lower extremity physical function over time. Gerontology. 2007;53(6):445-53. | CrossRef | PubMed |
Pugh MJ, Palmer RF, Parchman ML, Mortensen E, Markides K, Espino DV. Association of suboptimal prescribing and change in lower extremity physical function over time. Gerontology. 2007;53(6):445-53. | CrossRef | PubMed | Langeard A, Pothier K, Morello R, Lelong-Boulouard V, Lescure P, Bocca ML, et al. Polypharmacy Cut-Off for Gait and Cognitive Impairments. Front Pharmacol. 2016 Aug 31;7:296. | CrossRef | PubMed |
Langeard A, Pothier K, Morello R, Lelong-Boulouard V, Lescure P, Bocca ML, et al. Polypharmacy Cut-Off for Gait and Cognitive Impairments. Front Pharmacol. 2016 Aug 31;7:296. | CrossRef | PubMed | Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: I. Psychotropic drugs. J Am Geriatr Soc. 1999 Jan;47(1):30-9. | PubMed |
Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: I. Psychotropic drugs. J Am Geriatr Soc. 1999 Jan;47(1):30-9. | PubMed | Abreu HC de A, Reiners AAO, Azevedo RC de S, Silva AMC da, Abreu DR de OM, Oliveira AD de. Incidence and predicting factors of falls of older inpatients. Revista de Saúde Pública. FapUNIFESP (SciELO); 2015;49(0). | CrossRef |
Abreu HC de A, Reiners AAO, Azevedo RC de S, Silva AMC da, Abreu DR de OM, Oliveira AD de. Incidence and predicting factors of falls of older inpatients. Revista de Saúde Pública. FapUNIFESP (SciELO); 2015;49(0). | CrossRef | Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013 May;75(1):51-61. | CrossRef | PubMed |
Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013 May;75(1):51-61. | CrossRef | PubMed | Loram ID, Lakie M, Gawthrop PJ. Visual control of stable and unstable loads: what is the feedback delay and extent of linear time-invariant control? J Physiol. 2009 Mar 15;587(Pt 6):1343-65. | CrossRef | PubMed |
Loram ID, Lakie M, Gawthrop PJ. Visual control of stable and unstable loads: what is the feedback delay and extent of linear time-invariant control? J Physiol. 2009 Mar 15;587(Pt 6):1343-65. | CrossRef | PubMed | Alonso AC, Mochizuki L, Silva Luna NM, Ayama S, Canonica AC, Greve JMDA. Relation between the Sensory and Anthropometric Variables in the Quiet Standing Postural Control: Is the Inverted Pendulum Important for the Static Balance Control? BioMed Research International. Hindawi Limited; 2015;2015:1-5. | CrossRef |
Alonso AC, Mochizuki L, Silva Luna NM, Ayama S, Canonica AC, Greve JMDA. Relation between the Sensory and Anthropometric Variables in the Quiet Standing Postural Control: Is the Inverted Pendulum Important for the Static Balance Control? BioMed Research International. Hindawi Limited; 2015;2015:1-5. | CrossRef | Panossian LA, Veasey SC. Daytime sleepiness in obesity: mechanisms beyond obstructive sleep apnea--a review. Sleep. 2012 May 1;35(5):605-15. | CrossRef | PubMed |
Panossian LA, Veasey SC. Daytime sleepiness in obesity: mechanisms beyond obstructive sleep apnea--a review. Sleep. 2012 May 1;35(5):605-15. | CrossRef | PubMed | Pereira C, Silva RAD, de Oliveira MR, Souza RDN, Borges RJ, Vieira ER. Effect of body mass index and fat mass on balance force platform measurements during a one-legged stance in older adults. Aging Clin Exp Res. 2018 May;30(5):441-447. | CrossRef | PubMed |
Pereira C, Silva RAD, de Oliveira MR, Souza RDN, Borges RJ, Vieira ER. Effect of body mass index and fat mass on balance force platform measurements during a one-legged stance in older adults. Aging Clin Exp Res. 2018 May;30(5):441-447. | CrossRef | PubMed | Piovezan RD, Abucham J, Dos Santos RV, Mello MT, Tufik S, Poyares D. The impact of sleep on age-related sarcopenia: Possible connections and clinical implications. Ageing Res Rev. 2015 Sep;23(Pt B):210-20.
| CrossRef | PubMed |
Piovezan RD, Abucham J, Dos Santos RV, Mello MT, Tufik S, Poyares D. The impact of sleep on age-related sarcopenia: Possible connections and clinical implications. Ageing Res Rev. 2015 Sep;23(Pt B):210-20.
| CrossRef | PubMed | Takács J, Bódizs R, Ujma PP, Horváth K, Rajna P, Harmat L. Reliability and validity of the Hungarian version of the Pittsburgh Sleep Quality Index (PSQI-HUN): comparing psychiatric patients with control subjects. Sleep Breath. 2016 Sep;20(3):1045-51. | CrossRef | PubMed |
Takács J, Bódizs R, Ujma PP, Horváth K, Rajna P, Harmat L. Reliability and validity of the Hungarian version of the Pittsburgh Sleep Quality Index (PSQI-HUN): comparing psychiatric patients with control subjects. Sleep Breath. 2016 Sep;20(3):1045-51. | CrossRef | PubMed | Girschik J, Fritschi L, Heyworth J, Waters F. Validation of self-reported sleep against actigraphy. J Epidemiol. 2012;22(5):462-8. | CrossRef | PubMed |
Girschik J, Fritschi L, Heyworth J, Waters F. Validation of self-reported sleep against actigraphy. J Epidemiol. 2012;22(5):462-8. | CrossRef | PubMed | Ng ECW, Fisher AT. Understanding Well-Being in Multi-Levels: A review. Health, Culture and Society. University Library System, University of Pittsburgh; 2013 Nov 15;5(1):308-23. | CrossRef |
Ng ECW, Fisher AT. Understanding Well-Being in Multi-Levels: A review. Health, Culture and Society. University Library System, University of Pittsburgh; 2013 Nov 15;5(1):308-23. | CrossRef |