 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Palabras clave: naproxen, paracetamol, pamabrom, primary dysmenorrhea, pyrilamine
INTRODUCTION
Dysmenorrhea is caused by the discharge of prostaglandins into the uterine tissue; therefore, non-steroidal anti-inflammatory drugs (NSAIDs) are the established initial therapy for dysmenorrhea. Dysmenorrhea therapy may include the administration of drug monotherapy or combination therapy. However, clinical scientific evidence on the efficacy of medications with two or three drugs combined is scarce or nonexistent.
OBJECTIVE
To evaluate and compare the efficacy and safety of two oral fixed-dose combinations for the relief of the symptoms of primary dysmenorrhea among Mexican women. One of the combinations is widely used in Mexico (paracetamol, pyrilamine and pamabrom) and the selected comparison was a medication with naproxen sodium, paracetamol and pamabrom based on the pathophysiology of primary dysmenorrhea.
METHODS
This was a single-centre, double blind, experimental, parallel group, randomized trial. Female patients with primary dysmenorrhea, older than 17 years and with pain intensity greater than 45 mm on a visual analogue scale, were included. The patients were then randomized to receive tablets with naproxen sodium, paracetamol and pamabrom or tablets with paracetamol, pyrilamine and pamabrom for one menstrual cycle. Patient evaluations of symptomatology and pain intensity were recorded throughout one menstrual period. Descriptive and inferential statistical analyses were utilized.
RESULTS
An intention-to-treat population of 91 women, with a mean age of 21.3 ± 3.2 years, received paracetamol, pyrilamine and pamabrom tablets, and 98 participants, with a mean age of 21.0 ± 3.2 years, received naproxen sodium, paracetamol and pamabrom tablets. The participants’ assessments of pain on the Visual Analogue Scale during the menstrual cycle demonstrated a significant reduction in both treatment groups (p<0.05). There is no significant difference in efficacy between both groups (p>0.05).
CONCLUSIONS
The results showed that both drug combinations were not different in reducing dysmenorrheic pain. Likewise, both treatments were well tolerated. Therefore, both treatments may be used for the treatment of primary dysmenorrhea.
Dysmenorrhea is a chronic, cyclic pelvic pain associated with menstruation and may be associated with nausea, vomiting, diarrhea, headache, fatigue, back pain, and dizziness. The prevalence of dysmenorrhea ranges from 20% to 90% [1],[2],[3]. Primary dysmenorrhea (PD) is suggested to be caused by the release of prostaglandins into the uterine tissue [3],[4],[5]. Prostaglandins are derivatives of arachidonic acid metabolism by the enzyme cyclooxygenase (COX). Nonsteroidal anti-inflammatory drugs (NSAIDs) are a group of chemically different drugs, which inhibit cyclooxygenase enzyme causing a decrease in prostaglandin synthesis [5]. Nonsteroidal anti-inflammatory drugs have anti-inflammatory, analgesic and antipyretic effects. A very recent meta-analysis showed that nonsteroidal anti-inflammatory drugs are more effective than placebo in reducing pain in women with primary dysmenorrhea [5]. Therefore, nonsteroidal anti-inflammatory drugs such as naproxen, diclofenac, ibuprofen among others, are the initial established therapy for primary dysmenorrhea [1],[2],[3],[4],[5].
Oral contraceptives, analgesic-antipyretic such as paracetamol, among other drugs are other pharmacological therapeutic options for the treatment of pain in women with primary dysmenorrhea [1],[2],[3],[4]. However, its effectiveness in relieving pain in patients with primary dysmenorrhea is controversial [3],[6],[7],[8]. Some studies demonstrated the ability of paracetamol to decrease the production of F2α prostaglandin in menstrual fluid and symptoms in women with primary dysmenorrhea [7],[8]. However, meta-analysis studies showed that paracetamol was less effective than nonsteroidal anti-inflammatory drugs and as effective as placebo in relieving pain in patients with this condition [3],[5].
Although nonsteroidal anti-inflammatory drugs are the best choice for pain relief in patients with primary dysmenorrhea; there is evidence showing that these drugs administered alone may lead to therapeutic failure or to a weak analgesic effect [1],[2],[3],[5]. Furthermore, clinical studies of patients with primary dysmenorrhea found that nonsteroidal anti-inflammatory drugs have more gastrointestinal and neurological adverse reactions than placebo [3],[5]. Therefore, another treatment option for primary dysmenorrhea is the administration of a medication containing two or three different drugs, which have different, but complementary action mechanisms [9],[10]. What is expected of such combinations is causing better pain relief at lower doses (synergism) and fewer adverse reactions. For example, our group demonstrated that the most common medications prescribed by physicians and used in self-medication to treat dysmenorrheic pain in Mexican students were the medications Syncol® (a combination of paracetamol [analgesic], pamabrom [diuretic] and pyrilamine [antihistamine H1]) and Buscapina Compositum® (a combination of metamizole [NSAID] plus butylhyoscine [antimuscarinic]) [1],[2].
It is important to note that clinical scientific evidence on the efficacy of medications with two or three drugs combined (for example, paracetamol with naproxen sodium) is scarce or missing [1],[2],[6]. Therefore, well designed studies are mandatory to assess the effectiveness of medications that contain a combination of several drugs and are commonly used in the management of primary dysmenorrhea in some countries.
Therefore, the objective of the present study was to evaluate the efficacy and safety of two medications with different drug combinations: a) naproxen sodium + paracetamol + pamabrom (NPP; tested medication), and b) paracetamol + pyrilamine + pamabrom (PPP; medication of reference) on primary dysmenorrhea in Mexican women; and in particular demonstrate non-inferiority of the tested medication compared to the reference one.
Participants
According to the health legislation of Mexico, first of all, the study protocol was reviewed and approved by a local Ethics Committee (Universidad del Fútbol y Ciencias del Deporte, Hidalgo, Mexico). After that, the same study protocol was submitted for review and approval by independent Ethics and Research Committees pertaining to the Federal Commission for the Protection against Sanitary Risk (COFEPRIS, Mexico). The study was conducted in accordance with the Declaration of Helsinki.
Recruitment was performed from students of the Universidad Autónoma del Estado de Hidalgo, Mexico. The inclusion criteria were an agreement to participate in the research work and a signed informed consent, age over 17 years, satisfactory health, negative urine pregnancy test and, primary dysmenorrhea screened by a physician who also obtained a medical history and performed a physical examination. Each woman had a history of primary dysmenorrhea, and reported it as painful menstruation in the previous four months with pain intensity greater than
The exclusion criteria were: patients with dysmenorrhea secondary to organic pathology, chronic degenerative diseases and use of nonsteroidal anti-inflammatory drugs or oral contraceptives within three months prior to study entry. Patients with active peptic ulcer(s) or any gastrointestinal disease associated with clinically significant blood loss within the last two years.
Study design
This was a single-centre, double blind, prospective, experimental, parallel group, randomized study.
Randomization and blinding
A database with the names of the participants in spreadsheets Microsoft Excel 2010 was completed. Participants were numbered from 1 to 200. According to the experimental design, two groups of participants (group "A" for naproxen sodium, paracetamol and pamabrom of 100 participants and another group "B" for paracetamol, pyrilamine and pamabrom of 100 participants) were established. A random numbers scheme was performed with Microsoft Excel 2010. Each participant was randomly assigned to receive its respective treatment to either "A" or "B". Randomization and allocation was concealed to statistical and clinical evaluators. The two medications (naproxen, paracetamol and pamabrom or paracetamol, pyrilamine and pamabrom) were packed in bottles and labeled "A" (100 bottles) or "B" (100 bottles). Identification codes of the medications were also hidden to statistical and clinical evaluators. The opening of the identification codes was performed when the capture and verification of all data from the case report forms and diaries of patients was completed.
Pharmacological intervention phase
Once the participants were included, a checklist was used to collect the demographic data, menstrual history, and past medical and reproductive histories. Participants were randomly divided into two groups: a group that received tablets orally with a combination of naproxen sodium (220 mg), paracetamol (300 mg), and pamabrom (25 mg) (naproxen, paracetamol and pamabrom group, Analgen FEM®, Laboratorios Liomont, S.A. de C.V, Distrito Federal, Mexico) three times a day for one menstrual cycle and another group that received tablets orally with a combination of paracetamol (500 mg), pamabrom (25 mg) and pyrilamine (15 mg) (paracetamol, pamabrom and pyrilamine group, Syncol®, Laboratorios Sanfer, S. A. de C.V., Estado de Mexico, Mexico) three times a day for one menstrual cycle. Study medications were properly equipped. The study medications were provided in opaque white polyethylene bottles, labeled with letter "A" or "B", and were sufficient for 3 days of treatment (nine identical tablets per bottle for each of the medications; initiating treatment 24 hours before menstruation and up to 48 hours after starting menstruation). During the final evaluation, the participants returned their empty bottles.
Primary efficacy measures
The primary efficacy measure was the menstrual pain intensity reported in the patient’s case reports prior to taking the first dose of the study medication, which occurred at regular intervals (every eight hours), after the first dose and at the end of the study (72 hours). Pain intensity was determined by the visual analogue scores of pain severity (
Secondary efficacy measures included
A) The proportion of the patients who at the end of the treatment period reported a reduction of their baseline pain by at least 50%.
B) Symptoms of dysmenorrhea were evaluated and reported in the patient’s case reports prior to taking the first dose of study medication and at the end of the study (72 hours).
C) The patient’s global evaluation of the study medication was performed at the end of the study. Patients assessed the response to their study medication as: satisfactory response, moderate response, poor response and no response to treatment.
Treatment safety
Participants were informed to call or go to the principal investigator in the presence of any suspected adverse event produced by the medications throughout the study period. No adverse event report was received before the final study visit. A clinical interrogatory and complete physical examination at the final evaluation were performed. Adverse events were reported by the patients and were recorded in case report forms by the researchers. Adverse events were registered, evaluated and classified according to the event start date, severity, relationship to study medication, action and treatment, outcome and end date of the event.
Statistical analysis
Assuming a hypothesis of non-inferiority, the sample size was determined using a computerized software package nQuery Advisor®, version 7.0. A sample size of 174 participants was estimated to provide 80% power, with a margin of non-inferiority of 10 mm [11] between two groups in the evaluation of pain relief, using a visual analogue scale of 100 mm and assuming a significance level of 0.025 and a standard deviation of 23.4 mm. With a projected dropout rate of 10%, a minimum of 200 participants (100 per each treatment) were estimated to be required.
An analysis of the efficacy data was accomplished on the intention-to-treat population, which is defined as all of the randomized patients who fulfilled the inclusion criteria, had completed the treatments for the menstrual cycle and had one complete evaluable cycle
In the present study, parametric statistical analysis was applied to the scores of pain intensity from the visual analogue, because several sources of information concerning statistical analysis of data obtained with this scale justify their use [12],[13],[14],[15].
The pain intensity scores were analyzed using a repeated-measures ANOVA with the treatment group as a between-subject factor, time as a within-subject factor and the interaction between the treatment group and time.
The degrees of freedom of the F statistical associated with the effects of the treatment group and the interaction were corrected (statistical test more conservative) using the epsilon estimated (sphericity) of Greenhouse-Geisser, because this is a relatively small sample size and for deviations to the sphericity assumption applicable to repeated measures ANOVA [16].
The primary comparison to assess efficacy was pain intensity mean difference between the global (72-hour period) means of the treatment groups. The efficacy measures recorded in categorical scale (symptoms of dysmenorrhea and patient’s global evaluation of the study medication) were analyzed using the Fisher’s exact test. Sociodemographic continuous variables were analyzed using the t-test and the categorical variables were analyzed using the Fisher’s exact test. For all of the statistical tests, the type I error was fixed at 5% (α = 0.05). Stata® version 13 was used to generate all of the graphics and most of the analysis described in this report. NCSS 9® (NCSS, LLC. Kaysville, Utah, United States) was used for the repeated-measures ANOVA.
Sociodemographic and clinical characteristics of both groups
Two hundred patients were included in the study, and the patients were randomly assigned to the naproxen, paracetamol and pamabrom group (100 patients) or the paracetamol, pamabrom and pyrilamine group (100 patients). Nonetheless, 11 randomized participants who met the inclusion criteria and received their bottle of medicine could not be incorporated into the analysis of the intention-to-treat population; 10 were removed (without data from an evaluable complete cycle, because the patients did not return, nor could be reached, personally or by telephone) and one due to protocol deviation (not meeting a level of initial pain greater than 45 mm) (Figure 1). Consequently, there were 189 participants (naproxen, paracetamol and pamabrom group: n = 98 and paracetamol, pamabrom and pyrilamine group: n = 91) included in the intent-to-treat population. The baseline demographic, clinical data and dysmenorrheic symptoms of both groups are shown in Tables 1 and 2.
Figure 1. Flow diagram for randomized participants.
Table 1. Sociodemographic and clinical data of the 189 patients according to group.
Pain intensity
Figure 2 shows the pain intensity vs. time profiles for both treatment groups. The results of the repeated-measures ANOVA indicated that both treatment groups significantly reduced the pain intensity over time (P<0.001). A statistically significant reduction of pain intensity in both groups was observed after the first eight hours of treatment (P<0.01) (Figure 2).
The estimated mean difference between the group global means (NPP-PPP) was 3.25 mm, 95% CI (
The proportion of patients who reported a pain reduction by at least 50%
This proportion of patients was 80.6% (79/98) in the naproxen, paracetamol and pamabrom group and 87.9% (80/91) in the paracetamol, pamabrom and pyrilamine group. There was not a significant association between the treatment groups, regardless of whether the patients achieved a pain reduction of at least 50%, with respect to the baseline conditions (p = 0.2318).
Post-treatment symptoms of dysmenorrhea
Table 3 shows the post-treatment symptoms of dysmenorrhea by the treatment group. There was no significant association between the treatment group and the post-treatment symptoms of dysmenorrhea.
Table 3. Symptoms in women with dysmenorrhea at the end of treatment.
Patient’s global evaluation of the treatment effectiveness
Most women rated the effectiveness of their treatment as a “moderate response” and “satisfactory response” in both naproxen, paracetamol and pamabrom and paracetamol, pamabrom and pyrilamine groups (Table 4). Nevertheless, there was no significant association (p = 0.7096) between the patient’s global evaluation and the treatment groups.
Tabla 4. End clinical response to treatment.
Safety findings
During the study period and of the 200 women in the safety population, 2 (2.0%) patients experienced 3 adverse events during the paracetamol, pamabrom and pyrilamine treatment and 4 (4.0%) women experienced five adverse events during the naproxen, paracetamol and pamabrom treatment. The most commonly reported adverse events in the study were headache (one case) and abdominal pain (two cases) in the paracetamol, pamabrom and pyrilamine group. However, somnolence (one case), headache (one case), dizziness (one case), increased thirst (one case) and diarrhea (one case) were reported in the naproxen, paracetamol and pamabrom group. No serious adverse events were reported in this study.
Medications and alternative treatments are the main therapeutic strategies to alleviate the signs and symptoms caused by primary dysmenorrhea. Nonsteroidal anti-inflammatory drugs are the first drugs of choice in the treatment of primary dysmenorrhea, which is supported by findings that have shown that prostaglandins are the main substances involved in the pathogenesis of primary dysmenorrhea [3],[4],[7]. Patients suffering from primary dysmenorrhea usually do not go to the physician for care. Instead, they resort to non-drug remedies and self-medication. Several studies have found an important therapeutic failure or a small analgesic effect of nonsteroidal anti-inflammatory drugs [1],[2],[3],[5]. Therefore, it is necessary to resort to other therapeutic measures to eliminate or ameliorate the symptoms that women experience with primary dysmenorrhea. In our study and according to the analysis of the primary and secondary efficacy variables, we found that the medications with the naproxen, paracetamol and pamabrom combination and the paracetamol, pamabrom and pyrilamine combination significantly reduced pain intensity in a period of 72 hours (p <0.0001). In addition, no significant difference was found in the other secondary efficacy measures between the two treatment groups (p> 0.05).
The clinical use of combinations of analgesic and/or nonsteroidal anti-inflammatory drugs has increased significantly in the last decades. The purpose is to associate two or three drugs with different mechanisms of action to achieve a synergistic interaction, yielding a sufficient analgesic effect with low doses and therefore reduce the intensity and incidence of untoward effects [9],[10]. Previous studies have demonstrated the effectiveness of paracetamol in alleviating the symptoms caused by primary dysmenorrhea [7],[8].
There is evidence that paracetamol is a weak inhibitor of prostaglandin synthesis [17]. The ability of paracetamol to decrease the production of prostaglandins F2α in menstrual fluid and the symptoms in women with primary dysmenorrhea has been previously demonstrated [9],[10]. Likewise, paracetamol inhibits spinal cord nitric oxide synthesis and reinforces the serotonergic descending inhibitory pain pathways [18],[19]. Experimental data shows that the paracetamol metabolite N-arachidonoylaminephenol inhibits the uptake and degradation of anandamide, which is reported to mediate the analgesic action of acetaminophen via the CB1 receptor [20]. It is likely that these paracetamol are involved in it ability to reduce pain in patients with primary dysmenorrhea.
On the other hand, it has been reported that histamine produces contractile activity of pregnant human uterine strips, and this effect was blocked by the H1 histamine receptor antagonist pyrilamine [21],[22]. Recently, our group demonstrated that pyrilamine was able to block the contractile effect induced by KCl (
Alternatively, pamabrom, chemically 2-amino-2-methyl-propanol 8-bromo theophylline, is a weak diuretic that is effective in treating primary dysmenorrhea and premenstrual syndrome [24]. Theophylline, the active xanthine derivative of pamabrom, has been shown to alleviate the angina-like chest pain induced by adenosine, post-dural puncture headache and pain during experimental ischemia in humans [25],[26],[27]. There is experimental evidence to suggest that the analgesic activities produced by theophylline involve phosphodiesterase and adenosine receptors [25],[26]. Taken together, it is possible to suggest that the pamabrom-induced pharmacological effects were included with the activities produced for paracetamol and pyrilamine in the efficacy produced for the paracetamol, pamabrom and pyrilamine mix observed in the present study. The real participation of the different action mechanisms of each drug of the paracetamol, pamabrom and pyrilamine mix requires future elucidation.
Naproxen and naproxen sodium are very potent analgesic and anti-inflammatory drugs that are used for treating painful conditions such as arthritis and gout [28],[29]. The anti-inflammatory and analgesic properties of naproxen have been attributed to the inhibition of cyclooxygenase and the consequent inhibition of prostaglandin biosynthesis [28].
The analgesic efficacy of naproxen in women with primary dysmenorrhea has been previously demonstrated. Marjoribanks et al. [5] published a meta-analysis to compare nonsteroidal anti-inflammatory drugs used in the treatment of primary dysmenorrhea. The authors found that naproxen was significantly more effective than the placebo in producing moderate to excellent relief of dysmenorrheic pain (OR 3.67, 95 % IC: 2.94 to 4.58). In the same study, it was found that naproxen was significantly more effective than paracetamol in decreasing the symptomatology of primary dysmenorrhea [5].
In a previous study, we demonstrated that the self medication of naproxen was statistically more effective in alleviating dysmenorrheic pain than the paracetamol, pamabrom and pyrilamine mix (P=0.006) or the over-the-counter medication with metamizole (a nonsteroidal anti-inflammatory drug) plus butylhyoscine bromide (P=0.004) [1].
In our study, the paracetamol dose (300 mg) employed in the naproxen, paracetamol and pamabrom mix was less than the paracetamol dose (500 mg) used in the paracetamol, pamabrom and pyrilamine combination. Based on these results, we suggest that the analgesic effect produced by the naproxen, paracetamol and pamabrom combination could be synergistic. In addition, we propose that the efficacy of the naproxen, paracetamol and pamabrom combination in our study was due to the ability of naproxen to inhibit prostaglandin biosynthesis and the probable activation of the action mechanisms of paracetamol and pamabrom mentioned above.
With the experimental design used in this study, it is not possible to determine superiority or inferiority of individual drugs versus any of the combinations. In this sense, it would be of great importance to perform another clinical study in which the effectiveness and safety of any of the combinations (e.g. naproxen, paracetamol and pamabrom) were compared versus naproxen and paracetamol individually. Another limitation of this study is that a placebo group was not included to determine the intrinsic efficacy of both medications. This was because in its planning the use of placebo, being a study related to pain, was not considered as ethical. In addition, patients were selected within a range of age and other physiological conditions, according to inclusion and exclusion criteria of the protocol so that the results of this study may not be generalizable to the entire target population.
The results show that both drug combinations were not different in reducing the dysmenorrheic pain, therefore the medication tested (naproxen, paracetamol and pamabrom) is not inferior to the medication of reference (paracetamol, pamabrom and pyrilamine). For this reason, we suggest that the drug combinations naproxen, paracetamol and pamabrom and paracetamol, pamabrom and pyrilamine are effective and safe options for the treatment of primary dysmenorrhea.
From the editor
The authors originally submitted this article in Spanish and English. The Journal has not copyedited the English version.
Acknowledgement
This research and its publication were supported by Laboratorios Liomont, S.A. de C.V. Federal, Mexico.
Conflicts of interest
The authors completed the ICMJE conflicts of interest declaration form, and declare having received funds from Laboratorios Liomont, S.A. Ciudad de México, Mexico for the completion of this report. Mario I. Ortiz is a worker of the Universidad Autónoma del Estado de Hidalgo, Pachuca, Mexico; Gabriela Murguía-Cánovas is a worker of the Universidad del Futbol y Ciencias del Deporte, Pachuca, Hidalgo, Mexico; Rodolfo Silva is a worker of Laboratorios Liomont, S.A. Ciudad de México, Mexico; Mario González-de la Parra is a worker of Biokinetics, S.A. de C.V., Ciudad de México, Mexico. Forms can be requested to the responsible author or the editorial direction of the Journal.
Ethical aspects
According to the health legislation of Mexico, first of all, the study protocol was reviewed and approved by a local Ethics Committee (Universidad del Futbol y Ciencias del Deporte, Hidalgo, Mexico). After that, the same study protocol was submitted for review and approval by independent Ethics and Research Committees pertaining to the Federal Commission for the Protection against Sanitary Risk (COFEPRIS, Mexico). All procedures performed in the current study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Signed Informed consent was obtained from all individual participants included in the study.
Funding
This research and its publication were supported by Laboratorios Liomont, S.A. de C.V. Federal, Mexico.

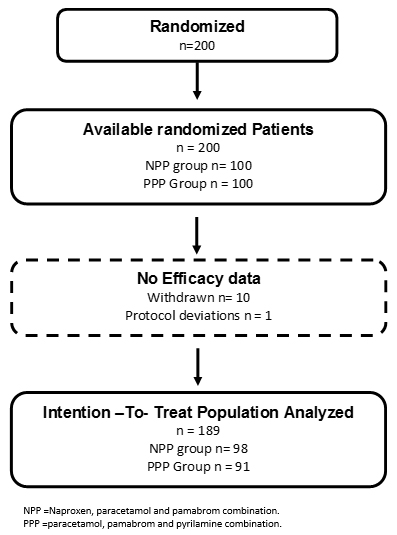 Figure 1. Flow diagram for randomized participants.
Figure 1. Flow diagram for randomized participants.

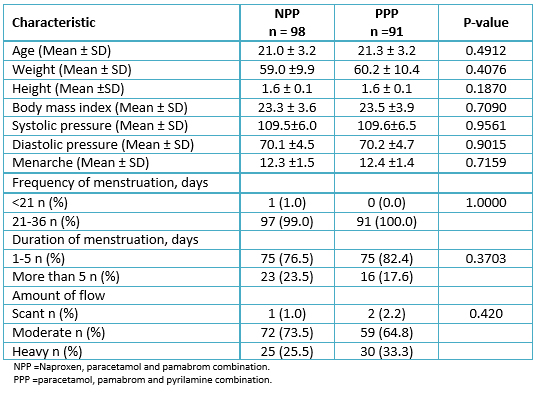 Table 1. Sociodemographic and clinical data of the 189 patients according to group.
Table 1. Sociodemographic and clinical data of the 189 patients according to group.

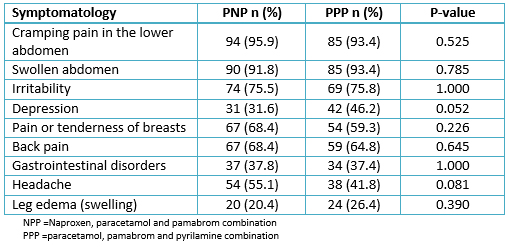 Table 2. Baseline characteristics of symptomatology in dysmenorrheic young women, who reported the presence of symptoms: NPP (98 patients) and PPP (91 patients)
Table 2. Baseline characteristics of symptomatology in dysmenorrheic young women, who reported the presence of symptoms: NPP (98 patients) and PPP (91 patients)

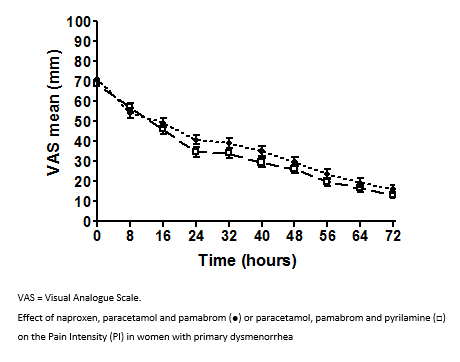 Figure 2. Effect of naproxen, paracetamol and pamabrom or paracetamol, pamabrom and pyrilamine on the Pain Intensity (PI) in women with primary dysmenorrhea.
Figure 2. Effect of naproxen, paracetamol and pamabrom or paracetamol, pamabrom and pyrilamine on the Pain Intensity (PI) in women with primary dysmenorrhea.

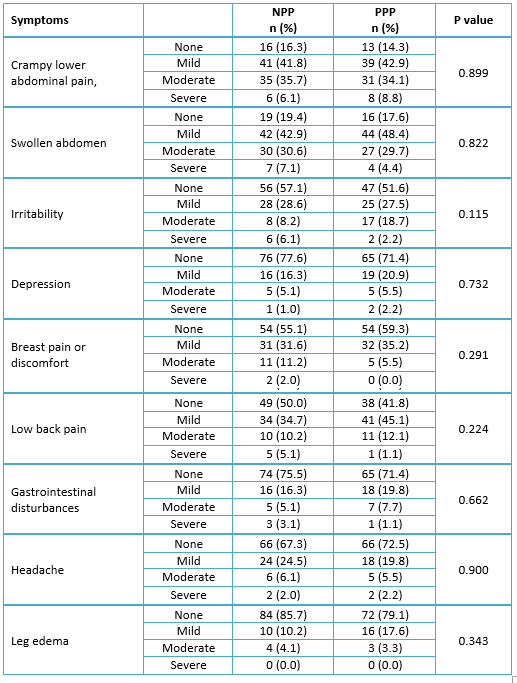 Table 3. Symptoms in women with dysmenorrhea at the end of treatment.
Table 3. Symptoms in women with dysmenorrhea at the end of treatment.

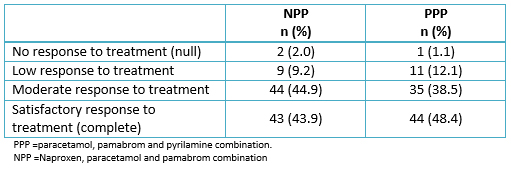 Tabla 4. End clinical response to treatment.
Tabla 4. End clinical response to treatment.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCTION
Dysmenorrhea is caused by the discharge of prostaglandins into the uterine tissue; therefore, non-steroidal anti-inflammatory drugs (NSAIDs) are the established initial therapy for dysmenorrhea. Dysmenorrhea therapy may include the administration of drug monotherapy or combination therapy. However, clinical scientific evidence on the efficacy of medications with two or three drugs combined is scarce or nonexistent.
OBJECTIVE
To evaluate and compare the efficacy and safety of two oral fixed-dose combinations for the relief of the symptoms of primary dysmenorrhea among Mexican women. One of the combinations is widely used in Mexico (paracetamol, pyrilamine and pamabrom) and the selected comparison was a medication with naproxen sodium, paracetamol and pamabrom based on the pathophysiology of primary dysmenorrhea.
METHODS
This was a single-centre, double blind, experimental, parallel group, randomized trial. Female patients with primary dysmenorrhea, older than 17 years and with pain intensity greater than 45 mm on a visual analogue scale, were included. The patients were then randomized to receive tablets with naproxen sodium, paracetamol and pamabrom or tablets with paracetamol, pyrilamine and pamabrom for one menstrual cycle. Patient evaluations of symptomatology and pain intensity were recorded throughout one menstrual period. Descriptive and inferential statistical analyses were utilized.
RESULTS
An intention-to-treat population of 91 women, with a mean age of 21.3 ± 3.2 years, received paracetamol, pyrilamine and pamabrom tablets, and 98 participants, with a mean age of 21.0 ± 3.2 years, received naproxen sodium, paracetamol and pamabrom tablets. The participants’ assessments of pain on the Visual Analogue Scale during the menstrual cycle demonstrated a significant reduction in both treatment groups (p<0.05). There is no significant difference in efficacy between both groups (p>0.05).
CONCLUSIONS
The results showed that both drug combinations were not different in reducing dysmenorrheic pain. Likewise, both treatments were well tolerated. Therefore, both treatments may be used for the treatment of primary dysmenorrhea.
 Autores:
Mario I. Ortiz[1], Gabriela Murguía-Cánovas[2], Laura C. Vargas-López[1], Rodolfo Silva[3], Mario González-de la Parra[4]
Autores:
Mario I. Ortiz[1], Gabriela Murguía-Cánovas[2], Laura C. Vargas-López[1], Rodolfo Silva[3], Mario González-de la Parra[4]

Citación: Ortiz M, Murguía-Cánovas G, Vargas-López LC, Silva R, González-de la Parra M. Naproxen, paracetamol and pamabrom versus paracetamol, pyrilamine and pamabrom in primary dysmenorrhea: a randomized, double-blind clinical trial. Medwave 2016 Oct;16(9):6587 doi: 10.5867/medwave.2016.09.6587
Fecha de envío: 15/5/2016
Fecha de aceptación: 12/9/2016
Fecha de publicación: 24/10/2016
Origen: no solicitado
Tipo de revisión: con revisión por tres pares revisores externos, a doble ciego

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Ortiz MI, Rangel-Flores E, Carrillo-Alarcón LC, Veras-Godoy HA. Prevalence and impact of primary dysmenorrhea among Mexican high school students. Int J Gynaecol Obstet. 2009 Dec;107(3):240-3. | CrossRef | PubMed |
Ortiz MI, Rangel-Flores E, Carrillo-Alarcón LC, Veras-Godoy HA. Prevalence and impact of primary dysmenorrhea among Mexican high school students. Int J Gynaecol Obstet. 2009 Dec;107(3):240-3. | CrossRef | PubMed | Ortiz MI. Primary dysmenorrhea among Mexican university students: prevalence, impact and treatment. Eur J Obstet Gynecol Reprod Biol. 2010 Sep;152(1):73-7. | CrossRef | PubMed |
Ortiz MI. Primary dysmenorrhea among Mexican university students: prevalence, impact and treatment. Eur J Obstet Gynecol Reprod Biol. 2010 Sep;152(1):73-7. | CrossRef | PubMed | Latthe PM, Champaneria R, Khan KS. Dysmenorrhoea. BMJ Clin Evid. 2011 Feb 21;2011. pii: 0813. | PubMed |
Latthe PM, Champaneria R, Khan KS. Dysmenorrhoea. BMJ Clin Evid. 2011 Feb 21;2011. pii: 0813. | PubMed | Zahradnik HP, Hanjalic-Beck A, Groth K. Nonsteroidal anti-inflammatory drugs and hormonal contraceptives for pain relief from dysmenorrhea: a review. Contraception. 2010 Mar;81(3):185-96. | CrossRef | PubMed |
Zahradnik HP, Hanjalic-Beck A, Groth K. Nonsteroidal anti-inflammatory drugs and hormonal contraceptives for pain relief from dysmenorrhea: a review. Contraception. 2010 Mar;81(3):185-96. | CrossRef | PubMed | Marjoribanks J, Ayeleke RO, Farquhar C, Proctor M. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2015 Jul 30;(7):CD001751. | CrossRef | PubMed |
Marjoribanks J, Ayeleke RO, Farquhar C, Proctor M. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2015 Jul 30;(7):CD001751. | CrossRef | PubMed | Di Girolamo G, Sánchez AJ, De Los Santos AR, González CD. Is acetaminophen, and its combination with pamabrom, an effective therapeutic option in primary dysmenorrhoea? Expert Opin Pharmacother. 2004 Mar;5(3):561-70. | PubMed |
Di Girolamo G, Sánchez AJ, De Los Santos AR, González CD. Is acetaminophen, and its combination with pamabrom, an effective therapeutic option in primary dysmenorrhoea? Expert Opin Pharmacother. 2004 Mar;5(3):561-70. | PubMed | Dawood MY, Khan-Dawood FS. Clinical efficacy and differential inhibition of menstrual fluid prostaglandin F2alpha in a randomized, double-blind, crossover treatment with placebo, acetaminophen, and ibuprofen in primary dysmenorrhea. Am J Obstet Gynecol. 2007 Jan;196(1):35.e1-5. | PubMed |
Dawood MY, Khan-Dawood FS. Clinical efficacy and differential inhibition of menstrual fluid prostaglandin F2alpha in a randomized, double-blind, crossover treatment with placebo, acetaminophen, and ibuprofen in primary dysmenorrhea. Am J Obstet Gynecol. 2007 Jan;196(1):35.e1-5. | PubMed | Ayan M, Taş U, Söğüt E, Arıcı S, Karaman S, Esen M, Demirtürk F. [Comparing efficiencies of diclofenac sodium and paracetamol in patients with primary dysmenorrhea pain by using Visual Analog Scale]. Agri. 2013;25(2):78-82. | CrossRef | PubMed |
Ayan M, Taş U, Söğüt E, Arıcı S, Karaman S, Esen M, Demirtürk F. [Comparing efficiencies of diclofenac sodium and paracetamol in patients with primary dysmenorrhea pain by using Visual Analog Scale]. Agri. 2013;25(2):78-82. | CrossRef | PubMed | Eisenberg E, Suzan E. Drug combinations in the treatment of neuropathic pain. Curr Pain Headache Rep. 2014 Dec;18(12):463.
| CrossRef | PubMed |
Eisenberg E, Suzan E. Drug combinations in the treatment of neuropathic pain. Curr Pain Headache Rep. 2014 Dec;18(12):463.
| CrossRef | PubMed | Dahl JB, Nielsen RV, Wetterslev J, Nikolajsen L, Hamunen K, Kontinen VK, et al. Post-operative analgesic effects of paracetamol, NSAIDs, glucocorticoids, gabapentinoids and their combinations: a topical review. Acta Anaesthesiol Scand. 2014 Nov;58(10):1165-81. | CrossRef | PubMed |
Dahl JB, Nielsen RV, Wetterslev J, Nikolajsen L, Hamunen K, Kontinen VK, et al. Post-operative analgesic effects of paracetamol, NSAIDs, glucocorticoids, gabapentinoids and their combinations: a topical review. Acta Anaesthesiol Scand. 2014 Nov;58(10):1165-81. | CrossRef | PubMed | Gerlinger C, Schumacher U, Faustmann T, Colligs A, Schmitz H, Seitz C. Defining a minimal clinically important difference for endometriosis-associated pelvic pain measured on a visual analog scale: analyses of two placebo-controlled, randomized trials. Health Qual Life Outcomes. 2010 Nov 24;8:138. | CrossRef | PubMed |
Gerlinger C, Schumacher U, Faustmann T, Colligs A, Schmitz H, Seitz C. Defining a minimal clinically important difference for endometriosis-associated pelvic pain measured on a visual analog scale: analyses of two placebo-controlled, randomized trials. Health Qual Life Outcomes. 2010 Nov 24;8:138. | CrossRef | PubMed | Philip BK. Parametric statistics for evaluation of the visual analog scale. Anesth Analg. 1990 Dec;71(6):710. | PubMed |
Philip BK. Parametric statistics for evaluation of the visual analog scale. Anesth Analg. 1990 Dec;71(6):710. | PubMed | Dexter F, Chestnut DH. Analysis of statistical tests to compare visual analog scale measurements among groups. Anesthesiology. 1995 Apr;82(4):896-902. | PubMed |
Dexter F, Chestnut DH. Analysis of statistical tests to compare visual analog scale measurements among groups. Anesthesiology. 1995 Apr;82(4):896-902. | PubMed | Myles PS, Troedel S, Boquest M, Reeves M. The pain visual analog scale: is it linear or nonlinear? Anesth Analg. 1999 Dec;89(6):1517-20. | PubMed |
Myles PS, Troedel S, Boquest M, Reeves M. The pain visual analog scale: is it linear or nonlinear? Anesth Analg. 1999 Dec;89(6):1517-20. | PubMed | Kannan S, Gowri S. Visual analog scale: Verify appropriate statistics. Perspect Clin Res. 2015 Apr-Jun;6(2):120. | CrossRef | PubMed |
Kannan S, Gowri S. Visual analog scale: Verify appropriate statistics. Perspect Clin Res. 2015 Apr-Jun;6(2):120. | CrossRef | PubMed | Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika 1959; 24(2):95-112.
| CrossRef |
Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika 1959; 24(2):95-112.
| CrossRef | Warner TD, Mitchell JA. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J. 2004 May;18(7):790-804.
| PubMed |
Warner TD, Mitchell JA. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J. 2004 May;18(7):790-804.
| PubMed | Godfrey L, Bailey I, Toms NJ, Clarke GD, Kitchen I, Hourani SM. Paracetamol inhibits nitric oxide synthesis in murine spinal cord slices. Eur J Pharmacol. 2007 May 7;562(1-2):68-71. | PubMed |
Godfrey L, Bailey I, Toms NJ, Clarke GD, Kitchen I, Hourani SM. Paracetamol inhibits nitric oxide synthesis in murine spinal cord slices. Eur J Pharmacol. 2007 May 7;562(1-2):68-71. | PubMed | Pickering G, Estève V, Loriot MA, Eschalier A, Dubray C. Acetaminophen reinforces descending inhibitory pain pathways. Clin Pharmacol Ther. 2008 Jul;84(1):47-51. Epub 2007 Oct 24. | PubMed |
Pickering G, Estève V, Loriot MA, Eschalier A, Dubray C. Acetaminophen reinforces descending inhibitory pain pathways. Clin Pharmacol Ther. 2008 Jul;84(1):47-51. Epub 2007 Oct 24. | PubMed | Umathe SN, Manna SS, Utturwar KS, Jain NS. Endocannabinoids mediate anxiolytic-like effect of acetaminophen via CB1 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2009 Oct 1;33(7):1191-9. | CrossRef | PubMed |
Umathe SN, Manna SS, Utturwar KS, Jain NS. Endocannabinoids mediate anxiolytic-like effect of acetaminophen via CB1 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2009 Oct 1;33(7):1191-9. | CrossRef | PubMed | Cruz MA, Gonzalez C, Acevedo CG, Sepulveda WH, Rudolph MI. Effects of histamine and serotonin on the contractility of isolated pregnant and nonpregnant human myometrium. Gynecol Obstet Invest. 1989;28(1):1-4. | PubMed |
Cruz MA, Gonzalez C, Acevedo CG, Sepulveda WH, Rudolph MI. Effects of histamine and serotonin on the contractility of isolated pregnant and nonpregnant human myometrium. Gynecol Obstet Invest. 1989;28(1):1-4. | PubMed | Bytautiene E, Vedernikov YP, Saade GR, Romero R, Garfield RE. Degranulation of uterine mast cell modifies contractility of isolated myometrium from pregnant women. Am J Obstet Gynecol. 2004 Nov;191(5):1705-10. | PubMed |
Bytautiene E, Vedernikov YP, Saade GR, Romero R, Garfield RE. Degranulation of uterine mast cell modifies contractility of isolated myometrium from pregnant women. Am J Obstet Gynecol. 2004 Nov;191(5):1705-10. | PubMed | Ortiz MI, Ponce-Monter HA, Mora-Rodríguez JA, Barragán-Ramírez G, Barrón-Guerrero BS. Synergistic relaxing effect of the paracetamol and pyrilamine combination in isolated human myometrium. Eur J Obstet Gynecol Reprod Biol. 2011 Jul;157(1):22-6. | CrossRef | PubMed |
Ortiz MI, Ponce-Monter HA, Mora-Rodríguez JA, Barragán-Ramírez G, Barrón-Guerrero BS. Synergistic relaxing effect of the paracetamol and pyrilamine combination in isolated human myometrium. Eur J Obstet Gynecol Reprod Biol. 2011 Jul;157(1):22-6. | CrossRef | PubMed | Seetman SC. Martindale: The Complete Drug Reference. 37th ed. London: The Pharmaceutical Press; 2011:112, 1499. | Link |
Seetman SC. Martindale: The Complete Drug Reference. 37th ed. London: The Pharmaceutical Press; 2011:112, 1499. | Link | Rao SS, Mudipalli RS, Remes-Troche JM, Utech CL, Zimmerman B. Theophylline improves esophageal chest pain--a randomized, placebo-controlled study. Am J Gastroenterol. 2007 May;102(5):930-8. | PubMed |
Rao SS, Mudipalli RS, Remes-Troche JM, Utech CL, Zimmerman B. Theophylline improves esophageal chest pain--a randomized, placebo-controlled study. Am J Gastroenterol. 2007 May;102(5):930-8. | PubMed | Mahoori A, Hassani E, Noroozinia H, Javaheri N, Hatami S. Theophylline versus acetaminophen in the treatment of post-dural puncture headache (PDPH). Middle East J Anaesthesiol. 2013 Oct;22(3):289-92. | PubMed |
Mahoori A, Hassani E, Noroozinia H, Javaheri N, Hatami S. Theophylline versus acetaminophen in the treatment of post-dural puncture headache (PDPH). Middle East J Anaesthesiol. 2013 Oct;22(3):289-92. | PubMed | Segerdahl M, Karelov A. Experimentally induced ischaemic pain in healthy humans is attenuated by the adenosine receptor antagonist theophylline. Acta Physiol Scand. 2004 Mar;180(3):301-6. | PubMed |
Segerdahl M, Karelov A. Experimentally induced ischaemic pain in healthy humans is attenuated by the adenosine receptor antagonist theophylline. Acta Physiol Scand. 2004 Mar;180(3):301-6. | PubMed | Duggan KC, Walters MJ, Musee J, Harp JM, Kiefer JR, Oates JA, Marnett LJ. Molecular basis for cyclooxygenase inhibition by the non-steroidal anti-inflammatory drug naproxen. J Biol Chem. 2010 Nov 5;285(45):34950-9. | CrossRef | PubMed |
Duggan KC, Walters MJ, Musee J, Harp JM, Kiefer JR, Oates JA, Marnett LJ. Molecular basis for cyclooxygenase inhibition by the non-steroidal anti-inflammatory drug naproxen. J Biol Chem. 2010 Nov 5;285(45):34950-9. | CrossRef | PubMed | Bjarnason I, Thjodleifsson B. Gastrointestinal toxicity of non-steroidal anti-inflammatory drugs: the effect of nimesulide compared with naproxen on the human gastrointestinal tract. Rheumatology (Oxford). 1999 May;38 Suppl 1:24-32. | PubMed |
Bjarnason I, Thjodleifsson B. Gastrointestinal toxicity of non-steroidal anti-inflammatory drugs: the effect of nimesulide compared with naproxen on the human gastrointestinal tract. Rheumatology (Oxford). 1999 May;38 Suppl 1:24-32. | PubMed |