 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Palabras clave: hypertension, high blood pressure, hypertensive cardiopathy, risk factors
INTRODUCTION
Among the conditions resulting from target organ damage by arterial hypertension, hypertensive cardiopathy is the one that exhibits the highest morbidity and mortality rates. Its prevention should be a target of all high blood pressure medical care programs.
OBJECTIVE
To identify risk factors for the development of hypertensive cardiopathy.
METHODS
A prospective cohort study was carried out in hypertensive patients assisted at the specialized arterial hypertension physicians’ offices of the “Carlos Manuel de Céspedes” Specialty Policlinic attached to the General University Hospital, Bayamo Municipality, Granma Province, Cuba, from January 1st, 2000 to December 31st, 2009.
RESULTS
Multivariate analysis done to estimate the hazard rate (HR) of developing hypertensive cardiopathy, showed significant independent statistic association for most factors. The first place was occupied by lack of blood pressure control (HR=2.022; 95% CI: 1.659-2.465; p<0.005), followed by hypertension stage 2 (HR=2.015; 95% CI: 1.715-2.366; p<0.005). Another factors with significant HRs were microalbuminuria (HR=1.9; 95% CI: 1.6-2.2) and age over 60 years (HR=1.6; 95% CI: 1.4-1.9).
CONCLUSIONS
Several risk factors must be considered for the prevention of hypertensive heart disease in high blood pressure patients.
Arterial hypertension stands out among cardiovascular diseases with a high prevalence in the population, particularly in individuals over 60 years of age, from both sexes. Besides, it is one of the main factors of cardiovascular risk and responsible for most of the mortality and morbidity in developed countries [1].
Arterial hypertension is related to the onset of cardiac ischemic disease, cardiac insufficiency, cerebrovascular disease, nephropathy, and other functional and organic disorders resulting from the microvascular lesions that it provokes [1],[2].
The damage to target organs caused by arterial hypertension with the highest morbidity and mortality is hypertensive cardiopathy. This condition can be defined as a complex and variable group of effects that cause a chronic elevation of blood pressure in the heart, and is characterized by the presence of anatomic and biochemical signs of left ventricular hypertrophy, diastolic or systolic ventricular dysfunction, myocardial ischemia, and heart rhythm disorders [1],[3],[4].
In addition to role played by the hemodynamic overload that arterial hypertension implies in the development of hypertensive cardiopathy, there are other important pathologic processes mediated by hormones, growing factors, cytokines and other molecules whose effects on the cardiomyocyte and the rest of the cellular and non-cellular of a hypertensive patient’s myocardium, can explain the development of left ventricular hypertrophy and myocardial remodeling [4].
Likewise, the etiology of cardiovascular risk factors is an issue of medical interest nowadays, since there is a variety of cultural, epidemiological and clinical patterns, which are responsible for the differences in different geographic regions and populations [1],[3],[4] and the doubts about the real influence of each of these factors.
For instance, controlling arterial hypertension is not always a guarantee to avoid hypertensive cardiopathy, which indicate the presence of a group of factors in hypertensive individuals capable of increasing the risk of developing hypertensive cardiopathy, which are independent of hypertension control [1],[2],[3].
Among these factors are: age, excessive sodium intake in the diet, dyslipidemias, smoking, obesity, sedentariness, and the like [1],[2],[3],[4],[5],[6]. It seems that the interaction of these factors provokes hypertensive cardiopathy, but their degree of influence and independence has not been clearly stated. The results differ from one study to another [1],[2],[3],[4],[5],[6] probably as a result of the differences in their methodological designs and the criteria followed for the diagnosis of hypertensive cardiopathy.
Despite the existence of research about the risk factors for hypertensive cardiopathy and their important contribution to the knowledge on this condition, there are grounds that justify continuing the research on the topic. Among these reasons are the high incidence and prevalence of hypertensive cardiopathy that increases mortality and the incapacity caused by cardiovascular diseases in developed and developing countries. Furthermore, there is also the need to understand the independent contribution of each risk factor for the development of cardiopathy in countries like Cuba.
For all that has been stated above, the objective of the present paper is to evaluate the influence of different risk factors in the development of hypertensive cardiopathy.
A cohort prospective study was carried out in hypertensive patients assisted at the hypertension outpatient department of the Specialty Policlinic attached to the “Carlos Manuel de Céspedes” Hospital, Bayamo Municipality, Granma, Cuba, from January 1st, 2000 to December 31st, 2009. These patients had four appointments a year.
Inclusion and exclusion criteria
The patients included in the study were patients over 18 years with a past history of essential hypertension over five years who did not have a diagnosis of hypertensive cardiopathy (considering the diagnostic criteria for hypertensive cardiopathy further described in the definition of the dependent variable were not present).
Patients with a diagnosis of ischemic cardiopathy were excluded from the study. In spite of its high frequency in hypertensive patients, this study was aimed at evaluating the effects of hypertension in isolation; therefore, the inclusion of patients with ischemic cardiopathy could bring about confusion. For the same reason patients with interventricular and auriculoventricular conduction disorders were also excluded; furthermore, it was also monitored that the included patients did not suffer from other conditions that could cause cardiopathy. So patients were excluded if they had a diagnosis of myocardiopathy (in any of its clinical forms), diabetes mellitus, thyroid diseases, chronic renal insufficiency, and chronic inflammatory intestinal or collagen diseases. Patients receiving treatment with cytostatic agents were also excluded from the study.
Patients’ typical track in the study
The cohort patients were chosen from the records of the hospital hypertension outpatient offices, provided that they met the inclusion criteria, over two years prior to the beginning of the study on January 1st, 2000. They had been referred to the hospital by specialists who work in different health areas (primary health care).
Every patient included in the study was interviewed and a thorough physical examination was performed to obtain the necessary information. Later on, clinical evaluations were done every three months during the first year. Echocardiographic and electrocardiographic studies were done every six months. After the second year and for ten years these patients had follow-up appointments every six months.
During the study all the patients received an initial uniform medical treatment based in the therapeutic protocol approved by the Hospital Research Ethics Committee, described in the Cuban guidelines for the treatment of arterial hypertension and valid since 1998. The treatment is customized according to the patient’s age, skin color, and other possible risk factors and contraindications. As a whole, the treatment protocol includes an inhibitor of angiotensin II-converting enzyme plus a diuretic; calcium antagonists alone or combined with a diuretic or a beta-blocker alone or combined with a diuretic; according to the patient’s grade of hypertension.
Sample characteristics
During the two years prior to the beginning or the cohort evaluation, 3,817 patients from Bayamo, capital city of Granma Province, were seen at the outpatient department, 2,646 (69.32%) of them came from urban areas and 1,171 (30.68%) came from rural areas. Out of these 3,817 patients, 2,551 patients that met the inclusion criteria were accepted in the study. During the 10-year follow-up, 237 patients died due to causes other than hypertensive cardiopathy and 211 were dropouts for other causes (did not attend the appointments, moved to another province, developed an excluding condition). Moreover, patients who had a diagnosis of hypertensive cardiopathy during the first year of the follow-up were also excluded from the study, on the basis that in these patients it was not possible to carry out an adequate evaluation of the effect of the factors under study. Finally, 2,103 individuals were included in the study.
The date taken as the beginning of the cohort was January 1st, 2000. After this date the cohort was considered closed and no more patients were included. Individual evaluations ceased if the patient developed hypertensive cardiopathy or after ten years in the study in patients who did not develop this condition.
Definition of variables
Dependent variable: two categories, developed hypertensive cardiopathy or not. This diagnosis was reserved for all hypertensive patients who during the follow-up period met the following criteria:
The echocardiogram was done by two cardiologists specialized in echocardiography and with more than 15 years of experience. An ASAOTE Caris PLUS machine was used using the American Society of Echocardiography guidelines.
Independent variables: factors whose influence on the development of hypertensive cardiopathy is being evaluated and which are described next. All variables were dichotomized: one category was called exposed (according to previous knowledge, there was a higher probability of developing cardiopathy); the other category was called non-exposed.
Age was quantified in years, patients over 60 years were considered exposed.
Sex was divided into male (exposed) and female.
Smoking was grouped into two categories: smokers (exposed) if they smoked cigarettes, cigars, or pipe daily or almost daily, regardless the number of units smoked, and ex-smokers who quit the habit less than a year before, and non-smokers, those who were not in the habit or those who had quit it more than a year before.
Alcoholism (exposed, if more than 1 ounce of pure alcohol was ingested daily, equivalent to 1 ounce (20 ml) of ethanol, 8 ounces (240 ml) of wine, 24 ounces (720 ml) of beer, 1 ½ ounce (45ml) of rum. In the case of women and low-weight patients (according to size), they were considered exposed if they consumed 15 ml or more per day of any kind of alcoholic beverage [11].
Obesity was established by calculating body mass index (BMI: weight in kilograms/height in m2). Every subject with a body mass index greater or equal to 30 or with a waist circumference greater or equal to 102 cm for men and 88 cm for women was considered exposed.
Sedentariness: a sedentary person is one that daily spends less than a given amount of time in leisure activities that consume four or more metabolic equivalents (physical activity equivalent in energy output to or greater than to walking briskly (more than 6 km/h) or riding a bicycle at a speed between 16‐19 km/h). The patient’s profession was also taken into account. For the definition of the variable, the patients were asked the questions below, based on the data obtained from studies that related sedentariness and cardiovascular diseases [12],[13]:
1. What kind of job do you do?
Possible answers:
a) A job that involves little or no physical activity: usually sits down or stands up all day long or walks very little.
b) A job that demands a high energy output from the patient (example: construction workers, farmers, stevedores, etc.).
2. What kind of physical activity do you do in your free time?
Possible answers:
a) I never exercise.
b) Some physical or sports activity (at least 25 minutes for women and 30 for men) several times a month.
c) Regular physical activity (at least 25 minutes for women and 30 for men) several times a week.
d) Daily physical training.
Patients who answered affirmatively items 1a and 2a were considered sedentary. The rest of them were considered non sedentary.
Excessive salt intake. Every subject who had an ingestion of salt greater than 5 gram/day (this is equivalent to a level teaspoonful, distributed among the dishes prepared for lunch and dinner) was considered exposed. As exposed were also included those patients who consumed bakery products or used table salt (three or more times a week) [11],[14]. To obtain accurate answers, the questions asked about consumed food, according to amount and frequency, the amount of sodium in the foods most frequently consumed, the amount of salt added while cooking and at the table, and the ingestion of foods with high sodium content; to obtain this information the following questions were used:
1. Do you (or anybody else at home) add more than a level teaspoonful of salt per person when cooking, distributed among the dishes prepared for lunch and dinner?
Possible answers:
a) Yes
b) No
2. Do you add salt to your food after it is cooked or do you use salt shakers?
Possible answers:
a) Yes, every day or almost every day
b) Occasionally (less than three times a week)
c) Never
3. Do you eat salty foods? Such as: preserved foods and sausages (bacon, ham, sardines, olives, canned meat, salami, hot dogs, spiced sausage, and the like); foods that contain salt (crackers, bread, corn flakes, peanut, among others, sauces and canned soups, cheese, butter, mayonnaise, foods preserved in salt); other foodstuffs (bottled or carbonated soft drinks, beers, pickles, artificial flavors) or other salty foods (soy, fish sauce, tomato sauce, sauces for local dishes).
Possible answers:
a) Yes, every day or almost every day.
b) Occasionally (less than three times a week).
c) Never.
Patients who answered affirmatively any of the items 1a, 2a or 3a were considered to have excessive salt intake (exposed).
The biomarkers selected as possible risk factors were cholesterol, uric acid, triglycerides, HDL-cholesterol, glycaemia, C reactive protein, creatinine, microalbuminuria and the cholesterol/HDL quotient. Blood samples for laboratory tests were obtained in the fasting state (8 to 12 hour), and they were centrifuged at room temperature at 2,000 rpm for 10 minutes. Creatinine, uric acid, cholesterol, HDL-cholesterol, triglycerides, and glycaemia were measured with a HITACHI 902® machine during the first 24 hours after extraction and the first two ones were expressed in µmol/l and the rest in mmol/l. Determination of all the studies were done by means of enzymatic methods.
The end-points used in the statistical, univariate, and multivariate analyses for the dichotomization of each laboratory variable, were established according to a method proposed in the literature to determine optimal end-points, which is explained below. In this way, the end-points that defined the “exposed” patients were established at the following values: serum cholesterol over 4.8 mmol/l, triglyceridemia greater than 1.7 mmol/l, HDL-cholesterol lower than 1.5 mmol/l, cholesterol/HDL quotient greater than 4, glycemia in fasting state greater than 5.4 mmol/l, creatinine greater than 80 µmol/l, and uric acid greater than 375 µmol/l. C reactive protein was determined by the turbidimetric quantitative determination method and patients with values over 4 mg/l were considered exposed. Microalbuminuria patients were considered exposed when their values ranged from 0.02 to 0.2 g/l in a 24-hour period [1] and it was quantified by means of the Microalb-Látex technique (measurement of the amount of this substance on first-void urine specimen). The value of the quantitative variables was based on the average of three results obtained in the first three appointments during the first year after the patient’s inclusion in the study.
Hypertension control. As controlled patients were defined those patients with blood pressure readings below 140 and 90 mmHg (systolic and diastolic respectively) in 100% of determinations at the appointments during each follow-up year until the onset of hypertensive cardiopathy or the study was concluded (at least four determinations per year) under medical treatment, and uncontrolled patients who did not meet the previous criteria. This definition was based on the Cuban hypertension guidelines [11],[15]. Other blood pressure determinations during other contacts with the health system out of the hospital outpatient department for whatever reason were also taken into account (for this purpose, the patients were instructed to be bring in writing their blood pressure values); to guarantee the authenticity of this variable, each patient was given a form demanding the following information: date, time, blood pressure values, and doctor’s signature and seal. This document should be presented to the hypertension specialist on the day of the appointment.
Finally, disease evolution time and hypertension stage were also considered. Concerning evolution time, the patients were grouped into two categories: patients with an evolution time between 5 and 15 years, and patients with more than 15 years of evolution (exposed). Hypertension stage was classified according to the NYHA (New York Heart Association) 7th report proposal [16].
For blood pressure determinations, aneroid and mercury sphygmomanometers previously calibrated by the Territorial Quality and Normalization Office, accredited for such purposes were used. The data were obtained at the interviews carried out by the authors during the appointments (every three months in the first year of the cohort and every six months during the next nine years), with previous patients’ awareness and consent.
Statistical analysis
The statistical analysis started with a characterization of the sample, which meant a description of all the variables. For the quantitative variables the means and standard deviations were determined, along with the minimum and maximum values of each distribution; whereas for the qualitative variables absolute and relative frequencies were obtained (percentages).
Univariate analysis
To evaluate the association between qualitative variables and the risk of developing hypertensive cardiopathy, Mantel’s chi-square test was used. The magnitude of the associations was estimated by means of the relative risk calculation (RR, exposed/non exposed) to acquire a hypertensive cardiopathy between 1 and 10 years. Estimations of the relative risks were obtained by points and by interval of confidence (95%). The hypothesis that the population relative risk equaled 1 with a significance level of 0.05 was proved for each variable.
The same procedure was followed for the univariate analysis of the quantitative variables. The variables were dichotomized looking for optimal end points. In the searching of possible end points the most extreme values of the variables on both ends were not considered, that is, under percentile 5 and above percentile 95. Likewise, due to the increased probability of type I error when using several hypothesis test, the following formula was used to correct it: p = −3.13 pmin(1 + 1.65 Ln(pmin)), where pmin is the minimum probability value obtained and p is the corrected value. The C value was chosen as optimal end point since it had the highest chi square result (that is, it was the one with the lowest p value) for all the values of the dichotomized variables. So, it was selected as end point for each variable the value that best differentiated patients who developed hypertensive cardiopathy and those who did not [17],[18].
Multivariate analysis
Cox’s proportional hazards model was used with all independent variables. The regression coefficient (β), and the standard error of each coefficient (S) were estimated. The significance of each coefficient was tested (null hypothesis β=0) using Wald’s and chi square test. Hazard ratios (HR) like exp (B) with interval of confidence (IC) of 95% were also estimated. Cox’s proportional hazards model allows modeling the risk of occurrence of hypertensive cardiopathy of the different covariables during the study. Hazard ratios (HR) permit to estimate how many times greater is the risk of developing hypertensive cardiopathy in exposed compared to the non-exposed in each variable, while the rest of the variables can be controlled. The adjustment of Cox’s regression function, which is equivalent to the estimation of its parameters, was done by the method of maximum likelihood (omnibus test in the SPSS statistical package). All the patients who did not develop hypertensive cardiopathy during the ten years that lasted the study cohort, although they were used in both the univariate and multivariate analysis) were considered censored cases. And those patients who met the exclusion criteria were also included in the multivariate analysis as censored data.
Ethical considerations
In this research all basic ethical principles of clinical epidemiological studies were observed. All the research process was carried out with the approval of the board of directors and the ethics committee of the hospital. All potentially eligible patients were informed about the study and their consent was requested. The privacy of the patients’ identities and personal information was also guaranteed. The patients received a fair and most respectful treatment throughout the study. All the patients chose to participate in the study voluntarily.
Of the 2,103 patients of the cohort, 701 developed hypertensive cardiopathy (33.33%).
Patients over 60 years represented 46.8% of the cases and there was a discrete prevalence of male patients (50, 6%). Most of the patients were diagnosed stage 1 hypertension (61.2%) and 38.8% of them showed no hypertension control. Table 1.
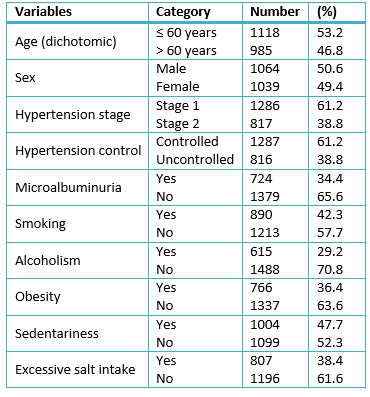
Table 1. Sample characterization. Categorical variables
Table 2 summarizes the data gathered from the quantitative variables. According to their variation coefficient, the variables with greatest variations were: C-reactive protein, time of evolution of hypertension, and triglycerides. The mean values of all variables were within normal limits.
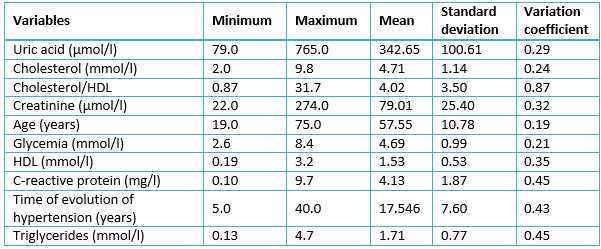
Table 2. Sample characterization. Quantitative variables
The univariate analysis of categorical variables is presented in Table 3. With the exception of alcoholism (RR= 1.114; p= 0.104) and the male sex (RR= 1.002; p= 0.975), all categorical variables showed a relative risk significantly greater than one. Lack of control of hypertension was noticeable since it increased the risk of developing hypertensive cardiopathy more than three times (RR= 3,030; p< 0,005) compared to controlled patients.
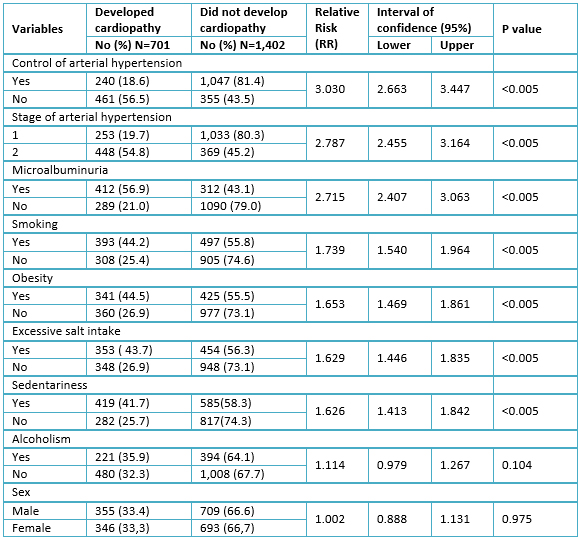
Table 3. Univariate analysis of qualitative variables
Table 4 shows the results of the univariate analysis of the quantitative variables; all of them reached a significant relative risk greater than 1. C reactive protein was the most important factor because it increased the risk of developing hypertensive cardiopathy 4.77 times (95% confidence interval= 3.528-4.946; p<0.005) in the exposed compared to the non-exposed; followed by age over 60 years (RR: 3.601; 95% confidence interval: 3.097-4.187; p<0.005) compared to those under 60 years.
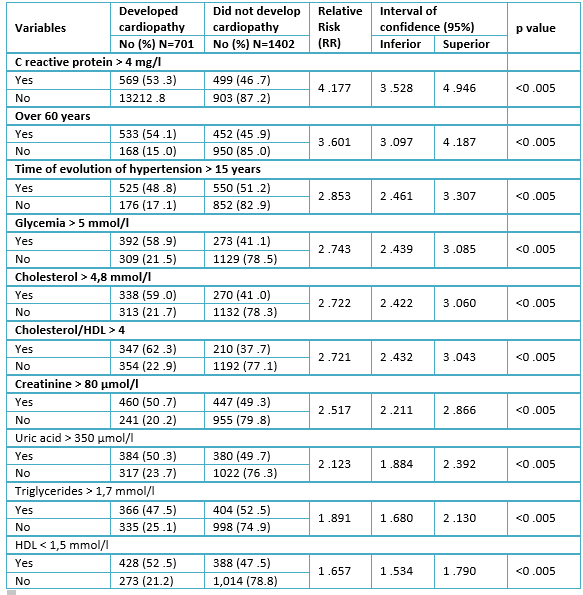
Table 4. Univariate analysis of quantitative variables, after being dichotomized
Table 5 shows the results of multivariate analysis, showing the relationship of each variable with the probability of developing hypertensive cardiopathy in years, after control of the rest of the variables. It can be seen that there was a significant statistical association among most risk factors and the development of hypertensive cardiopathy.
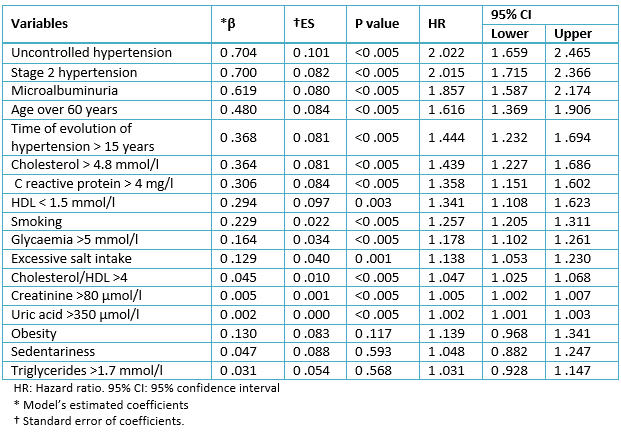
Table 5. Results of Cox’s multivariate model.
The most significant factor was the lack of control of arterial blood pressure (β: 0.704; HR: 2.022; 95% confidence interval: 1.659-2.465) followed by stage 2 hypertension (β: 0.700; HR: 2.015; 95% confidence interval: 1.715-2.366) and microalbuminuria (β: 0.619; HR: 1.857; 95% confidence interval: 1.587-2.174).
Because arterial hypertension is one of the most important cardiovascular risk factors, its control is paramount.
Similarly to the findings of the present study, Cuspidi et al. [19] demonstrated an important association between the left ventricular mass index (β =0.167, P< 0. 0001) and systolic hypertension over 140 mmHg. An American study [20] confirmed a mortality risk by cardiovascular disease 1. 89 (95% confidence interval: 1.48–2.40) times greater in uncontrolled hypertensive patients than in controlled ones. Likewise, Peralta et al. [21] found greater incidence of cardiovascular events in patients with arterial systolic hypertension over 150 mmHg.
Functional changes (cellular permeability changes, increase of intracellular and extracellular matrix protein synthesis) and structural (left ventricular hypertrophy, remodeling, fibrosis, diastolic dysfunction, and microcirculation to terminal cardiopathy of the myocardium) which take place in Hypertensive patients [3],[4],[8],[22] explain the high incidence of hypertensive cardiopathy in individuals with uncontrolled blood pressure, as it was seen in the present study.
As in the present study, other authors [19],[20],[21],[22] confirmed that patients with blood pressure readings of 160/100 mmHg or higher were more liable to experience ventricular growing, cardiac dysfunction, and death. In addition, León Álvarez and Pérez Caballero [23] in a national study demonstrated that more than 90% of patients with this factor eventually had cardiovascular complications.
These results confirm the existence of a continuous, consistent and independent association between arterial e hypertension and the risk of cardiovascular complications. Elevated blood pressure favors a greater likelihood of heart attack or cardiac insufficiency (the likelihood is twofold per each 20 mmHg increase in arterial systolic pressure) [15].
The effects of maintained chronic hypertension on the heart and blood vessels have been well demonstrated [4],[8],[24],[25]. For that reason, it is necessary to reduce arterial systolic pressure below 150 mmHg, particularly in patients over 60 years [26].
Microalbuminuria is an important factor to predict cardiovascular risk. It is evidenced by the fact that there is a high prevalence of concentric left ventricular hypertrophy and subclinical deterioration of the left ventricle in subjects with microalbuminuria [1],[27],[28]. These findings coincide with our study.
The results presented above, are explained by the close association of microalbuminuria with different physiopathologic changes such as: insulin resistance, endothelial dysfunction, dyslipidemias, salt sensitivity and increase of angiotensin II. As a result, an inflammatory process appears which is responsible for kidney and heart cell membrane dysfunction [28],[29].
Straina et al. [30] and Yeboah et al. [31] demonstrated a significant association (p < 0,001) between smoking and left ventricular mass increase and hypertrophy in patients with microvascular dysfunction, independent of blood pressure figures; while Halperin et al. [32] found a relative risk of 1, 15 (p= 0,006) of developing arterial hypertension and its complications in smokers, results that coincide with the present study. Active exposure to tobacco smoke can cause vasomotor dysfunction, atherogenesis, excitation of the sympathetic nervous system and poor response to treatment, [32],[33],[34] facts that can explain the findings obtained in this research.
In hypertensive and obese people the risk of left ventricular hypertrophy is twofold. Similarly to our results, Ärnlöv et al. [35] and Woodiwiss et al. [36] found greater risk of cardiovascular complications in obese patients, with independence of blood pressure values.
Although the mechanisms by which obesity induces the development of arterial hypertension and its complications are not very clear, there is a complex relationship between obesity and cardiovascular diseases, due to the different physiopathologic mechanisms which involve many factors which interact in a complex way; among them are sodium retention, insulin resistance, subclinical inflammation, neurohormonal activation, high leptin concentrations, increasedoxidation of free fat acids in the myocardium, fat deposit in the heart and the capacity of adipose tissue to synthesize great amounts of hormones and interleukins [36],[37],[38],[39],[40].
Resembling our results, other studies show an association between excessive salt in the diet and the development of arterial hypertension, uncontrolled hypertension, and the occurrence of hypertensive cardiopathy [41],[42],[43]. The relationship between a high salt intake in the diet and arterial hypertension and its complications is due to the fact that a high salt intake increases plasmatic activity of rennin and other components of the axis, reduces the cerebral natriuretic peptide and of creatinine clearing, as well as an increase in the retention of liquids and arterial rigidity [44].
On the other hand, there is an inverse relation between physical activity and blood pressure level, which is independent of adiposity; hypertension is more frequent in sedentary individuals. Physical activity decreases insulin resistance and favors coagulation and fibrinolysis, reduces oxidative stress, modifies the lipid profile, improves cardiac function and attenuates the development of hypertension and its complications [45]. These facts can explain our results.
Different from the results of this study, other authors quote a relationship between alcoholism and the risk of cardiovascular complications in hypertensive patients [46],[47],[48]. Most of the patients studied were moderate drinkers, which probably had a protective effect.
Sex was not a major risk to develop hypertensive cardiopathy. Although arterial hypertension appears in men earlier than in women and is associated to other cardiovascular risk factors, it has been observed that after menopause, the risk is similar for both sexes or even greater in female [43].
Since the importance of inflammation became evident in the stages of atherosclerosis and the genesis of cardiovascular events, C reactive protein determination has become a routine study in many health centers and it has been attributed a predictive value. As it happened in our study, different authors found association between C reactive protein and the risk of cardiovascular diseases [49],[50],[51],[52]. C reactive protein is a biomarker of active vascular processes, which have a direct effect on the morphological and cardiac functions and can contribute to the prognosis of a hypertensive patient [52],[53],[48].
Age plays a role in the different biological processes, since it is responsible for all the changes associated to aging; its predictive value has been recognized in almost all conditions, and hypertension and its complications are not exceptions. Coincident with the present study, Akasheh et al. [54] demonstrated a greater association (regression coefficient = 0.714; p= 0.0008) between the risk of left ventricular hypertrophy and diastolic hypertension in individuals over 57 years.
Another important factor in the genesis of hypertensive cardiopathy is the time of evolution of arterial hypertension, as it was shown in the present study. After ten years of a diagnosis of hypertension the risk of cardiovascular events increases from 15% to 30% and, if the subclinic organic damaged is added, this value can be considerable. Hemodynamic, humoral, and inflammatory changes observed in Hypertensive patients result from chronic blood pressure elevation, present even in controlled patients and are responsible for organic lesions, mainly of the myocardium, hence its predictive value [1],[4],[8].
So, due to the highly versatile character of arterial hypertension and its progressive impact, it is necessary to change the approach towards blood pressure for a better long-term hypertension overload profile [55].
In relation to glucose metabolism, Cuspidi et al. [19] showed that greater values than 5.2 mmol/L was an independent risk factor in non-diabetic fasting hypertensive individuals (OR= 1.28) for hypertensive cardiopathy, similarly to our study. Separately or associated with arterial hypertension, hyperglycemia is related to cardiac hypertrophy and interstitial fibrosis, arteriosclerosis and coronary endothelial dysfunction which predisposes to ischemia in the absence of stenosis of these arteries. Also alterations of the systolic and diastolic functions occurred in the absence of valvular or coronary heart disease [23],[56].
Lipid metabolism disorders, in general, have been associated for more than 70 years to the risk of cardiovascular diseases and their prognoses. Consistent with this study, Marshall [57] and Piskorz et al. [58] stated that elevated cholesterol values increase the risk of left ventricular hypertrophy significantly. Likewise, Barter et al. [59] quoted that the cholesterol/HDL quotient causes approximately a twofold elevation of the risk of cardiovascular complications. Meanwhile Ashen and Blumenthal [60] demonstrated that it is not possible to achieve a significant reduction of the atheromatous plaque in individuals with treatment to reduce the levels of total and LDL cholesterol if the HDL values are not increased.
In zones of the vascular walls with predisposition to atherosclerotic lesions, plasmatic hypercholesterolemia is associated to an increase of LDL transit throughout the vascular endothelium. LDL retention in the arterial intima is an important step in the onset and progress of the atherosclerotic lesion since it begins a local inflammatory process [61]. These events result from the imbalance between the cholesterol coming in and out of the arterial wall, where the first component predominates and the responsible for these exchanges are LDL and HDL cholesterols respectively [62]. In this way there is a worsening of the hemodynamic and non-hemodynamic disorders which take part in the genesis of hypertensive cardiopathy [4],[8]. These elements may account for the findings in this study.
As in our research, other authors found creatinine levels under the threshold of chronic renal insufficiency in patients with arterial hypertension and damage to target organs [63],[64]. The kidney is important for the development and maintenance of arterial hypertension, where complex and interrelated factors which include the rennin-angiotensin system, several inflammatory mediators, and oxygen reactive species cause histological and functional changes characteristic of the renal hypertensive lesion which, in early stages, cause microalbuminuria and less creatinine clearance. Finally, renal and cardiac tubulointerstitial fibrosis takes place and, as a consequence, dysfunction of both organs appears [4],[65].
The uric acid level, even under the threshold considered pathologic, was a risk factor for the occurrence of arterial hypertension; according to Leiba et al. [66]. Díaz Arce et al. [67] also showed a similar relation, while Cuspidi et al. [19] found a greater risk of left ventricular hypertrophy in patients with elevated uric acid. Considerable evidences support the role of uric acid and the development of hypertension and its complications; through inflammatory and vascular changes in renal microcirculation, activation of the rennin-angiotensin system and endothelial dysfunction [68],[69]. These facts might explain our results.
The present paper demonstrates the importance of the effects of arterial hypertension, the presence of microalbuminuria and advanced age as factors with greater association with the risk of developing hypertensive cardiopathy. Nevertheless, obesity, sedentariness and triglycerides were not independent risk factors. These facts suggest that, in spite of its great importance in the prognosis of the hypertensive patient, their close association with other factors caused that their independent pathologic effects were less visible than other factors with stronger association possibilities.
As limitations of the present study it can be pointed out that it was not possible to study new predictors of cardiovascular risk like hypersensitive C reactive protein, endostatin, homocysteine, among others. Another limitation consisted on not being able to measure urine sodium excretion to evaluate sodium ingestion in a more objective way. Since urine sodium measurements cannot be done on daily basis due to its high cost and the Cuban health system cannot afford to make such studies available in every health center of the country, which hinders its generalization. For that reason, a set of questions was elaborated, as it was explained in the method, to have at least an approximate idea of the amount of salt ingested by these patients in their diets.
From the editor
The authors originally submitted this article in Spanish and subsequently translated it into English. The Journal has not copyedited this version.
Ethical aspects
The Journal is aware that the Research Ethics Committee of Carlos Manuel de Céspedes University Hospital, in Bayamo, Granma, Cuba, was informed about this study and its possible publication in a biomedical journal.
Conflicts of interest
The authors completed the ICMJE conflict of interest declaration form, and declare not having received funding for the preparation of this report, not having any financial relationships with organizations that could have interests in the published article in the last three years, and not having other relations or activities that might influence the article´s content. Forms can be requested to the responsible author or the editorial direction of the Journal.
Funding
The authors declare that there was no funding coming from external sources.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCTION
Among the conditions resulting from target organ damage by arterial hypertension, hypertensive cardiopathy is the one that exhibits the highest morbidity and mortality rates. Its prevention should be a target of all high blood pressure medical care programs.
OBJECTIVE
To identify risk factors for the development of hypertensive cardiopathy.
METHODS
A prospective cohort study was carried out in hypertensive patients assisted at the specialized arterial hypertension physicians’ offices of the “Carlos Manuel de Céspedes” Specialty Policlinic attached to the General University Hospital, Bayamo Municipality, Granma Province, Cuba, from January 1st, 2000 to December 31st, 2009.
RESULTS
Multivariate analysis done to estimate the hazard rate (HR) of developing hypertensive cardiopathy, showed significant independent statistic association for most factors. The first place was occupied by lack of blood pressure control (HR=2.022; 95% CI: 1.659-2.465; p<0.005), followed by hypertension stage 2 (HR=2.015; 95% CI: 1.715-2.366; p<0.005). Another factors with significant HRs were microalbuminuria (HR=1.9; 95% CI: 1.6-2.2) and age over 60 years (HR=1.6; 95% CI: 1.4-1.9).
CONCLUSIONS
Several risk factors must be considered for the prevention of hypertensive heart disease in high blood pressure patients.
 Autores:
Alexis Álvarez Aliaga[1], Julio César González Aguilera[1], Liliana del Rosario Maceo Gómez[2]
Autores:
Alexis Álvarez Aliaga[1], Julio César González Aguilera[1], Liliana del Rosario Maceo Gómez[2]

Citación: Álvarez Aliaga A, González Aguilera JC, Maceo Gómez LR. Factors associated to hypertensive heart disease development: a prospective cohort study in Bayamo, Cuba. Medwave 2016 Jul;16(6):6492 doi: 10.5867/medwave.2016.06.6492
Fecha de envío: 29/2/2016
Fecha de aceptación: 21/6/2016
Fecha de publicación: 7/7/2016
Origen: no solicitado
Tipo de revisión: con revisión por dos pares revisores externos, a doble ciego

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. Guía de práctica clínica de la ESH/ESC 2013 para el manejo de la hipertensión arterial. Rev Esp Cardiol. 2013;66(10):880.e1-880.e64. | Link |
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. Guía de práctica clínica de la ESH/ESC 2013 para el manejo de la hipertensión arterial. Rev Esp Cardiol. 2013;66(10):880.e1-880.e64. | Link | James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014 Feb 5;311(5):507-20. | CrossRef | PubMed |
James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014 Feb 5;311(5):507-20. | CrossRef | PubMed | Diamond JA, Phillips RA. Hypertensive heart disease. Hypertens Res. 2005 Mar;28(3):191-202. | PubMed |
Diamond JA, Phillips RA. Hypertensive heart disease. Hypertens Res. 2005 Mar;28(3):191-202. | PubMed | Díez J, Frohlich ED. A translational approach to hypertensive heart disease. Hypertension. 2010; 55:1-8. | Link |
Díez J, Frohlich ED. A translational approach to hypertensive heart disease. Hypertension. 2010; 55:1-8. | Link | Martín Raymondi D. Características de la cardiopatía hipertensiva en pacientes con hipertensión arterial no tratados previamente. Medicina Clínica. 2005;125(9):321-4. | CrossRef |
Martín Raymondi D. Características de la cardiopatía hipertensiva en pacientes con hipertensión arterial no tratados previamente. Medicina Clínica. 2005;125(9):321-4. | CrossRef | García Iglesias A, Lozano Alonso JE, Álamo Sanz R, Vega Alonso T; Workgroup of the RECCyL Study. [Factors associated with control of hypertension in the cohort from the study of Cardiovascular Disease Risk in Castilla y León (RECCyL)]. Hipertens Riesgo Vasc. 2015 Apr-Jun;32(2):48-55. | CrossRef | PubMed |
García Iglesias A, Lozano Alonso JE, Álamo Sanz R, Vega Alonso T; Workgroup of the RECCyL Study. [Factors associated with control of hypertension in the cohort from the study of Cardiovascular Disease Risk in Castilla y León (RECCyL)]. Hipertens Riesgo Vasc. 2015 Apr-Jun;32(2):48-55. | CrossRef | PubMed | Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977Apr;55(4):613-8. | PubMed |
Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977Apr;55(4):613-8. | PubMed | Drazner MH. The progression of hypertensive heart disease. Circulation. 2011 Jan 25;123(3):327-34. | CrossRef | PubMed |
Drazner MH. The progression of hypertensive heart disease. Circulation. 2011 Jan 25;123(3):327-34. | CrossRef | PubMed | Khouri MG, Peshock RM, Ayers CR, de Lemos JA, Drazner MH. A 4-tiered classification of left ventricular hypertrophy based on left ventricular geometry: the Dallas heart study. Circ Cardiovasc Imaging. 2010 Mar;3(2):164-71. | CrossRef | PubMed |
Khouri MG, Peshock RM, Ayers CR, de Lemos JA, Drazner MH. A 4-tiered classification of left ventricular hypertrophy based on left ventricular geometry: the Dallas heart study. Circ Cardiovasc Imaging. 2010 Mar;3(2):164-71. | CrossRef | PubMed | Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2011;123:104-23. | Link |
Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2011;123:104-23. | Link | Pérez Caballero MD, Dueñas Herrera A, Alfonso Guerra JP, Vázquez Vigoa A, Navarro Despaigne D, Hernández Cueto M, et al. Hipertensión arterial. Guía para la prevención, diagnóstico y tratamiento. Comisión Nacional Técnica Asesora del Programa de Hipertensión Arterial. La Habana: Editorial Ciencias Médicas; 2008.
Pérez Caballero MD, Dueñas Herrera A, Alfonso Guerra JP, Vázquez Vigoa A, Navarro Despaigne D, Hernández Cueto M, et al. Hipertensión arterial. Guía para la prevención, diagnóstico y tratamiento. Comisión Nacional Técnica Asesora del Programa de Hipertensión Arterial. La Habana: Editorial Ciencias Médicas; 2008.  Whelton S, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2002 Apr2;136(7): 493-503. | PubMed |
Whelton S, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2002 Apr2;136(7): 493-503. | PubMed | Cabrera de León A, Rodríguez-Pérez C, Rodríguez-Benjumeda M, Anía-Lafuente B, Brito-Díaz B, Muros de Fuentes M, et al. Sedentarismo: tiempo de ocio activo frente a porcentaje del gasto energético. Rev Esp Cardiol. 2007;60(3):244-50. | Link |
Cabrera de León A, Rodríguez-Pérez C, Rodríguez-Benjumeda M, Anía-Lafuente B, Brito-Díaz B, Muros de Fuentes M, et al. Sedentarismo: tiempo de ocio activo frente a porcentaje del gasto energético. Rev Esp Cardiol. 2007;60(3):244-50. | Link | Zhao W, Hasegawa K, Chen J. Part A. Recent advances in dietary assessment tools. The use of food-frequency questionnaires for various purposes in China. Public Health Nutrition. 2002; 5(6A), 829-833.
Zhao W, Hasegawa K, Chen J. Part A. Recent advances in dietary assessment tools. The use of food-frequency questionnaires for various purposes in China. Public Health Nutrition. 2002; 5(6A), 829-833.  Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010 May 26;303(20):2043-50. | CrossRef | PubMed |
Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010 May 26;303(20):2043-50. | CrossRef | PubMed | Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003 Dec;42(6):1206-52. | PubMed |
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003 Dec;42(6):1206-52. | PubMed | Mazumdar M, Glassman JR. Categorizing a prognostic variable: review of methods, code for easy implementation and applications to decision-making about cancer treatments. Stat Med. 2000 Jan 15;19(1):113-32. | PubMed |
Mazumdar M, Glassman JR. Categorizing a prognostic variable: review of methods, code for easy implementation and applications to decision-making about cancer treatments. Stat Med. 2000 Jan 15;19(1):113-32. | PubMed | Baneshi MR, Talei AR. Dichotomisation of Continuous Data: Review of Methods, Advantages, and Disadvantages. Iran J Cancer Prev. 2011; l4(1):26-32.
Baneshi MR, Talei AR. Dichotomisation of Continuous Data: Review of Methods, Advantages, and Disadvantages. Iran J Cancer Prev. 2011; l4(1):26-32.  Cuspidi C, Giudici V, Negri F, Meani S, Sala C, Zanchetti A, et al. Improving cardiovascular risk stratification in essential hypertensive patients by indexing left ventricular mass to height(2.7). J Hypertens. 2009 Dec;27(12):2465-71. | CrossRef | PubMed |
Cuspidi C, Giudici V, Negri F, Meani S, Sala C, Zanchetti A, et al. Improving cardiovascular risk stratification in essential hypertensive patients by indexing left ventricular mass to height(2.7). J Hypertens. 2009 Dec;27(12):2465-71. | CrossRef | PubMed | Gu Q, Dillon CF, Burt VL, Gillum RF. Association of hypertension treatment and control with all-cause and cardiovascular disease mortality among US adults with hypertension. Am J Hypertens. 2010 Jan;23(1):38-45. | CrossRef | PubMed |
Gu Q, Dillon CF, Burt VL, Gillum RF. Association of hypertension treatment and control with all-cause and cardiovascular disease mortality among US adults with hypertension. Am J Hypertens. 2010 Jan;23(1):38-45. | CrossRef | PubMed | Peralta CA, Katz R, Newman AB, Psaty BM, Odden MC. Systolic and diastolic blood pressure, incident cardiovascular events, and death in elderly persons: the role of functional limitation in the Cardiovascular Health Study. Hypertension. 2014 Sep;64(3):472-80. | CrossRef | PubMed |
Peralta CA, Katz R, Newman AB, Psaty BM, Odden MC. Systolic and diastolic blood pressure, incident cardiovascular events, and death in elderly persons: the role of functional limitation in the Cardiovascular Health Study. Hypertension. 2014 Sep;64(3):472-80. | CrossRef | PubMed | Frohlich ED, González A, Díez J. Hypertensive left ventricular hypertrophy risk: beyond adaptive cardiomyocytic hypertrophy. J Hypertens. 2011;29:17-26. | CrossRef |
Frohlich ED, González A, Díez J. Hypertensive left ventricular hypertrophy risk: beyond adaptive cardiomyocytic hypertrophy. J Hypertens. 2011;29:17-26. | CrossRef | León Álvarez JL, Pérez Caballero MD. Experiencia con el paciente en la consulta especializada de hipertensión arterial complicada. Rev Cubana Med. 2009;48(4):182-92. | Link |
León Álvarez JL, Pérez Caballero MD. Experiencia con el paciente en la consulta especializada de hipertensión arterial complicada. Rev Cubana Med. 2009;48(4):182-92. | Link | Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009 Jun 23;119(24):3070-7. | CrossRef | PubMed |
Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009 Jun 23;119(24):3070-7. | CrossRef | PubMed | Martín-Rioboó E, García Criado E, Pérula DeTorres LA, Cea-Calvo L, Anguita Sánchez M, López Granados A, et al. Prevalencia de hipertrofia ventricular izquierda, fibrilación auricular y enfermedad cardiovascular en hipertensos de Andalucía. Estudio PREHVIA. Med Clin (Barc). 2009; 132(7):243-50. | Link |
Martín-Rioboó E, García Criado E, Pérula DeTorres LA, Cea-Calvo L, Anguita Sánchez M, López Granados A, et al. Prevalencia de hipertrofia ventricular izquierda, fibrilación auricular y enfermedad cardiovascular en hipertensos de Andalucía. Estudio PREHVIA. Med Clin (Barc). 2009; 132(7):243-50. | Link | Wright JT Jr, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014 Apr 1;160(7):499-503. | CrossRef | PubMed |
Wright JT Jr, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014 Apr 1;160(7):499-503. | CrossRef | PubMed | Maione A, Annemans L, Strippoli G. Proteinuria and clinical outcomes in hypertensive patients. Am J Hypertens. 2009 Nov;22(11):1137-47. | CrossRef | PubMed |
Maione A, Annemans L, Strippoli G. Proteinuria and clinical outcomes in hypertensive patients. Am J Hypertens. 2009 Nov;22(11):1137-47. | CrossRef | PubMed | Perkovic V, Verdon C, Ninomiya T, Barzi F, Cass A, Patel A, et al. The relationship between proteinuria and coronary risk: a systematic review and meta-analysis. PLoS Med. 2008 Oct 21;5(10):e207. | CrossRef | PubMed |
Perkovic V, Verdon C, Ninomiya T, Barzi F, Cass A, Patel A, et al. The relationship between proteinuria and coronary risk: a systematic review and meta-analysis. PLoS Med. 2008 Oct 21;5(10):e207. | CrossRef | PubMed | Zhang Z, Dzau VJ. Angiotensin II type 1 receptor-associated protein is an endogenous inhibitor of angiotensin II type 1 receptor action in cardiac hypertrophy: role in check and balance. Hypertension. 2010 May;55(5):1086-7. | CrossRef | PubMed |
Zhang Z, Dzau VJ. Angiotensin II type 1 receptor-associated protein is an endogenous inhibitor of angiotensin II type 1 receptor action in cardiac hypertrophy: role in check and balance. Hypertension. 2010 May;55(5):1086-7. | CrossRef | PubMed | Straina WD, Chaturvedib N, Hughesb A, Nihoyannopoulos P, Bulpittd CJ, Rajkumare C, et al. Associations between cardiac target organ damage and microvascular dysfunction: the role of blood pressure. J Hypertens. 2010;28:952-95. | CrossRef |
Straina WD, Chaturvedib N, Hughesb A, Nihoyannopoulos P, Bulpittd CJ, Rajkumare C, et al. Associations between cardiac target organ damage and microvascular dysfunction: the role of blood pressure. J Hypertens. 2010;28:952-95. | CrossRef | Yeboah J, Crouse JR, Bluemke DA, Lima JA, Polak JF, Burke GL, et al. Endothelial dysfunction is associated with left ventricular mass(assessed using MRI) in an adult population (MESA). J Hum Hypertens. 2011 Jan;25(1):25-31. | CrossRef | PubMed |
Yeboah J, Crouse JR, Bluemke DA, Lima JA, Polak JF, Burke GL, et al. Endothelial dysfunction is associated with left ventricular mass(assessed using MRI) in an adult population (MESA). J Hum Hypertens. 2011 Jan;25(1):25-31. | CrossRef | PubMed | Halperin RO, Gaziano JM, Sesso HD. Smoking and the risk of incident hypertension in middle-aged and older men. Am J Hypertens. 2008 Feb;21(2):148-52. | CrossRef | PubMed |
Halperin RO, Gaziano JM, Sesso HD. Smoking and the risk of incident hypertension in middle-aged and older men. Am J Hypertens. 2008 Feb;21(2):148-52. | CrossRef | PubMed | Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004 May 19;43(10):1731-7. | PubMed |
Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004 May 19;43(10):1731-7. | PubMed | Johnson HM, Gossett LK, Piper ME, Aeschlimann SE, Korcarz CE, Baker TB, et al. Effects of smoking and smoking cessation on endothelial function: 1-year outcomes from a randomized clinical trial. J Am Coll Cardiol. 2010 May 4;55(18):1988-95. | CrossRef | PubMed |
Johnson HM, Gossett LK, Piper ME, Aeschlimann SE, Korcarz CE, Baker TB, et al. Effects of smoking and smoking cessation on endothelial function: 1-year outcomes from a randomized clinical trial. J Am Coll Cardiol. 2010 May 4;55(18):1988-95. | CrossRef | PubMed | Arnlöv J, Ingelsson E, Sundström J, Lind L. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation. 2010 Jan 19;121(2):230-6. | CrossRef | PubMed |
Arnlöv J, Ingelsson E, Sundström J, Lind L. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation. 2010 Jan 19;121(2):230-6. | CrossRef | PubMed | Woodiwiss AJ, Libhaber CD, Majane OH, Libhaber E, Maseko M, Norton GR. Obesity promotes left ventricular concentric rather than eccentric geometric remodeling and hypertrophy independent of blood pressure. Am J Hypertens. 2008 Oct;21(10):1144-51. | CrossRef | PubMed |
Woodiwiss AJ, Libhaber CD, Majane OH, Libhaber E, Maseko M, Norton GR. Obesity promotes left ventricular concentric rather than eccentric geometric remodeling and hypertrophy independent of blood pressure. Am J Hypertens. 2008 Oct;21(10):1144-51. | CrossRef | PubMed | López-Jiménez F, Corté s-Bergoderi M. Obesidad y corazón. Rev Esp Cardiol. 2011; 64(2):140-9. | Link |
López-Jiménez F, Corté s-Bergoderi M. Obesidad y corazón. Rev Esp Cardiol. 2011; 64(2):140-9. | Link | Windham BG, Griswold ME, Farasat SM, Ling SM, Carlson O, Egan JM, et al. Influence of leptin, adiponectin, and resistin on the association between abdominal adiposity and arterial stiffness. Am J Hypertens. 2010 May;23(5):501-7. | CrossRef | PubMed |
Windham BG, Griswold ME, Farasat SM, Ling SM, Carlson O, Egan JM, et al. Influence of leptin, adiponectin, and resistin on the association between abdominal adiposity and arterial stiffness. Am J Hypertens. 2010 May;23(5):501-7. | CrossRef | PubMed | Zhang Y, Ren J. Role of Cardiac Steatosis and Lipotoxicity in Obesity Cardiomyopathy. Hypertension. 2011; 57;148-150. | Link |
Zhang Y, Ren J. Role of Cardiac Steatosis and Lipotoxicity in Obesity Cardiomyopathy. Hypertension. 2011; 57;148-150. | Link | Lohmeier TE, Iliescu R, Liu B, Henegar JR, Maric-Bilkan C, Irwin ED. Systemic and renal-specific sympathoinhibition in obesityc hypertension. Hypertension. 2012 Feb;59(2):331-8. | CrossRef | PubMed |
Lohmeier TE, Iliescu R, Liu B, Henegar JR, Maric-Bilkan C, Irwin ED. Systemic and renal-specific sympathoinhibition in obesityc hypertension. Hypertension. 2012 Feb;59(2):331-8. | CrossRef | PubMed | Qin Y, Li T, Lou P, Chang G, Zhang P, Chen P, et al. Salt intake, knowledge of salt intake, and blood pressure control in Chinese hypertensive patients. J Am Soc Hypertens. 2014 Dec;8(12):909-14. | CrossRef | PubMed |
Qin Y, Li T, Lou P, Chang G, Zhang P, Chen P, et al. Salt intake, knowledge of salt intake, and blood pressure control in Chinese hypertensive patients. J Am Soc Hypertens. 2014 Dec;8(12):909-14. | CrossRef | PubMed | Umesawa M, Iso H, Date C, Yamamoto A, Toyoshima H, Watanabe Y, et al. Relations between dietary sodium and potassium intakes and mortality from cardiovascular disease: the Japan Collaborative Cohort Study for Evaluation of Cancer Risks. Am J Clin Nutr. 2008 Jul;88(1):195-202. | PubMed |
Umesawa M, Iso H, Date C, Yamamoto A, Toyoshima H, Watanabe Y, et al. Relations between dietary sodium and potassium intakes and mortality from cardiovascular disease: the Japan Collaborative Cohort Study for Evaluation of Cancer Risks. Am J Clin Nutr. 2008 Jul;88(1):195-202. | PubMed | Álvarez Aliaga A, González Aguilera JC. Algunos factores de riesgo de la cardiopatía hipertensiva. Rev Cubana de Med. 2009;48(4):139-51. | Link |
Álvarez Aliaga A, González Aguilera JC. Algunos factores de riesgo de la cardiopatía hipertensiva. Rev Cubana de Med. 2009;48(4):139-51. | Link | Pimenta E, Gaddam KK, Oparil S, Aban I, Husain S, Dell'Italia LJ, et al. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension. 2009 Sep;54(3):475-81. | CrossRef | PubMed |
Pimenta E, Gaddam KK, Oparil S, Aban I, Husain S, Dell'Italia LJ, et al. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension. 2009 Sep;54(3):475-81. | CrossRef | PubMed | Owen A, Wiles J, Swaine I. Effect of isometric exercise on resting blood pressure: a meta analysis. J Hum Hypertens. 2010 Dec;24(12):796-800. | CrossRef | PubMed |
Owen A, Wiles J, Swaine I. Effect of isometric exercise on resting blood pressure: a meta analysis. J Hum Hypertens. 2010 Dec;24(12):796-800. | CrossRef | PubMed | Wildman RP, Gu D, Muntner P, Huang G, Chen J, Duan X, et al. Alcohol intake and hypertension subtypes in Chinese men. J Hypertens. 2005 Apr;23(4):737-43. | PubMed |
Wildman RP, Gu D, Muntner P, Huang G, Chen J, Duan X, et al. Alcohol intake and hypertension subtypes in Chinese men. J Hypertens. 2005 Apr;23(4):737-43. | PubMed | Núñez-Córdoba JM, Martínez-González MA, Bes-Rastrollo M, Toledo E, Beunza JJ, Alonso A. Consumo de alcohol e incidencia de hipertensión en una cohorte mediterránea: el estudio SUN. Rev Esp Cardiol. 2009;62(6):633-41. | Link |
Núñez-Córdoba JM, Martínez-González MA, Bes-Rastrollo M, Toledo E, Beunza JJ, Alonso A. Consumo de alcohol e incidencia de hipertensión en una cohorte mediterránea: el estudio SUN. Rev Esp Cardiol. 2009;62(6):633-41. | Link | Fernández-Solà J, Fatjó F, Sacanella E, Estruch R, Bosch X, Urbano-Márquez A, et al. Evidence of apoptosis in alcoholic cardiomyopathy. Hum Pathol. 2006 Aug;37(8):1100-10. | PubMed |
Fernández-Solà J, Fatjó F, Sacanella E, Estruch R, Bosch X, Urbano-Márquez A, et al. Evidence of apoptosis in alcoholic cardiomyopathy. Hum Pathol. 2006 Aug;37(8):1100-10. | PubMed | De Marco M, de Simone G, Roman MJ, Chinali M, Lee ET, Russell M, et al. Cardiovascular and metabolic predictors of progression of prehypertension into hypertension: the Strong Heart Study. Hypertension. 2009 Nov;54(5):974-80. | CrossRef | PubMed |
De Marco M, de Simone G, Roman MJ, Chinali M, Lee ET, Russell M, et al. Cardiovascular and metabolic predictors of progression of prehypertension into hypertension: the Strong Heart Study. Hypertension. 2009 Nov;54(5):974-80. | CrossRef | PubMed | Tanaka F, Makita S, Onoda T, Tanno K, Ohsawa M, Itai K, et al. Prehypertension subtype with elevated C-reactive protein: risk of ischemic stroke in a general Japanese population. Am J Hypertens. 2010 Oct;23(10):1108-13. | CrossRef | PubMed |
Tanaka F, Makita S, Onoda T, Tanno K, Ohsawa M, Itai K, et al. Prehypertension subtype with elevated C-reactive protein: risk of ischemic stroke in a general Japanese population. Am J Hypertens. 2010 Oct;23(10):1108-13. | CrossRef | PubMed | Zhang Y, Thompson AM, Tong W, Xu T, Chen J, Zhao L, et al. Biomarkers of inflammation and endothelial dysfunction and risk of hypertension among Inner Mongolians in China. J Hypertens. 2010 Jan;28(1):35-40. | CrossRef | PubMed |
Zhang Y, Thompson AM, Tong W, Xu T, Chen J, Zhao L, et al. Biomarkers of inflammation and endothelial dysfunction and risk of hypertension among Inner Mongolians in China. J Hypertens. 2010 Jan;28(1):35-40. | CrossRef | PubMed | Nagai T, Anzai T, Kaneko H, Mano Y, Anzai A, Maekawa Y, et al. C-reactive protein overexpression exacerbates pressure overload–induced cardiac remodeling through enhanced inflammatory response. Hypertension. 2011;57:208-15. | Link |
Nagai T, Anzai T, Kaneko H, Mano Y, Anzai A, Maekawa Y, et al. C-reactive protein overexpression exacerbates pressure overload–induced cardiac remodeling through enhanced inflammatory response. Hypertension. 2011;57:208-15. | Link | Schulz R, Heusch G. C-reactive protein: just a biomarker of inflammation or a pathophysiological player in myocardial function and morphology? Hypertension. 2011 Feb;57(2):151-3. | CrossRef | PubMed |
Schulz R, Heusch G. C-reactive protein: just a biomarker of inflammation or a pathophysiological player in myocardial function and morphology? Hypertension. 2011 Feb;57(2):151-3. | CrossRef | PubMed | Akasheh A, Wu Y, Li Y, Dustin LD, Wong ND, Gardin JM, et al. Association of blood pressure with left ventricular mass in untreated hypertensives in rural Yunnan Province. Am J Hypertens. 2009 Jul;22(7):730-4. | CrossRef | PubMed |
Akasheh A, Wu Y, Li Y, Dustin LD, Wong ND, Gardin JM, et al. Association of blood pressure with left ventricular mass in untreated hypertensives in rural Yunnan Province. Am J Hypertens. 2009 Jul;22(7):730-4. | CrossRef | PubMed | Wang TD, Sheu WH. From casual blood pressure measurement to long-term blood pressure burden: better elucidation of the association between versatile blood pressures and cardiovascular events. Hypertens Res. 2011 Jan;34(1):49-51. | CrossRef | PubMed |
Wang TD, Sheu WH. From casual blood pressure measurement to long-term blood pressure burden: better elucidation of the association between versatile blood pressures and cardiovascular events. Hypertens Res. 2011 Jan;34(1):49-51. | CrossRef | PubMed | Franjic B, Marwick TH. The diabetic, hypertensive heart: epidemiology and mechanisms of a very high-risk situation. J Hum Hypertens. 2009 Nov;23(11):709-17. | CrossRef | PubMed |
Franjic B, Marwick TH. The diabetic, hypertensive heart: epidemiology and mechanisms of a very high-risk situation. J Hum Hypertens. 2009 Nov;23(11):709-17. | CrossRef | PubMed | Marshall T. The effect of blood pressure and cholesterol variability on the precision of Framingham cardiovascular risk estimation: a simulation study. J Hum Hypertens. 2010 Oct;24(10):631-8. | CrossRef | PubMed |
Marshall T. The effect of blood pressure and cholesterol variability on the precision of Framingham cardiovascular risk estimation: a simulation study. J Hum Hypertens. 2010 Oct;24(10):631-8. | CrossRef | PubMed | Piskorz D, Quaglino M, Pigozzi F, Vitelleschi M. Importancia de las variables no hemodinámicas en el desarrollo de hipertrofia ventricular izquierda en hipertensión. Rev Fed Arg Cardiol. 2010;39(4):288-93. | Link |
Piskorz D, Quaglino M, Pigozzi F, Vitelleschi M. Importancia de las variables no hemodinámicas en el desarrollo de hipertrofia ventricular izquierda en hipertensión. Rev Fed Arg Cardiol. 2010;39(4):288-93. | Link | Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007 Sep 27;357(13):1301-10. | PubMed |
Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007 Sep 27;357(13):1301-10. | PubMed | Ashen MD, Blumenthal RS. Clinical practice. Low HDL cholesterol levels. N Engl J Med. 2005 Sep 22;353(12):1252-60. Review. Erratum in: N Engl J Med. 2006 Jan 12;354(2):215. | PubMed |
Ashen MD, Blumenthal RS. Clinical practice. Low HDL cholesterol levels. N Engl J Med. 2005 Sep 22;353(12):1252-60. Review. Erratum in: N Engl J Med. 2006 Jan 12;354(2):215. | PubMed | Badimón L, Vilahur G, Padró Y. Lipoproteínas, plaquetas y aterotrombosis. Rev Esp Cardiol. 2009; 62(10):1161-78. | Link |
Badimón L, Vilahur G, Padró Y. Lipoproteínas, plaquetas y aterotrombosis. Rev Esp Cardiol. 2009; 62(10):1161-78. | Link | Badimón L, Vilahur G, Padró T. Lipoproteins, platelets and atherothrombosis. Rev Esp Cardiol. 2009 Oct;62(10):1161-78. | PubMed |
Badimón L, Vilahur G, Padró T. Lipoproteins, platelets and atherothrombosis. Rev Esp Cardiol. 2009 Oct;62(10):1161-78. | PubMed | Xu JZ, Zhang Y, Wu SN, Niu WQ, Zhu DL, Gao PJ. Impaired endothelial function in hypertensive patients with target organ damage. J Hum Hypertens. 2009 Nov;23(11):751-7. | CrossRef | PubMed |
Xu JZ, Zhang Y, Wu SN, Niu WQ, Zhu DL, Gao PJ. Impaired endothelial function in hypertensive patients with target organ damage. J Hum Hypertens. 2009 Nov;23(11):751-7. | CrossRef | PubMed | Oliveras A, Armario P, Hernández-Del Rey R, Arroyo JA, Poch E, Larrousse M, et al. Urinary albumin excretion is associated with true resistant hypertension. J Hum Hypertens. 2010 Jan;24(1):27-33. | CrossRef | PubMed |
Oliveras A, Armario P, Hernández-Del Rey R, Arroyo JA, Poch E, Larrousse M, et al. Urinary albumin excretion is associated with true resistant hypertension. J Hum Hypertens. 2010 Jan;24(1):27-33. | CrossRef | PubMed | Miguel-Carrasco JL, Mate A, Monserrat MT, Arias JL, Aramburu O, Vázquez CM. The role of inflammatory markers in the cardioprotective effect of L-carnitine in L-NAME-induced hypertension. Am J Hypertens. 2008 Nov;21(11):1231-7. | CrossRef | PubMed |
Miguel-Carrasco JL, Mate A, Monserrat MT, Arias JL, Aramburu O, Vázquez CM. The role of inflammatory markers in the cardioprotective effect of L-carnitine in L-NAME-induced hypertension. Am J Hypertens. 2008 Nov;21(11):1231-7. | CrossRef | PubMed | Leiba A, Vinker S, Dinour D, Holtzman EJ, Shani M. Uric acid levels within the normal range predict increased risk of hypertension: a cohort study. J Am Soc Hypertens. 2015 Aug;9(8):600-9. | CrossRef | PubMed |
Leiba A, Vinker S, Dinour D, Holtzman EJ, Shani M. Uric acid levels within the normal range predict increased risk of hypertension: a cohort study. J Am Soc Hypertens. 2015 Aug;9(8):600-9. | CrossRef | PubMed | Díaz Arce D, Cardellá Rosales LL, Cabada Pérez F, Fiterre Lancis I, Montenegro Valencia MM, Souto Rodríguez Y. Hiperuricemia y factores de riesgo cardiovascular en pacientes hospitalizados. Panorama Cuba y Salud. 2010;5(2):5-12. | Link |
Díaz Arce D, Cardellá Rosales LL, Cabada Pérez F, Fiterre Lancis I, Montenegro Valencia MM, Souto Rodríguez Y. Hiperuricemia y factores de riesgo cardiovascular en pacientes hospitalizados. Panorama Cuba y Salud. 2010;5(2):5-12. | Link |