 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Palabras clave: drug-induced liver injury, tuberculosis
INTRODUCTION
Hepatotoxicity is a serious adverse effect of tuberculosis treatment.
OBJECTIVES
The aim of this study was to estimate the prevalence, forms of presentation and clinical course of patients with hepatotoxicity secondary to antituberculosis drugs.
METHODS
We performed a descriptive and observational study using medical records from patients older than 16 years between Jaunary 1, 2011 and June 30, 2014 in the Medical Clinic of the Hospital Angela I. de Llano, Corrientes, Argentina.
RESULTS
During the study period 118 patients were diagnosed with tuberculosis; 7.6% (nine patients: six men and three women) developed hepatotoxicity. Six had hepatocellular characteristics and three had cholestatic characteristics. The mean age was 34.6 ± 14.3 years. All patients received triple-association medication plus ethambutol on a daily basis. They were hospitalized for an average of 16 days (range: 4-37). Four were asymptomatic, three had anorexia, nausea and vomiting, and two were jaundiced. The interval between the beginning of treatment and the appearance of clinical manifestations was on average 9.6 days (range 2-23). The interval between the onset and cessation of treatment was on average 15.2 days (range 3-48). No patients required liver transplantation and no deaths were recorded.
CONCLUSIONS
Hepatotoxicity of antituberculosis drugs has been associated with factors such as age over 35 years, female gender, pregnancy, malnutrition, alcoholism, human immunodeficiency virus, preexisting liver disease, daily treatment, diabetes, renal failure, and combined treatment. Since we lack a regional registry, this casuistry could be the kickoff for the creation of regional and/or national records of anti-tuberculosis drugs adverse effects and pharmacologic vigilance. Also, there is a need for programs to actively seek this complication, and the development of guidelines for unifying concepts and treatment protocols.
Tuberculosis is an infectious ancient disease produced by Mycobacterium Tuberculosis. It represents a marker disease of poverty and a serious problem in public health; 95% of cases occur in developing countries, where 98% of deaths are due to this illness [1]. According to the World Health Organization (WHO) Global Report on Tuberculosis 2014, 9 million people [2] have been ill in 2013. In the American region 280,000 new cases were reported in 2012. The estimated incidence rate was 29 cases per 100,000 inhabitants [3]. In 2012, in Argentina, which has a median incidence of this disease [1], 9,070 new cases were reported, with a reporting rate of 22 cases per 100,000 inhabitants [4],[5],[6]. Corrientes is one of the four provinces which integrates the Northeast Argentinean region, and it belongs to an endemic tuberculosis area. It has a rate of 23.4 cases per 100,000 inhabitants, this figure which, together with those of other seven jurisdictions out of the 24 that are part of the national territory, is above the national average [4],[5],[6].
Once tuberculosis is detected, the treatment generally consists in the administration of four drugs for at least six months, depending on each particular case. Adverse effects to anti- tuberculosis drugs decrease treatment fulfilling, lead to treatment failure and increase multi-drug-resistant tuberculosis incidence [7]. These effects range from mild self-limiting to severe with fatal consequences including rash - pruritus (16%), gastrointestinal intolerance (9%), arthralgia (1.8%), acne (1.8%), polyneuropathy (0.5 %) or hepatotoxicity with variable frequency; according to the consulted casuistry it varies between 4.3 and 19% [8][9].
Hepatotoxicity is a serious, disturbing and sometimes fatal side effect that may occur once these patients start treatment with specific drugs. Slight increases in liver function markers are expected in up to 20% of patients at treatment onset but generally tend to return to normal over time. On occasions these markers evolve into hepatotoxicity that may occur with a pattern of hepatocellular necrosis, cholestasis or a combination of both [10].
On account of the wide range reported in the literature and the lack of our own casuistry about this serious adverse effect, the aim of this study was to estimate the prevalence of hepatotoxicity due to tuberculosis treatment, and describe the presentation and outcome of patients with liver damage produced by tuberculosis drugs.
An observational and descriptive study was carried out.
Inclusion criteria: all patients over 16 years old hospitalized from January 1st, 2011 to June 30th, 2014 who were diagnosed with tuberculosis at the Medical Clinic Department of Angela I. Llano Hospital from Corrientes - Argentina.
Settings: Angela I. Llano Hospital, historically a hospital that treats patients diagnosed with tuberculosis, is one of the three referral hospitals in the city of Corrientes – Argentina. It receives all suspected tuberculosis cases referred from less complex centers that are located north of Corrientes River, making diagnosis and monitoring all tuberculosis cases. It has an influence area of about 450,000 inhabitants. The Clinical Medicine Department has a 30 bed general room, 6 of which correspond to the Phthisiology sector (3 for men and 3 for women).
Data Sources: we retrospectively analyzed medical records of hospitalized patients diagnosed with tuberculosis from January 1st, 2011 to June 30th, 2014.
Definition and variables: we defined hepatotoxicity of hepatocellular character as the increase of alanine aminotransferase and aspartate aminotransferase liver enzymes five times the value accepted as normal in the absence of clinical symptoms or the increase of alanine aminotransferase and aspartate aminotransferase liver enzymes three or more times the value considered normal in the presence of clinical symptoms. We understood as hepatotoxicity of cholestatic nature, the increase of alkaline phosphatase and bilirubin above normal values with or without clinical symptoms [10].
Selection of medical records was made after performing a survey of patients admitted to the Phthisiology sector during the studied period. Once the medical records were collected, data were stored in an Excel spreadsheet.
Before starting data collection, due to the absence of an Ethics Committee in the hospital, we requested approval for data search from the Teaching and Research Department. Data were processed and analyzed using the software EPI - INFO version 3.5.1.
From January 2011 to June 2014, 263 medical records were collected, 118 patients were diagnosed with tuberculosis. The mean age of these patients was 18.04 ± 41.4. The age range was from 17 to 94. Table 1 depicts the absolute number (N) and percentage (%) of the sample clinical and demographic characteristics.
Out of the patients diagnosed with tuberculosis who started anti-tuberculosis treatment, 97.4% (115) had as an initial scheme triple association (rifampicin, isoniazid, pyrazinamide) and ethambutol and 2.6% (three patients) initiated triple association, plus ethambutol and streptomycin because they were previously treated cases.
Nine patients (7.6%) developed hepatotoxicity being of hepatocellular cholestatic character in six and cholestatic in three of them.
Of the nine patients affected, six were men and three women. The average age of patients who developed hepatotoxicity was 34.6 ± 14.3, age range from 18 to 55 years old. All patients lived in the province; six patients were from the capital city and three from the interior of the province.
Eight patients had renal failure (creatinine clearance ≤60 ml/min), seven were malnourished (body mass index ≤ 20), four were older than 35 years old, and three patients had a history of alcoholism. None of the women who had hepatotoxicity were pregnant, at the beginning or during treatment; one patient had a diagnosis of systemic erythematosus lupus (untreated). None of the patients had diabetes mellitus, co-infection with human immunodeficiency virus, solid organ onco-hematological malignancy, or were under immunosuppressive treatment.
All patients who developed hepatotoxicity had pulmonary tuberculosis. Hundred percent of patients who developed hepatotoxicity had received triple association and ethambutol on a daily basis and according to a dose adjusted to body weight as therapeutic scheme.
In case of hepatotoxicity the adopted course of action was to admit patients, to suspend the initiated scheme, to request at least a weekly liver function lab test, to rotate through an alternative scheme (due to drug availability of the Tuberculosis Provincial Program in Corrientes, the scheme included an aminoglycoside, a fluoroquinolone, and ethambutol). Once the laboratory results were normal, rifampin, isoniazid and pyrazinamide were successively reintroduced. These patients underwent abdominal ultrasound, serology for hepatotropic viruses and, if the case justified it, special serologies were requested [10].
In patients with hepatocellular hepatitis the average values of the alanine aminotransferase and aspartate aminotransferase liver enzymes was 1,002 ± 875 U/l and 617U/l ± 506, representing an increase of 25 and 15 times above the upper normal limit respectively. In those cases that developed cholestatic hepatitis the average alkaline phosphatase value was 1,571 U/l ± 1,031 and those of total bilirubin were 10 mg/dl ± 2.36, which represents an increase of five and ten times above the limit considered normal respectively. The creatinine and albumin mean values of hepatotoxicity cases were 1.12 mg/dl ± 0.66 and 2.41 g/dl ± 1.13 respectively.
From this group of nine patients, eight of them immediately suspended tuberculosis treatment; one patient continued taking the medication and developed disseminated intravascular coagulation which evolved towards healing with treatment for this complication.
All patients were hospitalized an average of 16 days with a range from 4 to 37 days. Four of these patients were asymptomatic, three had anorexia, nausea and vomiting and two consulted for jaundice. The interval between the beginning of treatment and clinical manifestation, in patients who presented any sign or symptom, was an average of 9.6 days, with a range of 2-23 days. The interval between the beginning and the suspension of treatment for tuberculosis was on average 15.2 days with a range between 3 and 48 days. There was an exception, an asymptomatic patient who was in the maintenance phase 20 days prior to end the treatment which was suspended.
No relevant findings were reported in abdominal ultrasounds, serology for hepatotropic viruses and special serologies. A patient underwent liver biopsy for suspected liver granuloma, being the pathologic anatomy study non conclusive for this diagnosis. None of the affected patients required liver transplant and no death was recorded.
Drug-induced liver injury is one of the most complex liver disorders because of its potential severity, similar to other liver disease presentation and the absence of specific biomarkers. This leads to base diagnosis certainty on clinical suspicion and to the exclusion of alternative causes [1],[3],[7],[10],[11].
The survey described in this clinical context found a 7.6% prevalence of hepatotoxicity caused by antiphimic drugs that represents a total of nine cases.
Other authors have found different values in a range of 1.7 to 21% [9],[12],[13],[14]. Thus Berrouet et al. [9] in a retrospective analysis of 224 cases of tuberculosis found 21% of hepatotoxicity, a figure which the authors associate with the high number of tuberculosis / human immunodeficiency virus cases. The series presented features 6%, this percentage is within the regional incidence mean [2],[4].
Although none of the patients with tuberculosis/human immunodeficiency virus developed hepatotoxicity, it is difficult and complex to blame only one drug in particular for liver damage on account of the high number of drugs that these patients usually take. Shakya et al. [12] found 8% of hepatotoxicity more often in young people (odds ratio 2.75), which coincides with the casuistic collected in this study. The same happened in women, a fact that differs from this series. Tost et al. [13] found 2.3% of hepatotoxicity in their study after analyzing 4,348 cases of tuberculosis
. Sixty three percent of those patients who developed hepatotoxicity showed no risk factors; however they found that creatinine values higher than 1.5 mg / dl were associated with poor prognosis (OR 30.5, 95% CI 3-257, p = 0.002). These values differ from those found in these cases of hepatotoxicity. Dalbies et al. [14], on a national level, after analyzing 2,333 cases of tuberculosis in Jujuy province – Argentina between 2000 and 2004, showed 1.7% of liver toxicity cases due to antiphimic drugs. Similar to the population in our study where men predominated, 80% of patients had received triple association as part of the therapeutic scheme, this fact is similar in our report. In contrast these patients were older in average than our patients and a 10% mortality rate was found. This fact is opposed to our study group since none of our patients died.
Hepatotoxicity by antiphimic drugs has been associated with several risk factors such as age over 35, female sex, pregnancy, malnutrition, alcoholism [16], human immunodeficiency virus co-infection, previous liver disease [17], daily treatment [18], hepatotoxic drugs, diabetes mellitus, renal failure and combined treatment with several drugs [15],[19]. In the studied population no cases of hepatotoxicity were observed in patients with diabetes mellitus, co-infection with human immunodeficiency virus, pregnancy, hepatotropic viruses or other hepatotoxic drugs, and in those cases in which there was liver damage associated with any risk factors (older than 35, female sex, alcoholism, malnutrition, renal failure) our ability to assess these associations was limited due to the small number of patients included and a larger number of cases would be needed to make more accurate estimates as well as the design of a study in order to evaluate such links.
Although our survey shows a low number of cases with respect to other series and that we cannot establish connections between developing hepatotoxicity and risk factors, it is possible to say that this could be the starting point for the creation of regional and/or national records of anti-tuberculosis drugs adverse effects. The aim of this study would be to know more precisely the prevalence of the minor and major adverse effects to anti- tuberculosis drugs, since there is not enough information about it. Likewise pharmaco-vigilance programs which actively seek this complication and develop guides to unify concepts and targeted treatment protocols based on the characteristics of the population could be established.
Tuberculosis remains a serious public health problem in Argentina and in the world. Its treatment can lead to complications including the development of hepatotoxicity. Due to the fact that there is no alternative medication to first-line drugs with similar efficacy and less ability of liver damage, these facts highlight the importance of regular medical follow-up in patients who have or do not have risk factors. Thus, patient education with regard to adverse reactions to tuberculosis drugs is important, so that potentially serious or death cases can be avoided.
Acknowledgements
The authors thank Judit Rodríguez, secretary of the Clinical Medicine Interns of the Clinical Medicine Department, Angela I. Llano Hospital, for her excellent disposition and for making doctors´ complex matters, simple; to Sebastián Genero MD, for collaborating in the correction and submission of this manuscript; Hernán Doval MD and Carlos Tajer MD, GEDIC Directors, for giving us the necessary tools to start this path; Claudia Romano MD, Director of the Provincial Tuberculosis Program, for providing epidemiological data.
Ethical issues
The Journal certifies that this study has been approved by the Teaching and Research Department of Angela Iglesia de Llano Hospital, Corrientes- Argentina.
Conflicts of Interests
The authors have completed the conflict of interests declaration form from the ICMJE, which has been translated into Spanish by Medwave, and they declare that they have not received any funding whatsoever to write this article, nor have they any conflict of interests with the matter dealt herein. Forms can be requested to the responsible author or the editorial direction of the Journal.

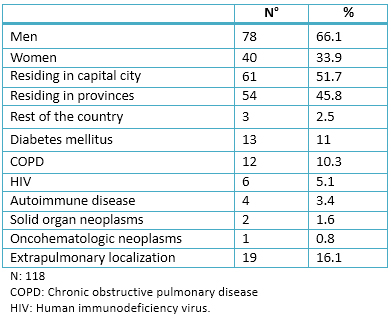 Table 1. Clinical and demographic characteristics of patients with tuberculosis in the Clinical Medicine Department.
Table 1. Clinical and demographic characteristics of patients with tuberculosis in the Clinical Medicine Department.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCTION
Hepatotoxicity is a serious adverse effect of tuberculosis treatment.
OBJECTIVES
The aim of this study was to estimate the prevalence, forms of presentation and clinical course of patients with hepatotoxicity secondary to antituberculosis drugs.
METHODS
We performed a descriptive and observational study using medical records from patients older than 16 years between Jaunary 1, 2011 and June 30, 2014 in the Medical Clinic of the Hospital Angela I. de Llano, Corrientes, Argentina.
RESULTS
During the study period 118 patients were diagnosed with tuberculosis; 7.6% (nine patients: six men and three women) developed hepatotoxicity. Six had hepatocellular characteristics and three had cholestatic characteristics. The mean age was 34.6 ± 14.3 years. All patients received triple-association medication plus ethambutol on a daily basis. They were hospitalized for an average of 16 days (range: 4-37). Four were asymptomatic, three had anorexia, nausea and vomiting, and two were jaundiced. The interval between the beginning of treatment and the appearance of clinical manifestations was on average 9.6 days (range 2-23). The interval between the onset and cessation of treatment was on average 15.2 days (range 3-48). No patients required liver transplantation and no deaths were recorded.
CONCLUSIONS
Hepatotoxicity of antituberculosis drugs has been associated with factors such as age over 35 years, female gender, pregnancy, malnutrition, alcoholism, human immunodeficiency virus, preexisting liver disease, daily treatment, diabetes, renal failure, and combined treatment. Since we lack a regional registry, this casuistry could be the kickoff for the creation of regional and/or national records of anti-tuberculosis drugs adverse effects and pharmacologic vigilance. Also, there is a need for programs to actively seek this complication, and the development of guidelines for unifying concepts and treatment protocols.
 Autores:
Alfredo Sebastián Golemba[1], Francisco Gastón Emmanuel Ferreyra[1], Ricardo Enrique Martearena[1], Fernando Ramón Achinelli[2], Gloria Beatriz Rovai[1]
Autores:
Alfredo Sebastián Golemba[1], Francisco Gastón Emmanuel Ferreyra[1], Ricardo Enrique Martearena[1], Fernando Ramón Achinelli[2], Gloria Beatriz Rovai[1]

Citación: Golemba AS, Ferreyra FGE, Martearena RE, Achinelli FR, Rovai GB. Drug-induced hepatotoxicity and tuberculosis in a hospital from the Argentinian northeast: cross-sectional study. Medwave 2015 May;15(4):e6135 doi: 10.5867/medwave.2015.04.6135
Fecha de envío: 3/2/2015
Fecha de aceptación: 14/4/2015
Fecha de publicación: 4/5/2015
Origen: no solicitado
Tipo de revisión: con revisión por cuatro pares revisores externos, a doble ciego

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Brian MC, Gaitán C, Pelaya E, Sáenz C. Consenso Argentino de tuberculosis. Asociación Argentina de Medicina Respiratoria, 2009. | Link |
Brian MC, Gaitán C, Pelaya E, Sáenz C. Consenso Argentino de tuberculosis. Asociación Argentina de Medicina Respiratoria, 2009. | Link | Organización Panamericana de la Salud. La tuberculosis en la Región de las Américas: Informe Regional 2012. Epidemiología, Control y Financiamiento. Washington, DC: OPS, 2013. | Link |
Organización Panamericana de la Salud. La tuberculosis en la Región de las Américas: Informe Regional 2012. Epidemiología, Control y Financiamiento. Washington, DC: OPS, 2013. | Link | Fernández HR, Arias SJ, Garcilazo DA. Situación de la Tuberculosis. Argentina, 2011-2012. Buenos Aires, Argentina: Instituto Nacional de Enfermedades Respiratorias “Dr. Emilio Coni”, 2013. | Link |
Fernández HR, Arias SJ, Garcilazo DA. Situación de la Tuberculosis. Argentina, 2011-2012. Buenos Aires, Argentina: Instituto Nacional de Enfermedades Respiratorias “Dr. Emilio Coni”, 2013. | Link | Ministerio de Salud de la Nación. Administración Nacional de Laboratorios e Institutos de Salud (ANLIS), con base en los datos de los Programas de Control de las 24 Jurisdicciones del país. Instituto Nacional de Enfermedades Respiratorias (INER) “Emilio Coni”, 2013. | Link |
Ministerio de Salud de la Nación. Administración Nacional de Laboratorios e Institutos de Salud (ANLIS), con base en los datos de los Programas de Control de las 24 Jurisdicciones del país. Instituto Nacional de Enfermedades Respiratorias (INER) “Emilio Coni”, 2013. | Link | Zerbini EV. Programa Nacional de Control de la Tuberculosis: Normas Técnicas 2013. 4ta ed. Santa Fe: Instituto Nacional de Enfermedades Respiratorias Dr. Emilio Coni, 2013. | Link |
Zerbini EV. Programa Nacional de Control de la Tuberculosis: Normas Técnicas 2013. 4ta ed. Santa Fe: Instituto Nacional de Enfermedades Respiratorias Dr. Emilio Coni, 2013. | Link | World Health Organization/IUATLD. Anti tuberculosis Drug Resistance in the World. Third Global Report. WHO/HTM/TB/2004.343. Geneva: World Health Organization, 2004. | Link |
World Health Organization/IUATLD. Anti tuberculosis Drug Resistance in the World. Third Global Report. WHO/HTM/TB/2004.343. Geneva: World Health Organization, 2004. | Link | García Rodríguez JF. Manejo de los Efectos Adversos del Tratamiento Antituberculoso. Galicia Clin. 2008; 69 (1):21-28 | Link |
García Rodríguez JF. Manejo de los Efectos Adversos del Tratamiento Antituberculoso. Galicia Clin. 2008; 69 (1):21-28 | Link | Berrouet Mejía MC, Escobar Toledo IE, Gómez Calzada UE, Agudelo Berruecos Y, Valencia Acosta NY, Ospina S. Hepatotoxicidad por antituberculosos en el Hospital Universitario San Vicente de Paúl, Medellín, 2005-2007. Iatreia. 2008 Dic;21(4):S-35. | Link |
Berrouet Mejía MC, Escobar Toledo IE, Gómez Calzada UE, Agudelo Berruecos Y, Valencia Acosta NY, Ospina S. Hepatotoxicidad por antituberculosos en el Hospital Universitario San Vicente de Paúl, Medellín, 2005-2007. Iatreia. 2008 Dic;21(4):S-35. | Link | Abbate EH, Palmero DJ, Castagnino J, Cufre M, Doval A, Estevan R, et al. Tratamiento de la tuberculosis. Guía práctica elaborada por la sección tuberculosis - Asociación Argentina de Medicina Respiratoria. MEDICINA (Buenos Aires) 2007; 67: 295-305. | Link |
Abbate EH, Palmero DJ, Castagnino J, Cufre M, Doval A, Estevan R, et al. Tratamiento de la tuberculosis. Guía práctica elaborada por la sección tuberculosis - Asociación Argentina de Medicina Respiratoria. MEDICINA (Buenos Aires) 2007; 67: 295-305. | Link | Aguayo M, Rodríguez JP. Hígado y terapia antituberculosa. Rev Chil Enf Respir. 2011;27(1):53-57. | CrossRef |
Aguayo M, Rodríguez JP. Hígado y terapia antituberculosa. Rev Chil Enf Respir. 2011;27(1):53-57. | CrossRef | Shakya R, Rao BS, Shrestha B. Incidence of hepatotoxicity due to antitubercular medicines and assessment of risk factors. Ann Pharmacother. 2004 Jun;38(6):1074-9. | PubMed |
Shakya R, Rao BS, Shrestha B. Incidence of hepatotoxicity due to antitubercular medicines and assessment of risk factors. Ann Pharmacother. 2004 Jun;38(6):1074-9. | PubMed | Tost J, Vidal R, Caylà J. Hepatotoxicidad por fármacos antituberculosos en España 1997-2001. Agència de Salut Pública de Barcelona, Hospital Valle Hebrón. Barcelona. | Link |
Tost J, Vidal R, Caylà J. Hepatotoxicidad por fármacos antituberculosos en España 1997-2001. Agència de Salut Pública de Barcelona, Hospital Valle Hebrón. Barcelona. | Link | Mariela D, Liliana F. Hepatotoxicidad por drogas antituberculosas 2000-2004. Hospital Dr Oscar Orias, Libertador Gral San Martin Jujuy, Mayo 2004. | Link |
Mariela D, Liliana F. Hepatotoxicidad por drogas antituberculosas 2000-2004. Hospital Dr Oscar Orias, Libertador Gral San Martin Jujuy, Mayo 2004. | Link | Devarbhavi H. Antituberculous drug-induced liver injury: current perspective. Trop Gastroenterol. 2011 Jul-Sep;32(3):167-74. | PubMed |
Devarbhavi H. Antituberculous drug-induced liver injury: current perspective. Trop Gastroenterol. 2011 Jul-Sep;32(3):167-74. | PubMed | Tost JR, Vidal R, Caylà J, Díaz-Cabanela D, Jiménez A, Broquetas JM. Severe hepatotoxicity due to anti-tuberculosis drugs in Spain. Int J Tuberc Lung Dis. 2005 May;9(5):534-40. | PubMed |
Tost JR, Vidal R, Caylà J, Díaz-Cabanela D, Jiménez A, Broquetas JM. Severe hepatotoxicity due to anti-tuberculosis drugs in Spain. Int J Tuberc Lung Dis. 2005 May;9(5):534-40. | PubMed | Fernández-Villar A, Sopeña B, Fernández-Villar J, Vázquez-Gallardo R, Ulloa F, Leiro V, et al. The influence of risk factors on the severity of anti-tuberculosis drug-induced hepatotoxicity. Int J Tuberc Lung Dis. 2004 Dec;8(12):1499-505. | PubMed |
Fernández-Villar A, Sopeña B, Fernández-Villar J, Vázquez-Gallardo R, Ulloa F, Leiro V, et al. The influence of risk factors on the severity of anti-tuberculosis drug-induced hepatotoxicity. Int J Tuberc Lung Dis. 2004 Dec;8(12):1499-505. | PubMed |