 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Palabras clave: reliability, questionnaires, Sleep Respiratory Disorders
Introduction: The use of sleep questionnaires constitutes undeniable resources for the study of Sleep Related Respiratory Disorders (SRRD) as clinical tools for the selection of patients that need to be developed lab tests and in the second case for the very useful epidemiological investigations. Objective: To evaluate the reliability of TuCASA questionnaire to find out the RDAD in the Cuban infant population. Design: Study of transverse cut during the period September-November 2009, in 113 children from 6-11 years of Camilo Cienfuegos Semi-Boarding School in Moa. To determine the degree of concordance we use the global agreement percentage, Square Chi and Index of Kappa and for the degree of internal consistence we use the coefficient á of Combrach. Results: The degree of global internal consistence got was 0.8028 while the subscales taking into account the daily and night symptoms were 0.8233 and 0.7823 respectively. In 11 (84.61%) of the items, there was concordance in the test-retest, 8 (72.73%) complied with the three criteria and only two (27.27 %) complied with two. Conclusions: The evaluated instrument is reliable to be used in our context for the selection of patients and epidemiological studies related to RDAD.
The Sleep Related Respiratory Disorders (SRRD) include a wide group of sicknesses that encompass the snoring children with scarce difficulty for the air pass by the superior air path and apparent innocuousness (primary snorer), and the children with clinical manifestations with the Syndrome of Obstructive Sleep Apnea (OSA), entity characterized by ventilating alterations, hypoxemia, hypercapnea and variations in the sleep normal structure with the subsequent somatic and neurocognoscitive (1, 2).
For the most of the authors the existing prevalence of SRRD is imprecise, affecting approximately from 10-12% of children and teenagers, observing similarly in both sexes, more common from 2-6 years. According to some existing epidemiological data the 12% of children snore, of them 10% are primary snorers and 2% with Syndrome of OSA (1, 3, 4, 5, 6).
The clinical manifestations of this entity vary frequently, encompassing practically the whole organism system, mainly constituting the true reasons of consult, treated by different specialists (Neurologists, Pneumologists, Clinics, Psychiatrists, Psychologists, Dieticians, Endocrinologists, Orthodontists), without any consensus in our context in the diagnosis and integrated management of them (1, 4, 5).
The untreated SRRD can cause significant morbidity that not only alter the children sleep quality, but also affects several organs and systems; some of these consequences can be completely reversed with the posterior treatment (1, 4, 7).
The SRRD in children, well-established now include: behaviour disorders, learning disorders, growth delay and dysfunction of the right ventriculum with pulmonary hypertension. There have been some children on a milk diet with aborted clinical forms of sudden death that after develop a syndrome of OSA (4, 7).
The precocious diagnosis of SRRD and its etiology allows reducing in more than the 80% of the cases the clinical manifestations and the complications mainly associated with the children, than can receive an adequate therapy, considered as a potentially curable chronic pathology (1).
The gold standard for the diagnosis is the Nocturnal Polysomnography (NPSG), allowing to evaluate the neurophysiologic and cardio respiratory parameters, as well as to know the children phases where SRRD is produced. Opposite we have to say that it is a hospitable test, uncomfortable for the children, hardworking, complex and very expensive (1, 3, 8).
Nowadays we insist in the Respiratory Polygraphy (RP), abbreviated method where cardio respiratory parameters are exclusively monitored. It is more economic, and very single to interpret than NPSG. It can also be developed in the patients’ house, more comfortable for children, allowing a more physiological sleep (1, 9, 10).
The initial problem for the diagnosis is in recognizing the children with normal snore (SRRD) among those getting habitual snore. In spite of that, the clinical history is not sensitive and specific enough to distinguish the primary snore of SRRD in children; the snore is present in 85-100% of children with obstruction in the superior air path. In this way, if the snore reliable prevalence were known, the prevalence superior limit of SRRD could be calculated (10, 11).
The use of sleep questionnaires constitutes undeniable resources for the study of SRRD for two reasons: Firs, as a clinical tool for the patient’s selection needing lab tests and in the second case, for the epidemiological investigation, considered a very useful instrument (12).
There have been different published scales for the sleep evaluation in infants, constructed with different and guided objectives to different age groups within the paediatric age and not all validated. There are not references in Cuba about the use of constructed or adapted questionnaires to determine the prevalence of SRRD in the infant population, however, the development reached in the health system and the frequency of nocturnal snore with or without clinical manifestations with Syndrome of OSA, establish the need to create or adapt an instrument that allows to evaluate the magnitude of this health problem in our context and to focus its diagnosis and management.
The development of a questionnaire is a hardworking process taking months before finding a definitive version that please the provided expectative. That’s why there is a general trend to use questionnaires that have showed their usefulness in other studies, that will also allow to compare results (13).
We have selected a questionnaire of 13 questions or items that evaluate the sleep habits, previously used in the study of Sleep Apnea Evaluation of Tucson (TuCASA) that has been originally used in Peru showing that can be applied in other contexts (14, 15).
The snore and related symptoms prevalence related to SRRD in children is unknown in our environment, skating this present study, in order to evaluate the reliability of TuCASA questionnaire that will constitute a very useful instrument for the future investigations about SRRD and in our infant population.
There was a transverse cut study with the universe of children form 6-12 years systematically going to Camilo Cienfuegos Semi-Boarding School in Rolo Monterrey, Municipality of Moa, Holguin Province, Cuba, from September-November 2009.
After checking the national and international bibliography about the SRRD, as well as the existing instruments to evaluate its prevalence, TuCASA questionnaire was selected (Attachment 1) developed and validated in Tucson infant population, Arizona (USA) applied in a representative sample that included 45,4% of Hispano children (14).
The universe of our study was with 439 children form 6-11 years systematically going to Camilo Cienfuegos Semi-Boarding School, where they receive their learning activities. The consent of the school was authorized to carry out the investigation, taking the children particular addresses who were visited by the authors.
During the home visits, families were explained the objective of the study, granting the reliability of the compiled information, asking for the approval of a new contact four weeks after (reminding period, avoiding the slant for memory effect), time where the instrument will be applied to the same father or tutor, taking after the informed consent in a written form.
The initial sample was 131 children, selected at random (30% of the universe). The definitive sample was constituted by 113 (86.25%) students, excluding 18 due to the no consent of their parents, amount that did not answer the questionnaire completely, or did not answer for the second time.
The version in Spanish of the questionnaire applied was used by Doctor. Lily Gutiérrez Mantari in a study developed to determine the prevalence of SRRD in children of elementary education of a public school in Lima-Peru in 2005, previous authorization of Dr. James L Goodwin author of the original instrument (11).
The info in process of evaluation is composed of 13 questions or items. There are verbal analogical answers in the code following a scale of punctuation with six possibilities of answers which are: ‘I don’t know’, ‘never’, ‘rarely’, ‘occasionally’, ‘frequently’, ‘almost always’. We only accepted as positives, the clinical manifestations, if the answers were ‘frequently’ or ‘almost always’.
For the evaluation of the scale reliability the internal consistency and the test-retest stability were determined, based on the pilot study, keeping in mind the international recommendations about the characteristics these instruments should have (13, 16, 17, 18).
To determine the internal characteristics the items were grouped in three dimensions, allowing us to measure the night snore (question 6), the night manifestations (questions 1-5) and the daily manifestations (questions 7-13). Statistically, we used the global and specific Cronbach alpha coefficient for the dimensions evaluated for more than one item (night and daily symptoms) calculated in the first poll and according with the bibliography used, it was considered that an Alpha of Cronbach less than 0.5 and 0.6 is a poor level, between 0.6 and 0.7 a weak level, and between 0.7 and 0.8 acceptable, between 0.8 and 0.9 good and levels more than 0.9 excellent (13, 19, 20, 21).
For the test-retest, two opinion polls answered for the same parents (30 days of average period with a range fluctuating between 27 and 33 days) were used. The aspects where there was similitude in the answers given by the parents or tutors in the different items of the questionnaires were considered as valid cases. The degree of concordance between the questions was evaluated using the following parameters/methods:
To establish the degree of concordance of the different items or questions, the following criteria were used.
There was one question with a good concordance in the two passes of the questionnaire, when it is presented at least two of the three criteria mentioned before (13, 16, 17).
Table 1 shows the main info characteristics used with 13 items, distributed in three dimensions that evaluate the night symptoms associated to SRRD in the questions 7-13. The scale of the answers is verbal, defining as positive the symptom if the answer is ‘frequently’ or ‘almost always’.
The way to administer the instrument was auto-complied in two occasions or phases of study. The time the parents delayed in answering the questionnaire was 4.58 minutes with a DE of ± 1.26. The parents or tutors were explained what the study consisted of, they were asked for the informed consent and orientations were given to fill the forms.
Table 1 Questionnaire Features
Source: Questionnaire and informed consent.
To evaluate the internal consistency (Table 2) of the measured instrument during the first step of the study, there was a global coefficient á of Combrach with 0.8028. Regarding the subscales with the daily and night symptoms the results were 0.8233 and 0.7823, respectively.
Table 2: Instrument Internal Consistency Evaluation according to Combrach coefficient alpha
Source: Questionnaire.
To determine the measured consistency between the two tests according to the test-retest method (Table 3) it was found that in 11 of the measured items (84.61%) there was consistency in the answers given during the two passes of the questionnaire to fulfil at least two of the three criteria previously established. Eight (72.73%) complied the three criteria and only two (27.27%) complied two criteria.
Table 3: Evaluation of Concordance of the Different Instrument Items according to Test-Retest.
Source: Questionnaire.
* Parameters not complying with the required criteria for concordance.
There was no concordance in the questions 5 and 11 of the questionnaire, where the first belonged to the subscale in charge of the nocturnal symptoms and the second to the subscale in charge of the daily symptoms.
The reliability was evaluated keeping in mind the international recommendations about the characteristics this kind of instrument should measure. The level of internal consistency for the night symptoms is global and good. This aspect states that the instrument is homogeneous, i.e, that the different items that compose each subscale are related and the three subscales have a good level of correlation between them. The references to the subject tend to be reliable to the info when calculating the coefficient á of Combrach, getting higher results to 0.7 (13, 16, 19).
Regarding to the repetition or reliability of the test-retest most of the items (84.61%) there is a good concordance of the questionnaire phases, considering it as right. This indicates that there is stability in the given answers by those supplying information in different times. We should point out that the average time between the two tests was 30 days, avoiding in this way the lack of the learning effect, one of the most frequent in this kind of study (13, 16, 21).
These results state according to most of the authors checked that the instrument measure with a good accuracy and short margin of error the aspects used for our population finding in general results higher than 70% in the correlation and concordance (13, 16, 17).
Conclusions
The evaluated instrument is reliable to be used in our context for the selection of patients and epidemiological studies related to SRRD.

 Table 1 Questionnaire Features
Table 1 Questionnaire Features

 Table 2: Instrument Internal Consistency Evaluation according to Combrach coefficient alpha
Table 2: Instrument Internal Consistency Evaluation according to Combrach coefficient alpha

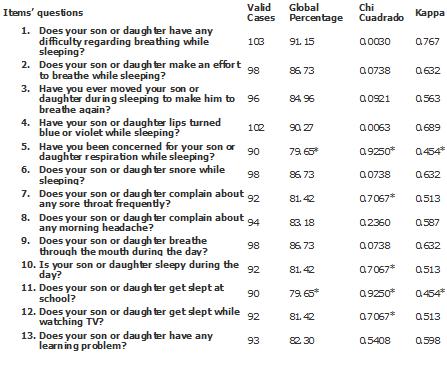 Table 3: Evaluation of Concordance of the Different Instrument Items according to Test-Retest.
Table 3: Evaluation of Concordance of the Different Instrument Items according to Test-Retest.

 Figure 1
Figure 1
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Introduction: The use of sleep questionnaires constitutes undeniable resources for the study of Sleep Related Respiratory Disorders (SRRD) as clinical tools for the selection of patients that need to be developed lab tests and in the second case for the very useful epidemiological investigations. Objective: To evaluate the reliability of TuCASA questionnaire to find out the RDAD in the Cuban infant population. Design: Study of transverse cut during the period September-November 2009, in 113 children from 6-11 years of Camilo Cienfuegos Semi-Boarding School in Moa. To determine the degree of concordance we use the global agreement percentage, Square Chi and Index of Kappa and for the degree of internal consistence we use the coefficient á of Combrach. Results: The degree of global internal consistence got was 0.8028 while the subscales taking into account the daily and night symptoms were 0.8233 and 0.7823 respectively. In 11 (84.61%) of the items, there was concordance in the test-retest, 8 (72.73%) complied with the three criteria and only two (27.27 %) complied with two. Conclusions: The evaluated instrument is reliable to be used in our context for the selection of patients and epidemiological studies related to RDAD.
 Autores:
Alexander Torres Molina[1]
Autores:
Alexander Torres Molina[1]

Citación: Torres A. . Medwave 2010 Nov;10(10):e4791 doi: 10.5867/medwave.2010.10.4791
Fecha de envío: 5/10/2010
Fecha de aceptación: 16/10/2010
Fecha de publicación: 1/11/2010
Origen: no solicitado, ingresado por FTS
Tipo de revisión: con revisión interna por miembros del consejo editorial

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Jordi Coromina, Eduard Estivil. El niño roncador. El niño con síndrome
de apnea obstructiva del sueño. 2006 Jordi Coromina y Eduard Estivill
Edita: Editores Médicos. S.A. (EDIMSA) | Link |
Jordi Coromina, Eduard Estivil. El niño roncador. El niño con síndrome
de apnea obstructiva del sueño. 2006 Jordi Coromina y Eduard Estivill
Edita: Editores Médicos. S.A. (EDIMSA) | Link | Puertas Cuesta FJ, Pin Arboledas G, Santa María Cano J, Durán-Cantolla J. Consenso Nacional sobre el Síndrome de Apneas-Hipopneas del Sueño (SAHS). Grupo Español de Sueño (GES). Arch Bronconeumol. 2005;41(Supl 4):3-110. | Link |
Puertas Cuesta FJ, Pin Arboledas G, Santa María Cano J, Durán-Cantolla J. Consenso Nacional sobre el Síndrome de Apneas-Hipopneas del Sueño (SAHS). Grupo Español de Sueño (GES). Arch Bronconeumol. 2005;41(Supl 4):3-110. | Link | Muzumdar H, Arens R. Diagnostic issues in pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008 Feb 15;5(2):263-73. | CrossRef | PubMed | PMC |
Muzumdar H, Arens R. Diagnostic issues in pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008 Feb 15;5(2):263-73. | CrossRef | PubMed | PMC | Katz ES, D'Ambrosio CM. Pathophysiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008 Feb 15;5(2):253-62. | CrossRef | PubMed | PMC |
Katz ES, D'Ambrosio CM. Pathophysiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008 Feb 15;5(2):253-62. | CrossRef | PubMed | PMC | Rey de Castro J M. El síndrome de apneas-hipopneas del sueño en la población pediátrica. Rev Peru Pediatr. v.60 n.3 Lima sep./dic. 2007. | Link |
Rey de Castro J M. El síndrome de apneas-hipopneas del sueño en la población pediátrica. Rev Peru Pediatr. v.60 n.3 Lima sep./dic. 2007. | Link | Gutiérrez M L, Peña VA, Villavicencio MM, Cáceda PN. Tratarnos respiratorios asociados al sueño en niños de educación primaria de un colegio público de Lima-Perú. Paediatrica 7(2) 2005. | Link |
Gutiérrez M L, Peña VA, Villavicencio MM, Cáceda PN. Tratarnos respiratorios asociados al sueño en niños de educación primaria de un colegio público de Lima-Perú. Paediatrica 7(2) 2005. | Link | Rey de Castro J M. Síndrome de Apneas Hipopneas del Sueño.¿Asesino silencioso? Revisión de la literatura. Rev Med Hered 2007;18(4). | Link |
Rey de Castro J M. Síndrome de Apneas Hipopneas del Sueño.¿Asesino silencioso? Revisión de la literatura. Rev Med Hered 2007;18(4). | Link | Alonso Alvarez ML, Terán Santos J, Cordero Guevara JA, Navazo Egüia AI, Ordax Carbajo E, Masa Jiménez JF, Pelayo R. [Reliability of respiratory polygraphy for the diagnosis of sleep apnea-hypopnea syndrome in children]. Arch Bronconeumol. 2008 Jun;44(6):318-23. | PubMed |
Alonso Alvarez ML, Terán Santos J, Cordero Guevara JA, Navazo Egüia AI, Ordax Carbajo E, Masa Jiménez JF, Pelayo R. [Reliability of respiratory polygraphy for the diagnosis of sleep apnea-hypopnea syndrome in children]. Arch Bronconeumol. 2008 Jun;44(6):318-23. | PubMed | Grupo Español del Sueño (GES). Documento de consenso nacional sobre el Síndrome de Apneas-Hipopneas del Sueño (SAHS). Septiembre 2005. | Link |
Grupo Español del Sueño (GES). Documento de consenso nacional sobre el Síndrome de Apneas-Hipopneas del Sueño (SAHS). Septiembre 2005. | Link | Villaasensi J.R. Síndrome de apneas hipopneas de sueño (SAHS) en el niño. Protocolos de Patología respiratoria. Bolpediat 2007; 47(SUPL. 2):14-22. | Link |
Villaasensi J.R. Síndrome de apneas hipopneas de sueño (SAHS) en el niño. Protocolos de Patología respiratoria. Bolpediat 2007; 47(SUPL. 2):14-22. | Link | Gutiérrez Mantari L, Peña V A, Villavicencio MM, Cáceda NP. Trastornos respiratorios asociados al sueño en niños de educación primaria de un colegio público de Lima-Perú. Paediatrica 2005;7(2). | Link |
Gutiérrez Mantari L, Peña V A, Villavicencio MM, Cáceda NP. Trastornos respiratorios asociados al sueño en niños de educación primaria de un colegio público de Lima-Perú. Paediatrica 2005;7(2). | Link | Tomás Vila M, Miralles Torres A, Beseler Soto B. [Spanish version of the Pediatric Sleep Questionnaire (PSQ). A useful instrument in investigation of sleep disturbances in childhood. Reliability analysis]. An Pediatr (Barc). 2007 Feb;66(2):121-8. | PubMed |
Tomás Vila M, Miralles Torres A, Beseler Soto B. [Spanish version of the Pediatric Sleep Questionnaire (PSQ). A useful instrument in investigation of sleep disturbances in childhood. Reliability analysis]. An Pediatr (Barc). 2007 Feb;66(2):121-8. | PubMed | García de Yébenes Prous MA, Rodríguez Salvanés F, Carmona Ortells L. [Validation of questionnaires]. Reumatol Clin. 2009 Jul-Aug;5(4):171-7. Epub 2009 May 20. | PubMed |
García de Yébenes Prous MA, Rodríguez Salvanés F, Carmona Ortells L. [Validation of questionnaires]. Reumatol Clin. 2009 Jul-Aug;5(4):171-7. Epub 2009 May 20. | PubMed | Goodwin JL, Babar SI, Kaemingk KL, Rosen GM, Morgan WJ, Sherrill DL, Quan SF; Tucson Children's Assessment of Sleep Apnea Study. Symptoms related to sleep-disordered breathing in white and Hispanic children: the Tucson Children's Assessment of Sleep Apnea Study. Chest. 2003 Jul;124(1):196-203. | CrossRef | PubMed |
Goodwin JL, Babar SI, Kaemingk KL, Rosen GM, Morgan WJ, Sherrill DL, Quan SF; Tucson Children's Assessment of Sleep Apnea Study. Symptoms related to sleep-disordered breathing in white and Hispanic children: the Tucson Children's Assessment of Sleep Apnea Study. Chest. 2003 Jul;124(1):196-203. | CrossRef | PubMed | Goodwin JL, Kaemingk KL, Mulvaney SA, Morgan WJ, Quan SF. Clinical screening of school children for polysomnography to detect sleep-disordered breathing--the Tucson Children's Assessment of Sleep Apnea study (TuCASA). J Clin Sleep Med. 2005 Jul 15;1(3):247-54. | PubMed | PMC |
Goodwin JL, Kaemingk KL, Mulvaney SA, Morgan WJ, Quan SF. Clinical screening of school children for polysomnography to detect sleep-disordered breathing--the Tucson Children's Assessment of Sleep Apnea study (TuCASA). J Clin Sleep Med. 2005 Jul 15;1(3):247-54. | PubMed | PMC | Prieto L, Lamarca R, Casado A. [Assessment of the reliability of clinical findings: the intraclass correlation coefficient]. Med Clin (Barc). 1998 Feb 7;110(4):142-5. | PubMed |
Prieto L, Lamarca R, Casado A. [Assessment of the reliability of clinical findings: the intraclass correlation coefficient]. Med Clin (Barc). 1998 Feb 7;110(4):142-5. | PubMed | Martin MC. Diseño y validación de cuestionarios. Matronas Profesión 2004;5(17):23-29.
Martin MC. Diseño y validación de cuestionarios. Matronas Profesión 2004;5(17):23-29.  Josep María Argimón Pallás, Argimon, Josep Jiménez Villa. Métodos de investigación clínica y epidemiológica. Elsevier España, 2004 - 393 páginas. | Link |
Josep María Argimón Pallás, Argimon, Josep Jiménez Villa. Métodos de investigación clínica y epidemiológica. Elsevier España, 2004 - 393 páginas. | Link |