 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Palabras clave: Atrioventricular Block, Arrhythmias, Cardiac, Atrioventricular Node, Antibodies
Atrioventricular blocks are chronotropic abnormalities produced by multifactorial alterations in the cardiac innervation system, specifically between the internodal pathways between the sinus node and the atrioventricular node. These bradyarrhythmias represent 2.3% of cardiac alterations in intrauterine life, registering one case for every 20 000 to 25 000 live births. However, its occurrence in childhood and adolescence is unknown. Likewise, the percentage of idiopathic atrioventricular blocks in this group in Colombia is unknown to date. Among the possible etiol-ogies, congenital and acquired causes have been documented. Some examples are isolated cases found in pregnancies with maternal isoimmunization, from carriers with lupus, and in coexis-tence with structural alterations, such as anomalies of the interventricular septum due to cardiac tumors and defects in the ostium and septation of the cardiac chambers. Atrioventricular blocks are also associated with respiratory syncytial virus infection and concomitant viral myocarditis and with cardiomyopathies of immune, rheumatic, infectious, tumoral, and structural origin, in addition to mitochondrial diseases such as Kearns Sayre syndrome, presenting with multi- organ involvement. These etiologies lead to chronic inflammation with fibrotic repair in the cardiac conduction system, which alters the transmission of the action potential and bradycardia with atrioventricular asynchrony. Idiopathic causes described in the onset of primary dysautonomia have also been reported. We present the case of an 11- year- old patient with a headache crisis and paroxysmal vegetative symptoms associated with repeated fainting, with subsequent studies where no structural alterations or autoimmune findings were identified. The patient was diag-nosed with idiopathic complete atrioventricular block and received expectant management by the electrophysiology service.
|
Main Messages
|
The cardiac conduction system consists of specialized nerve fibers organized in cellular groups and the myocardial muscle. In this network, the sinus node normally sends signals to the atrioventricular node, passing through the His bundle’s right and left collateral branches to the Purkinje fibers. Cardiac action potentials, with a duration between 500 and 800 milliseconds, are generated automatically in this system, where the propagation of this potential from its origin in the sinus node to the atrioventricular node has a physiological delay in the internodal fibers. Such pathways comprise the anterior fascicle of Bachman, the medial fascicle of Wenkebach, and the posterior fascicle of Torel. The latter two generate a delay in the conduction of 120 and 200 milliseconds, respectively [1]. When there is a block in the atrioventricular communication, this is identified by electrocardiographic changes, including a PR interval greater than 200 milliseconds or an absence of a QRS complex following atrial depolarization (P wave) [2].
Type two second- degree blocks (also known as Mobitz II) and complete atrioventricular dissociation (third- degree blocks) are the most dangerous and generate dysautonomic or vegetative symptoms, including nausea, headache, diaphoresis, lipothymia, and syncope. On the other hand, type one second- degree blocks (also known as Mobitz I) occur approximately 1- 2% in healthy and young people, especially during sleep. The prevalence of third- degree atrioventricular block is only 0.2% in the United States and 0.04% worldwide, with an increasing incidence at older ages but with an early peak in neonates and infants due to congenital blocks [2].
Some atrioventricular blocks are idiopathic and manifest during early and late childhood without structural, immunological, or infectious diseases. The scientific literature is scarce in these cases and considers that many pregnant women with autoimmune diseases are asymptomatic, and less than one- third of them are seropositive for autoantibodies. Therefore, many pregnant women may have an underdiagnosed autoimmune disease [3],[4] but present a good prognosis compared to other etiologies. In this context, a pure dysautonomic phenomenon may cause atrioventricular blocks. Autonomic dysfunction maintains a C- fiber anomaly based on alterations in mechano and baroreceptors of the left ventricle and carotid glomus, leading to reflex vasodilatation with excessive vagotonic discharge after a vigorous heartbeat (known as Bezold-J arisch reflex), which generates extreme bradycardia, diaphoresis and syncope [5].
Case reports on atrioventricular blocks discuss the experience of pacemaker implantation, and therapeutics in congenital complete blocks, among other indications. However, the avail-able information is scarce. To date, there are no clinical practice guidelines on conduction blocks in Colombia’s pediatric population, nor is there an expert consensus on managing atrioventricular blocks.
The following case report describes the primary care approach of a pediatric patient with suspected conduction disorder and discusses etiologies, pathophysiology, clinical relevance, and management of this heart disease.
A female patient of 11 years old, schoolgirl, without a pathological or surgical history, product of the first and only pregnancy of a healthy mother, born by euthyroid vaginal delivery at 38 weeks of gestation, consulted to the emergency department. The patient had recurrent non- pulsatile holocraneal headaches without photo- sonophobia and dysautonomic symptoms, including nausea, profuse diaphoresis, emesis, and lipothymia. On admission, she was stable and had a blood pressure of 110/80 millimeters of mercury, a heart rate of 60 beats per minute, oxygen saturation by pulse oximetry of 94% in room air, a temperature of 36.5 degrees Celsius, and respiratory rate of 26 breaths per minute. She had bradycardic and normofonetic sounds on auscultation, and a glucometry showed 98 micrograms per deciliter glucose level. A complete neurological examination was normal. With this symptomatology, the patient had a presumptive diagnosis of an uncomplicated migraine cri-sis and had outpatient management with a prokinetic (metoclopramide) plus analgesic (acetaminophen) with subsequent medical follow- up.
Twenty- four hours later, the patient was readmitted to the emergency department due to a syncopal episode secondary to physical activity. For this reason, non- invasive hemodynamic monitoring was performed, finding a heart rate of 49 beats per minute, a blood pressure of 90/70 millimeters of mercury, and irregular heart sounds without audible murmurs. A 12- lead electrocardiogram (Figure 1) showed a third- degree atrioventricular block with a calculated heart rate of 53 beats per minute, with no other alterations or dynamic ST- segment changes. However, QRS complexes 3° and 6° (left to right DI) appeared to have some degree of conduction since there was an advancement in the aforementioned QRS complexes. Sheldon et al. [6] score was used to evaluate the patient, ruling out a neurogenic background. Therefore, syncope of cardiogenic origin was assumed. Blood chemistry, an echocardiogram, and a 24- hour electrocardiographic recording were ordered, deferring management with an external pacemaker. Figure2 shows the chest X- ray with no pathological findings.
Laboratory tests included thyroid function (TSH), serum electrolytes (sodium, potassium, chloride, and calcium), a complete blood count, and C- reactive protein, all of which were normal. A transthoracic echocardiogram (Figure 3A and B) showed no structural alterations in the four cavity projections, a normal pericardial sac, a left ventricular ejection fraction of 65%, and a pulmonary artery systolic pressure of 19 millimeters of mercury.
The cardiac Holter evidenced atrial arrhythmia with a ventricular extension of isolated behavior, a maximum heart rate of 149 beats per minute, day and night episodes of Mobitz I, and third- grade atrioventricular block, with a minimum frequency of 40 beats per minute of nocturnal predominance, without ST- segment changes and average PR interval of 190 milliseconds (Figure 4A, B, C and D). However, some complexes showed a 2:1 atrioventricular block pattern (Figure 4C). Complementary anti- nuclear, anti- Ro, and anti- La antibodies were assessed in the mother, being all negative.
The patient was transferred to the pediatric intensive care unit, where invasive hemodynamic monitoring with an arterial line and continuous electrocardiographic surveillance was per-formed. α- and β-mimetic management was deferred since she remained asymptomatic at rest, with no hemodynamic alterations. A stress test was performed to determine the need for pacemaker implantation. The test was satisfactory, with adequate chronotropic response to exercise, reaching a metabolic task index of seven METS. Subsequently, she was evaluated by electrophysiology and pediatric cardiology. These units concluded that the patient had idiopathic and intermittent high- grade atrioventricular block secondary to vagotonic discharge and dysautonomic syndrome due to increased parasympatho-mimetic response, leading to exercise intolerance. Expectant outpatient management was indicated with lifestyle recommendations such as limiting intense physical activity, a normosodic diet and exercises to strengthen the muscle pump in the pro-drome. In addition, strict follow- up was prescribed, not ruling out the need for a permanent pacemaker.
At the time of writing, the patient is without pharmacological management. She persisted with lipothymia but improved symptoms with the recommendations and had a follow- up by pediatrics every three months and electrophysiology every six months. Likewise, a Holter monitoring every three months was prescribed to evaluate the paroxysms during wakefulness.
An ethics committee approved this case report, and the patient’s parents signed informed consent.
Figure 1. Admission electrocardiogram in prolonged DII lead.
Figure 2. Anteroposterior chest X-ray.
Figure 3A. Transthoracic echocardiogram 2D mode.
Figure 3B. Transthoracic echocardiogram color Doppler mode and M-mode.
Figure 4A. Holter test with increased PR interval and Wenkebach’s phenomenon.
Figure 4B. Holter test with supraventricular arrhythmia paroxysms.
Figure 4C. Holter test with Mobitz I and third-degree atrioventricular block.
Figure 4D. Heart rate as a function of time.
Atrioventricular block has been found in intrauterine life as early as 18 to 28 weeks of gestation. In pregnancy, this pathology is usually suspected through unsatisfactory echocardiography, evidencing fetal bradycardia, and confirmed by mechanical measurement of the PR interval. Atrioventricular blocks can be found in fetuses with pericardial and pleural effusion and severe hydrops [7]. Disease after birth is variable, and death may be caused by dilated heart disease with congestive heart failure and severe mitral insufficiency. However, many patients remain asymptomatic even into adulthood, presenting only heart rate declines during REM sleep.
This disorder has a congenital presentation in one per 15 000 to 20 000 live births due to various etiologies. Causes include alterations in the abnormal embryonic development of the atrio-ventricular node due to fibrosis in autoimmune diseases. Other causes include ventricular septal defect, including right heart anomalies like Ebstein’s anomaly, which debuts with prolonged PR intervals [8]. Similarly, exposure to maternal anti- Sjögren’s- syndrome- related antigen A autoantibodies (anti- SSA/Ro) and anti- Sjögren’s- syndrome- related antigen B autoantibodies (anti- SSB/La) in pregnancy has been associated with atrioventricular blocks in children of mothers with autoimmune entities, mainly mixed connective tissue disease and systemic lupus erythematosus (70% of cases). More rarely, it has been linked to other diseases such as rheumatoid arthritis, systemic sclerosis, or dermatomyositis. These gamma globulins internalize calcium channels and generate inflammation, degradation, and calcification of the cardiac conduction system by effector T cell attack, leading to fibrotic repair. The atrioventricular block is one of the first manifestations of the disease and may even be detected in perinatal intrauterine life [2],[3],[9]. Moreover, atrioventricular blocks are associated with primary myocardial tumors, such as tuberous rhabdomyosarcoma of septal location, with an incidence of 0.0017% [10], and Hunter syndrome in type II mucopolysaccharidosis. Cases have also been described as secondary to respiratory syncytial virus infection and concomitant viral myocarditis and cardiomyopathies of immune, rheumatic, infectious, tumor, and structural origin. Mitochondrial diseases of cardiomyocytes, such as the Kearn Sayre syndrome (present-ing with multi- organ involvement), have also been associated [11].
Finally, healthy children and young adults with atrioventricular conduction disturbances may simulate congenital heart block due to infectious processes such as myocarditis – mainly due to respiratory syncytial virus (18% of cases). The association between acute rheumatic carditis in Kawasaki disease, Lyme disease, and Chagas disease (endemic in Latin American countries) has also been found [2],[4].
We found no similar case reports in pediatric patients in a literature review. Díaz et al. [12] published in 2008 a case of a 31- week premature Colombian patient with non- immune hydrops diagnosed with congenital complete atrioventricular block secondary to maternal lupus confirmed by positive anti- nuclear and anti- Ro and anti- La antibodies. The patient was managed with inotropic support and subsequent pacemaker implantation, and ultimately, the patient resolved her heart failure. This clinical case differs from ours in the age of presentation, etiology, management, and outcome of the atrio-ventricular block.
Soria et al. [13], and Puga et al. [14], reported cases of sporadic dysautonomic symptoms associated with electrocardiographic alterations, including high-grade heart blocks in pediatric patients, captured only in routine controls. In such events, complementary studies ruled out a structural, immune, endocrine, metabolic, or infectious etiology, establishing the entity as isolated or idiopathic. In addition, class IA recommendations were given for the early implantation of definitive chronotropic sup-port devices. These cases differ from ours due to the wide spectrum of electrocardiographic alterations and the clinical and therapeutic approaches.
Moreover, Ouelugo et al. published in 2021 a case associated with respiratory syncytial virus infection, which had no link to a primary or secondary alteration of the heart other than acquired viral myocarditis. In this case, they decided to defer specific treatment and perform expectant surveillance until clearance of viremia to avoid sequelae and improve prognosis [15].
High- grade atrioventricular block in the absence of structural, immunologic, or infectious diseases (also called idiopathic) may be associated with genetic markers, channelopathies, and auto-nomic dysfunction in 4% to 20% of all congenital blocks. Generally, the prognosis is good, especially for those diagnosed in late childhood. Such blocks are not mediated by antibodies and have a better long- term prognosis as they have a lower risk of progressing to dilated cardiomyopathy and maintain better left ventricular function [2],[4],[16].
According to the natural history of atrioventricular blocks in adulthood, all patients (whether symptomatic or asymptomatic) should be treated with a pacemaker at diagnosis due to a high risk of sudden death [4],[8]. However, expectant management is valid in patients with paroxysmal symptoms and without hemodynamic instability or functional class compromise in the pediatric population. The etiology may be purely dysautonomic in these cases but requires in- depth studies [17]. The literature defines as indications for definitive pace-maker the presence of escape rhythm with wide complexes, ectopy and ventricular dysfunction, prolonged QT interval, cardiomegaly, right atriotomy with inotropic alteration, and persistent dysautonomic symptoms due to low cardiac rhythms [18],[19],[20].
The asymptomatic pediatric population or with clinical manifestations such as syncope may suffer physiological consequences of bradycardia, taking into account that the ventricular rate tends to decrease slowly with age. This bradyarrhythmia may result in loss of atrioventricular synchrony and a higher systolic volume as compensation for left ventricular dilatation, which in some cases may lead to dilated cardiomyopathy and heart failure [4].
Although the literature describes permanent pacemaker implantation in patients with congenital complete atrioventricular block, the 2012 American Society of Cardiology guidelines mainly refers to pediatric patients. However, limited information is available in this regard. For this reason, although there are publications with good results in managing children and adolescents with this pathology, studies addressing high- grade block in the pediatric population with higher methodological quality are required to define indications of invasive and non- invasive management clearly.
At the time of writing, the American College of Cardiology, the American Heart Association, and the Heart Rhythm Society (ACC, AHA, HRS) do not recommend any pharmacological treatment in the pediatric population to manage bradycardia [18]. According to J. Scaglione in his book on arrhythmias in pediatrics, the diagnosis of cardiac rhythm disturbances in the pediatric population should be made by identifying abnormalities in the electrocardiogram. In a complementary manner, and when there is diagnostic suspicion in a child with syncope, a 24- hour Holter study and a transthoracic echocardiogram should be performed to rule out structural alterations [21].
As idiopathic atrioventricular blocks are rare in childhood, the treating team struggled with the scarce evidence and clinical practice guidelines to complement clinicians' judgment in primary care. The management of these entities is determined by expert consensus, taking into account three factors: structural abnormality requiring surgical correction, low cardiac rhythms with persistent symptoms or syncope, and ventricular dysfunction.
High- grade atrioventricular blocks are unusual arrhythmias in childhood, adolescence, and young adulthood, and their prevalence has been increasing due to better access to technologies. However, finding its cause remains a challenge.
The indication for pacemaker implantation in symptomatic patients is clear for those with severe circulatory and chrono-tropic complications associated with exercise intolerance and sudden death.
In young adults and children, expectant management is recommended in patients with paroxysmal vagal discharges, which do not cause fatal hemodynamic alterations.
It is suggested that future research should be directed to identify epidemiological profiles and clinical criteria based on statistics and measures of occurrence in order to establish criteria of suspicion to timely diagnose this uncommon pathology.
Contributor roles
JMGG: conceptualization, data collection. MFEP: data collection, writing. FABA: writing, supervision. JDTL: literature review, methodology. CBA: conceptualization, literature review, visualization.
Competing interests
The authors declare that they have no competing interests.
Funding
The authors of this study financed the publication.
Ethics
Endorsement was obtained from the ethics committee of the Institución Universitaria Visión de Las Américas. Informed consent was obtained for this publication.
Provenance and peer review
Not commissioned. Externally peer- reviewed by four reviewers, double- blind.
Language of submission
Spanish.

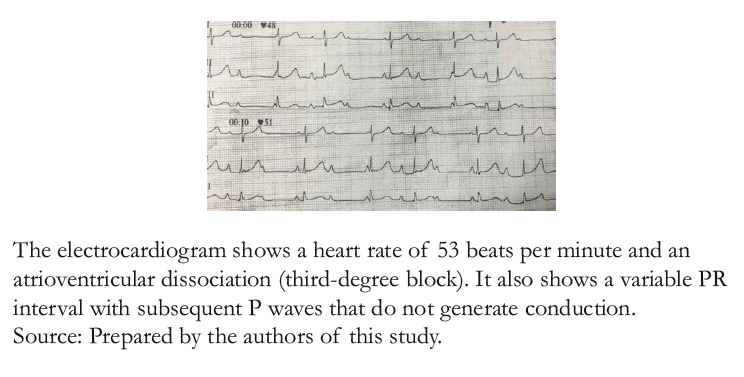 Figure 1. Admission electrocardiogram in prolonged DII lead.
Figure 1. Admission electrocardiogram in prolonged DII lead.

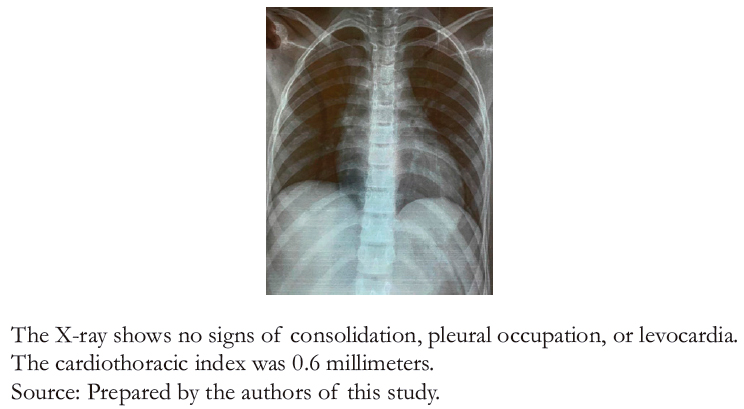 Figure 2. Anteroposterior chest X-ray.
Figure 2. Anteroposterior chest X-ray.

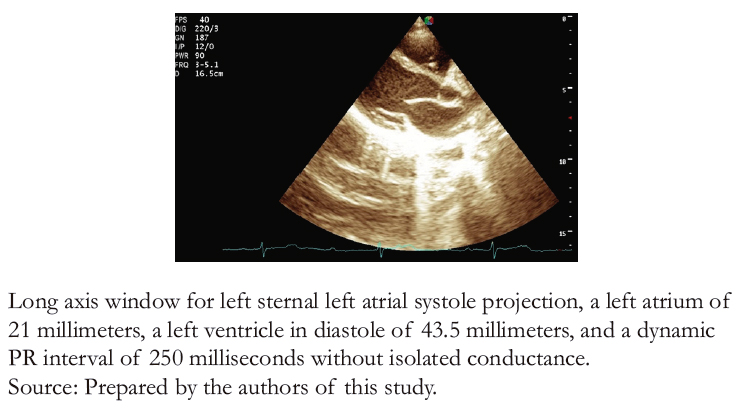 Figure 3A. Transthoracic echocardiogram 2D mode.
Figure 3A. Transthoracic echocardiogram 2D mode.

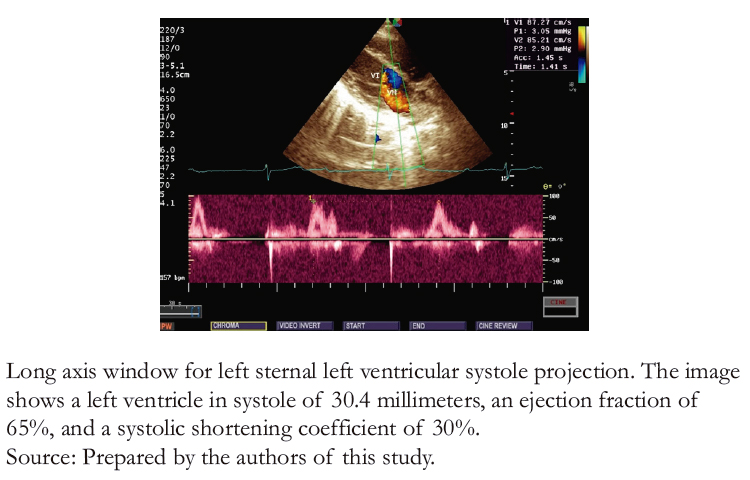 Figure 3B. Transthoracic echocardiogram color Doppler mode and M-mode.
Figure 3B. Transthoracic echocardiogram color Doppler mode and M-mode.

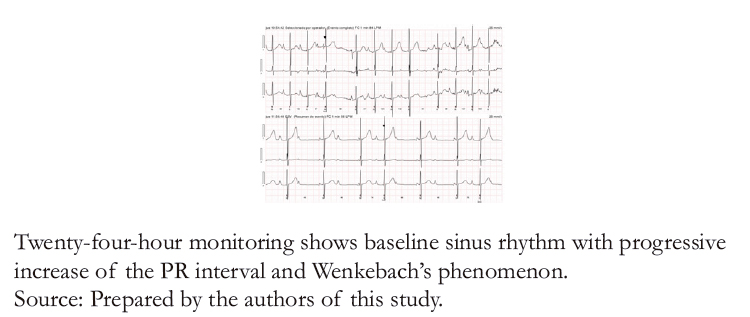 Figure 4A. Holter test with increased PR interval and Wenkebach’s phenomenon.
Figure 4A. Holter test with increased PR interval and Wenkebach’s phenomenon.

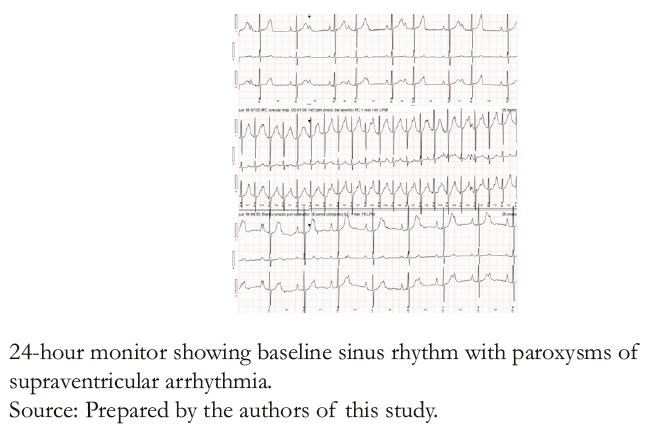 Figure 4B. Holter test with supraventricular arrhythmia paroxysms.
Figure 4B. Holter test with supraventricular arrhythmia paroxysms.

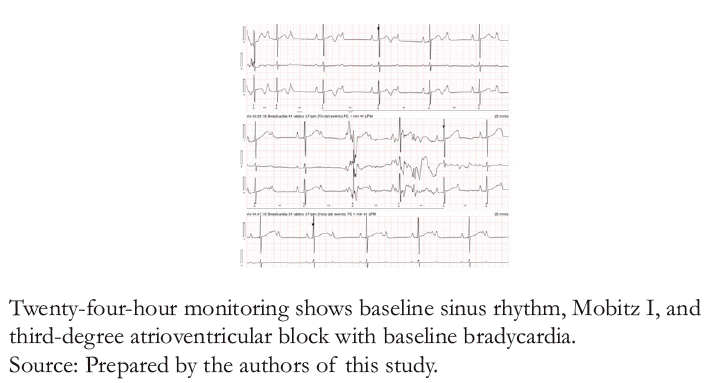 Figure 4C. Holter test with Mobitz I and third-degree atrioventricular block.
Figure 4C. Holter test with Mobitz I and third-degree atrioventricular block.

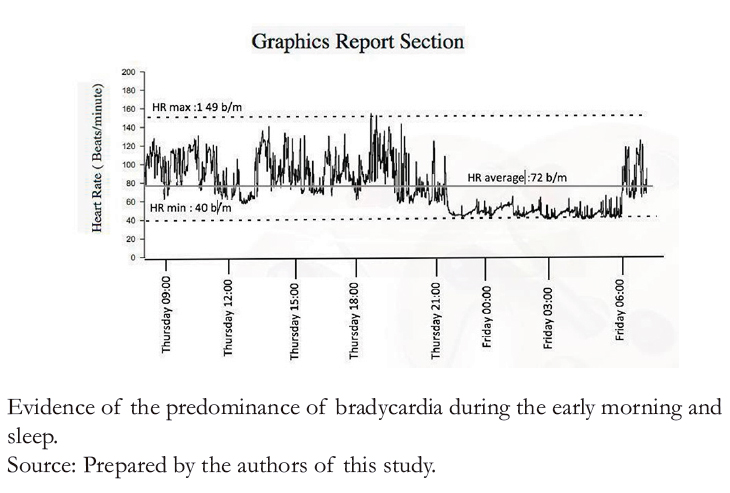 Figure 4D. Heart rate as a function of time.
Figure 4D. Heart rate as a function of time.

Atrioventricular blocks are chronotropic abnormalities produced by multifactorial alterations in the cardiac innervation system, specifically between the internodal pathways between the sinus node and the atrioventricular node. These bradyarrhythmias represent 2.3% of cardiac alterations in intrauterine life, registering one case for every 20 000 to 25 000 live births. However, its occurrence in childhood and adolescence is unknown. Likewise, the percentage of idiopathic atrioventricular blocks in this group in Colombia is unknown to date. Among the possible etiol-ogies, congenital and acquired causes have been documented. Some examples are isolated cases found in pregnancies with maternal isoimmunization, from carriers with lupus, and in coexis-tence with structural alterations, such as anomalies of the interventricular septum due to cardiac tumors and defects in the ostium and septation of the cardiac chambers. Atrioventricular blocks are also associated with respiratory syncytial virus infection and concomitant viral myocarditis and with cardiomyopathies of immune, rheumatic, infectious, tumoral, and structural origin, in addition to mitochondrial diseases such as Kearns Sayre syndrome, presenting with multi- organ involvement. These etiologies lead to chronic inflammation with fibrotic repair in the cardiac conduction system, which alters the transmission of the action potential and bradycardia with atrioventricular asynchrony. Idiopathic causes described in the onset of primary dysautonomia have also been reported. We present the case of an 11- year- old patient with a headache crisis and paroxysmal vegetative symptoms associated with repeated fainting, with subsequent studies where no structural alterations or autoimmune findings were identified. The patient was diag-nosed with idiopathic complete atrioventricular block and received expectant management by the electrophysiology service.
 Autores:
José Miguel Gutiérrez-Gallego[1], María Fernanda Estrada-Perez[2], Freddy Andrés Barrios-Arroyave[3], Juan Diego Torrente-López[3], Adoniran Correal-Barrios[4]
Autores:
José Miguel Gutiérrez-Gallego[1], María Fernanda Estrada-Perez[2], Freddy Andrés Barrios-Arroyave[3], Juan Diego Torrente-López[3], Adoniran Correal-Barrios[4]

Citación: Gutiérrez-Gallego JM, Estrada-Perez MF, Barrios-Arroyave FA, Torrente-López JD, Correal-Barrios A. High-grade idiopathic atrioventricular block in childhood: Case report and literature review. Medwave 2022;22(5):e002535 doi: 10.5867/medwave.2022.05.002535
Fecha de envío: 18/10/2021
Fecha de aceptación: 12/5/2022
Fecha de publicación: 15/6/2022
Origen: No solicitado
Tipo de revisión: Con revisión por pares externa, por cuatro árbitros a doble ciego

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Barra SNC, Providência R, Paiva L, Nascimento J, Marques AL. A review on advanced atrioventricular block in young or middle- aged adults. Pacing Clin Electrophysiol. 2012;35: 1395–405. | CrossRef | Link |
Barra SNC, Providência R, Paiva L, Nascimento J, Marques AL. A review on advanced atrioventricular block in young or middle- aged adults. Pacing Clin Electrophysiol. 2012;35: 1395–405. | CrossRef | Link | Cortés JM, Cortés JM, Cortés RA, Reyes BJ, Salazar A, Carrillo SI, et al. Revista mexicana de cardiología. 2013;24: 144–6. | Link |
Cortés JM, Cortés JM, Cortés RA, Reyes BJ, Salazar A, Carrillo SI, et al. Revista mexicana de cardiología. 2013;24: 144–6. | Link | Manolis AA, Manolis TA, Melita H, Manolis AS. Congenital heart block: Pace earlier (Childhood) than later (Adulthood). Trends Cardiovasc Med. 2020;30: 275–286. | CrossRef | Link |
Manolis AA, Manolis TA, Melita H, Manolis AS. Congenital heart block: Pace earlier (Childhood) than later (Adulthood). Trends Cardiovasc Med. 2020;30: 275–286. | CrossRef | Link | Sheldon R, Rose S, Ritchie D, Connolly SJ, Koshman M- L, Lee MA, et al. Historical criteria that distinguish syncope from seizures. J Am Coll Cardiol. 2002;40: 142–8. | CrossRef | Link |
Sheldon R, Rose S, Ritchie D, Connolly SJ, Koshman M- L, Lee MA, et al. Historical criteria that distinguish syncope from seizures. J Am Coll Cardiol. 2002;40: 142–8. | CrossRef | Link | Díaz A, Serrano A, Guzmán M, Ruz M. Bloque auriculoventricular congénito completo: reporte de un caso y revisión de la literatura. Revista colombiana de cardiología SCC. 2007;15: 35–41. | Link |
Díaz A, Serrano A, Guzmán M, Ruz M. Bloque auriculoventricular congénito completo: reporte de un caso y revisión de la literatura. Revista colombiana de cardiología SCC. 2007;15: 35–41. | Link | Peñaloza- Wandurraga JE, Prada- Motta S, Otero- Pinto JC. Prevalencia de hallazgos anormales en ecocardiogramas fetales en el Hospital Universitario de Santander, Bucaramanga (Colombia), 2007- 2013. Rev Colomb Obstet Ginecol. 2015;66: 110. | Link |
Peñaloza- Wandurraga JE, Prada- Motta S, Otero- Pinto JC. Prevalencia de hallazgos anormales en ecocardiogramas fetales en el Hospital Universitario de Santander, Bucaramanga (Colombia), 2007- 2013. Rev Colomb Obstet Ginecol. 2015;66: 110. | Link | Baruteau A- E, Pass RH, Thambo J- B, Behaghel A, Le Pennec S, Perdreau E, et al. Congenital and childhood atrioventricular blocks: pathophysiology and contemporary management. Eur J Pediatr. 2016;175: 1235–1248. | CrossRef | Link |
Baruteau A- E, Pass RH, Thambo J- B, Behaghel A, Le Pennec S, Perdreau E, et al. Congenital and childhood atrioventricular blocks: pathophysiology and contemporary management. Eur J Pediatr. 2016;175: 1235–1248. | CrossRef | Link | Lince R, Mesa C, Arteaga A, Montoya J, Vásquez L. Cardiac rhabdomyoma as manifestation of tuberous sclerosis: Presentation of two cases and literature review. Rev Colomb Cardiol. 2009;16: 224–8. | Link |
Lince R, Mesa C, Arteaga A, Montoya J, Vásquez L. Cardiac rhabdomyoma as manifestation of tuberous sclerosis: Presentation of two cases and literature review. Rev Colomb Cardiol. 2009;16: 224–8. | Link | Chlebowski MM, Heese BA, Malloy- Walton LE. Early childhood onset of high- grade atrioventricular block in Hunter syndrome. Cardiol Young. 2018;28: 786–787. | CrossRef | Link |
Chlebowski MM, Heese BA, Malloy- Walton LE. Early childhood onset of high- grade atrioventricular block in Hunter syndrome. Cardiol Young. 2018;28: 786–787. | CrossRef | Link | Díaz DA, SerranoGA, GuzmánBM, Ruz MM. Bloqueo aurículo- ventricular congénito completo: Reporte de un caso y revisión de la literatura. Rev Colomb Cardiol. 2008;15: 35–42. | Link |
Díaz DA, SerranoGA, GuzmánBM, Ruz MM. Bloqueo aurículo- ventricular congénito completo: Reporte de un caso y revisión de la literatura. Rev Colomb Cardiol. 2008;15: 35–42. | Link | Fernández Soria MT, Pérez- Lescure Picarzo FJ. Casos clínicos en Cardiología (n.o Casos clínicos en Cardiología (n. Rev Pediatr Aten Primaria. 2012;14: 243–247. | CrossRef | Link |
Fernández Soria MT, Pérez- Lescure Picarzo FJ. Casos clínicos en Cardiología (n.o Casos clínicos en Cardiología (n. Rev Pediatr Aten Primaria. 2012;14: 243–247. | CrossRef | Link | Puga Bravo MV, Jimbo Jimbo AC, Gallardo Medina Y, Mora Orellana EJ, Puga Bravo EE. Bloqueo aurículoventricular completo aislado: a propósito de un caso. Rev Pediatr Electrón. 2018; 23–8. | Link |
Puga Bravo MV, Jimbo Jimbo AC, Gallardo Medina Y, Mora Orellana EJ, Puga Bravo EE. Bloqueo aurículoventricular completo aislado: a propósito de un caso. Rev Pediatr Electrón. 2018; 23–8. | Link | Oulego- Erroz I, de Castro-V ecino P, Ocaña- Alcober C, Gutiérrez- Marqués S, Martínez- Badás JP, Centeno- Jiménez M. Bloqueo auriculoventricular completo asociado a infección por virus respiratorio sincitial: presentación de un caso y revisión de la literatura. Anales de Pediatría. 2021;94: 417–419. | CrossRef | Link |
Oulego- Erroz I, de Castro-V ecino P, Ocaña- Alcober C, Gutiérrez- Marqués S, Martínez- Badás JP, Centeno- Jiménez M. Bloqueo auriculoventricular completo asociado a infección por virus respiratorio sincitial: presentación de un caso y revisión de la literatura. Anales de Pediatría. 2021;94: 417–419. | CrossRef | Link | Villain E, Coastedoat- Chalumeau N, Marijon E, Boudjemline Y, Piette JC, Bonnet D. Presentation and prognosis of complete atrioventricular block in childhood, according to maternal antibody status. J Am Coll Cardiol. 2006;48: 1682–7. | CrossRef | Link |
Villain E, Coastedoat- Chalumeau N, Marijon E, Boudjemline Y, Piette JC, Bonnet D. Presentation and prognosis of complete atrioventricular block in childhood, according to maternal antibody status. J Am Coll Cardiol. 2006;48: 1682–7. | CrossRef | Link | Caprotta CG. Estado actual de la estimulación eléctrica cardíaca en pediatría. Arch. argent. pediatr. 2001;99: 41–7. | Link |
Caprotta CG. Estado actual de la estimulación eléctrica cardíaca en pediatría. Arch. argent. pediatr. 2001;99: 41–7. | Link | Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MR, etal. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;140: e382–e482. | CrossRef |
Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MR, etal. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;140: e382–e482. | CrossRef | Fredi M, Andreoli L, Bacco B, Bertero T, Bortoluzzi A, Breda S, et al. First Report of the Italian Registry on Immune- Mediated Congenital Heart Block (Lu.Ne Registry). Front Cardiovasc Med. 2019;6: 11. | CrossRef | Link |
Fredi M, Andreoli L, Bacco B, Bertero T, Bortoluzzi A, Breda S, et al. First Report of the Italian Registry on Immune- Mediated Congenital Heart Block (Lu.Ne Registry). Front Cardiovasc Med. 2019;6: 11. | CrossRef | Link | Jaeggi ET, Hamilton RM, Silverman ED, Zamora SA, Hornberger LK. Outcome of children with fetal, neonatal or childhood diagnosis of isolated congenital atrioventricular block. A single institution’s experience of 30 years. J Am Coll Cardiol. 2002;39: 130–7. | CrossRef | Link |
Jaeggi ET, Hamilton RM, Silverman ED, Zamora SA, Hornberger LK. Outcome of children with fetal, neonatal or childhood diagnosis of isolated congenital atrioventricular block. A single institution’s experience of 30 years. J Am Coll Cardiol. 2002;39: 130–7. | CrossRef | Link | Scaglione J. Trastornos de conducción. Argentina. Silver House publicaciones médicas; 2012. | Link |
Scaglione J. Trastornos de conducción. Argentina. Silver House publicaciones médicas; 2012. | Link |