 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Palabras clave: Klebsiella pneumoniae, drug resistance, urinary infections
Carbapenemases are one of the major mechanisms of antimicrobial resistance, usually due to the indiscriminate use of antibiotics. The expansion of this mechanism of resistance at world level is imminent and control measures are limited. In the region of the Central Sierra of Peru - Huancayo, we report a case of carbapenemase-producing Klebsiella pneumoniae, with the purpose of discussing the problems related to this emerging mechanism of antibiotic resistance.
We are heading towards the post-antibiotic era, where the infections that are currently treatable will become deadly [1]. This alarming fact is rapidly expanding worldwide and our country is not on the sidelines of this situation. An example of this are bacteria from the Carbapenem resistant Enterobacteriaceae family, which are associated with high mortality rates due to limited therapeutic options [1],[2]. Despite the multiple strategies used to reduce this problem, the only control measures that have proven effective are proper hand washing, strict contact precautions and the rational use of antimicrobials [3].
Carbapenems, belong to the last line of antimicrobials within the family of beta-lactams. These are used in situations where there is resistance to cephalosporins, aminopenicillins, ureidopenicillins or other commonly used antimicrobials. The carbapenemases are a group of enzymes capable of hydrolyzing this group of antimicrobials
In general terms, beta-lactamases can be classified into two types [4]:
Classifications
Beta-lactamases have been classified in different ways by different authors. Nowadays, the most used classification is Ambler´s [4]. According to Ambler, betalactamases are divided into four classes A, B, C and D. Classes A and D, are serine beta-lactamases, class B metallo-beta-lactamases, and class C cephalosporinases [4]. This classification was stablished in the 1980s when analyzing samples of beta-lactamases capable of hydrolyzing carbapenems that could not be inhibited by ethylenediaminetertraacetic acid (EDTA), calling them serino-beta-lactamases, o differentiate them from those that could be inhibited by this acid, as metallo-beta-lactamases.
From this classification, the carbapenemases are found within group A, B and D, so that classes A and D belong to serine-carbapenemases, and class B metallo-carbapenemases.
In some cases the overexpression of resistance genes that do not hydrolyze carbapenems can lead to carbapenemase activity. The latter happens with the class C carbapenemase. This class of enzymes belong rather to the overproduction of the AmpC enzyme, which usually does not hydrolyze carbapenems, but when its self-regulation mechanisms are altered, it produces a overexpression of the ampC gene with consequent overexpression of the AmpC enzyme, which leads to the degradation of carbapenems. Figure 1 shows the classification of carbapenemases according to Ambler.
Figure 1. Classification of carbapenemases according to Ambler.
Another classification used is the one of Bush et al. [5], which classifies beta-lactamases according to their functional characteristics, grouping them into four groups:
-Group 1: cephalosporinases.
-Group 2: serinobetalactamases.
-Group 3: metallobetalactamases.
-Group 4: beta-lactamases not fully characterized.
From this classification, the carbapenemases belong to groups 1, group 2 (2df, 2f) and group 3 (3a, 3b and 3c).
The first cases of carbapenemase producing Klebsiella pneumoniae were reported in the United States in 1996; the first case of carbapenemase producing Klebsiella pneumoniae in Latin America (Medellin - Colombia) was reported in 2005, later a case was reported in Chile in 2012 and in 2013 in Lima-Peru [6],[7]. Table 1 shows the integration between the classifications [5] of Ambler and Bush.
Table 1. Carbapenemases classification according to Ambler & Bush, adapted from Bush et al. [5]
The objective of this case report is to discuss the rational use of antimicrobials, and the real need to treat asymptomatic urinary tract infections through the presentation of a clinical case. Even when the resistance phenotype tempts the physician to eliminate the microorganism, it must always be kept in mind that this could be part of a microbiota that colonizes the patient, so treating such colonization can lead to the expression of various mechanisms of resistance in microorganisms that naturally do not express them, and that do not cause illness.
A 36-year-old male, from Huancayo – Peru, with a history of spinal cord injury associated with paraplegia, the reason why he undergoes motor kinesiotherapy and uses a Foley catheter intermittently.
In the last 5 years, the patient has presented five episodes of urinary tract infections, receiving in two episodes treatment with ciprofloxacin on an outpatient basis. Later, the last three episodes of urinary tract infections have required hospitalization. Ceftriaxone was the treatment used in the first hospitalization and imipenem in the last two hospitalizations.
In August 28, 2016, the patient was hospitalized after having a urine culture result with development of Klebsiella pneumoniae resistant to carbapenems (see table 2), receiving in this opportunity antimicrobial treatment with imipenem until completing seven days, awaiting assessment by the Infectology team.
At the same time, contact precautions for the patient were established, in addition to the active search for inhospital and intra-domiciliary contacts. During the hospital period this pathogen was not isolated in any other sample (28 beds, wife and child), and the patients who were in contact did not present any acute infection during their hospitalization.
Months later, two control urine cultures were performed on the patient, with an interval of 15 days apart, showing the presence of the same microorganism and resistance phenotype as in previous stages, all these in the absence of urinary symptoms, this was a reason to consider this as a colonization.
Currently, one year after the strain was investigated, the patient is stable, does not have urinary symptoms and continues receiving motor kinesiotherapy for the sequelae of spinal cord injury.
Regarding the microbiological diagnosis, the first sample for urine culture was taken in the emergency service (August 28, 2016), proceeding to the identification and study of antimicrobial susceptibility by automated system Vitek®2 Compact. The strain of Klebsiella pneumoniae with phenotype of resistance to carbapenems was identified.
Diagnostic confirmation of carbapenemase production was carried out through the Kirby Bauer method, according to the Clinical Laboratory Standards Institute 2015 standards and the manual of procedures for the phenotypic detection of resistance mechanisms of the Peruvian microbiology society (2014). Similarly, the Hodge phenotypic test was carried out.
Finally, on September 20, 2016, the strain was sent to the National Institute of Health - Peru, confirming on October 3, 2016 by conventional polymerase chain reaction (PCR) the finding of Carbapenemase producing Klebsiella pneumoniae (KPC).
Table 2. Antibiogram for Klebsiella pneumoniae*
Carbapenemases are a group of enzymes which have the ability to hydrolyze carbapenemic antibiotics. The carbapenemases of the type KPC (Klebsiella pneumoniae carbapenemase) belong to the class A of Ambler and group 2f of Bush; these enzymes are the most frequently isolated worldwide [5].
Factors for its acquisition include: immunosuppression, previous exposure to broad-spectrum antibiotics, presence of a urinary catheter and prolonged stay in the intensive care unit [8],[9]. Peru has been conducting epidemiological surveillance of antimicrobial resistance for six years [10]. Thus, in 2013 and 2014, the first cases of carabapenemases were reported in the capital of our country (Lima-Peru). After these events, these resistance profiles have not been reported to date in other cities. Unfortunately, the two cases mentioned above were deadly, unlike this case, which represents a colonization by K. pneumoniae producing KPC carbapenemases.
In 2009, the Centers for Disease Control and Prevention (CDC) disseminated strategies for the control of infections caused by carbapenemase-producing enterobacteria. Taking as main points: the isolation of the index case, the implementation of detection protocols for carbapenemic resistant strains and the weekly monitoring of index case contacts [11]. Likewise, a study of patients colonized with by Klebsiella pneumoniae producing KPC carbapenemases reported that the establishment of biosafety measures in hospitals of high prevalence decrease the incidence of this microorganism [12].
Another key point is to evaluate the colonization by carbapenemase producing Klebsiella pneumoniae. One study showed that the duration of colonization lasts less than four weeks in 17% of patients; while 83% of patients remain colonized for more than four weeks with an average duration of 270 days [13]. In the present case, the patient persists colonized by carbapenemase producing Klebsiella pneumoniae after one year.
Regarding the measures for the management of carbapenemase producing Klebisella pneumoniae infections, these are based on two main axes. First of all, it is essential to control and contain the index case, in order to reduce the transmissibility of the microorganism, these control measures consist of strict clinical hand washing, contact precautions and daily disinfection of the surfaces in the unit where the patient is located.
Considering that Klebsiella pneumoniae is part of the intestinal microbiota, some authors suggest the performance of routine rectal swabs [14] in order to detect early Klebsiella pneumoniae carriers with XDR resistance phenotypes, either beta-lactamase spectrum type extended (ESBL) or carbapenemase type KPC .
It is important to keep in mind that carbapenemases produced by Klebsiella pneumoniae are plasmidial, so if the control measures are not established early, the risk of plasmidial transfer between different bacterial genera is high, being able to generate the same phenotype of resistance in other microorganisms. which are found either in coinfection in the same patient or in other clinical units, constituting healthcare-associated infections (HAI).
Regarding the pharmacological management, once the strain has been detected, it must be assessed that this is not colonization. Subsequently, already defined that the microorganism is causing disease in the patient, the recommended scheme consists of colistin (polymyxin E) associated with meropenem or imipenem, or the use of ceftazidime-avibactam [15].
Other authors have described total remission of Klebsiella pneumoniae infections in blood cultures with the use of high dose oral fosfomycin (9 g every every 8 hrs) associated with other antibiotics according to antibiogram, with negative blood cultures results after two days of started the scheme [16].
The use of fosfomycin monotherapy at high doses (8 g every 8 hrs) intravenously is another option too, resulting in negative cultures at 24 hours since the start of therapy [17]. It should be noted that the bioavailability of fosfomycin is approximately 30% when administered orally, therefore, the use of oral fosfomycin must be at high doses in order to simulate the recommended intravenous administration dose of 3 g every eight hours. The oral administration of this antimicrobial is due to the fact that many countries don’t have yet intravenous presentation being forced to use other routes for the administration of the drug taking into account their pharmacokinetic parameters.
As mentioned above, keeping in mind the pharmacokinetics of each antimicrobial [18],[19] is crucial in predicting the desired antibiotic concentration in different extravascular compartments. The pharmacokinetic parameters of the antimicrobials indicated in Klebsiella pneumoniae KPC infection are presented in Table 3
Table 3. Pharmacokinetic parameters of antimicrobials [16] indicated for K. pneumoniae KPC.
Regarding the impact on mortality associated with this pathogen, an associated mortality of 32.1% was observed in a hospital in the United States for Klebsiella pneumoniae type KPC, compared to 9.9% for sensitive strains [20]. There are no studies addressing mortality by KPC in our country, due to the low incidence reported.
In retrospective, detailing the clinical history, the irrational use of antimicrobials was found as risk factors for the acquisition of this strain, as well as the chronic use of intermittent catheterization by Foley catheter. No other data were obtained that contribute to the acquisition of a KPC strain.
Unfortunately until now, our hospital does not have specific regulations for the management of these cases. Our action responded to the alarming fact of isolating a multidrug-resistant germ and its probable repercussion within our hospital.
The risk factors involved in the acquisition of carbapenemic-resistant strains in the present clinical case were the indiscriminate use of antimicrobials in previous hospitalizations together with the chronic use of intermittent Foley catheter.
Based on the information presented in the case, is a priority the creation of specific protocols for the management of cases of enterobacteria resistant to carbapenems, considering that these antimicrobials are the last line in the management of serious infections.
Active surveillance by rectal swabs in hospitalized patients has given favorable results for the early detection of XDR resistance phenotypes, whether they are extended-spectrum beta-lactamases (ESBLs) such as KPC-type carbapenemase.
The use of colistin associated with meropenem or imipenem is indicated as long as the organism is causing disease, and is not part of the patient's microbiota as colonization. Considering that 83% of patients remain colonized for more than four weeks. This translates into not initiating antimicrobial chemotherapy in patients with asymptomatic urinary tract infections.
The use of fosfomycin in cases of Klebsiella pneumoniae infections resistant to carbapenems is shown as a good alternative when the first line schemes have been refractory.
The unnecessary taking of urine cultures tends to cause confusion regarding the management of patients with asymptomatic bacteriuria, tempting the clinician to indicate antimicrobial schemes to microorganisms that are part of the patient's microbiota, thus generating positive selection and the expression of mechanisms of resistance.
From the editor
The authors originally submitted this article in Spanish and subsequently translated it into English. The Journal has not copyedited this version.
Ethical aspects
The authors declare that the privacy of the patient was respected according to the CIOMS rules, regarding the privacy of the data collected.
Statement of conflicts of interest
The authors have completed the declaration of conflicts of interest form of the ICMJE, and declare not having received financing for the realization of the report; not have financial relationships with organizations that could have interests in the published article, in the last three years; and not having other relationships or activities that could influence the published article. The forms can be requested by contacting the responsible author or the editorial direction of the Journal.
Financing
The authors declare that there were no sources of external financing.
Acknowledgement
To the National Institute of Health - Peru, for the collaboration in the confirmation of the strain.

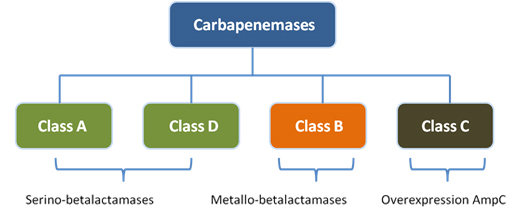 Figure 1. Classification of carbapenemases according to Ambler.
Figure 1. Classification of carbapenemases according to Ambler.

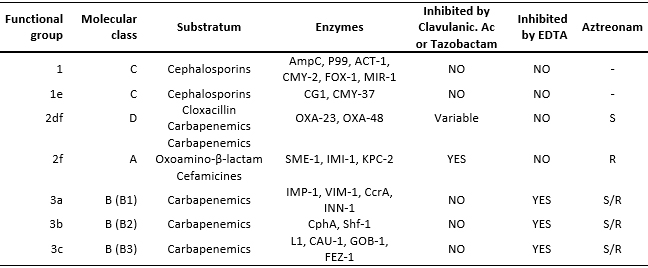 Table 1. Carbapenemases classification according to Ambler & Bush, adapted from Bush et al. [5]
Table 1. Carbapenemases classification according to Ambler & Bush, adapted from Bush et al. [5]

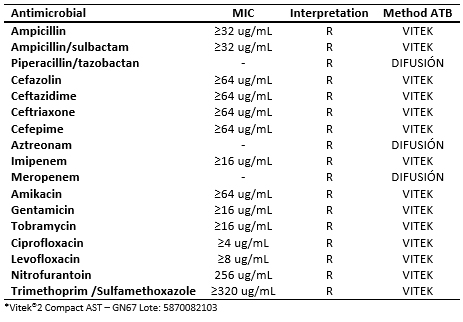 Table 2. Antibiogram for Klebsiella pneumoniae*
Table 2. Antibiogram for Klebsiella pneumoniae*

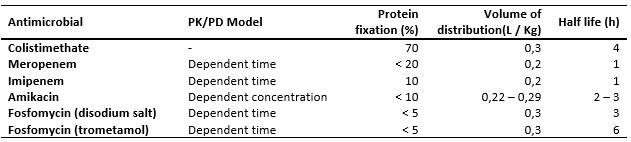 Table 3. Pharmacokinetic parameters of antimicrobials [16] indicated for K. pneumoniae KPC.
Table 3. Pharmacokinetic parameters of antimicrobials [16] indicated for K. pneumoniae KPC.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Carbapenemases are one of the major mechanisms of antimicrobial resistance, usually due to the indiscriminate use of antibiotics. The expansion of this mechanism of resistance at world level is imminent and control measures are limited. In the region of the Central Sierra of Peru - Huancayo, we report a case of carbapenemase-producing Klebsiella pneumoniae, with the purpose of discussing the problems related to this emerging mechanism of antibiotic resistance.
 Autores:
Jhosef Franck Quispe Pari[1,2], Jacqueline Olimpia Ingaruca Rojas[3], Abel Moises Castro Mucha[2], Marivel Liz Castro Ortega[2], Francklin Jhordy Ccoicca Hinojosa[2], Raul Montalvo Otivo[4], Alfonso Agustín Prieto Pozo[5], Francisco Luis Daniel Salvador Sagüez[5,6]
Autores:
Jhosef Franck Quispe Pari[1,2], Jacqueline Olimpia Ingaruca Rojas[3], Abel Moises Castro Mucha[2], Marivel Liz Castro Ortega[2], Francklin Jhordy Ccoicca Hinojosa[2], Raul Montalvo Otivo[4], Alfonso Agustín Prieto Pozo[5], Francisco Luis Daniel Salvador Sagüez[5,6]

Citación: Quispe Pari JF, Ingaruca Rojas JO, Castro Mucha AM, Castro Ortega ML, Ccoicca Hinojosa FJ, Montalvo Otivo R, et al. Carbapenemase producing Klebsiella pneumoniae in Peru: a case report and antimicrobial resistance discussion . Medwave 2018 Mar-Abr;18(2):e7191 doi: 10.5867/medwave.2018.02.7191
Fecha de envío: 11/11/2017
Fecha de aceptación: 29/1/2018
Fecha de publicación: 3/4/2018
Origen: no solicitado
Tipo de revisión: con revisión por un par revisor externo, a doble ciego

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Nombre/name: Jose Armando Gonzales
Fecha/date: 2018-05-07 17:15:31
Comentario/comment:
Klebsiella pneumoniae productora de carbapenemasas en Perú: ¿Existe la necesidad de más estudios fenotÃpicos y genotÃpicos?
Estimada editora:
He leÃdo con gran interés la publicación de Quispe Pari et al. acerca del aislamiento de Klebsiella pneumoniae productora de carbapenemasa en Perú [1]. Los autores describieron el interesante caso de un paciente de 36 años de Huancayo Perú que tuvo colonización por Klebsiella pneumoniae productora de KPC. Los autores usaron el Sistema automatizado Vitek 2 para la identificación y sensibilidad a antimicrobianos, lo cual les alertó de la presencia de resistencia a carbapenems. También mencionaron que el método de Kirby Bauer fue usado para confirmar la producción de carbapenemasas. A mi parecer esto es impreciso, ya que la detección de carbapenemasas no es posible con el uso de esta técnica. El método de Kirby Bauer solo evalúa resistencia a carbapenem, pero no proporciona información sobre el mecanismo de resistencia, el cual puede ser secundario no solo a carbapenemasas, sino también a mutaciones de porinas o bombas de expulsión.
Para una evaluación más detallada de la producción de carbapenemasas, los autores usaron el test modificado de Hodge, el cual es un buen estudio para detectar la mayorÃa de carbapenemasas. Sin embargo, falsos negativos han sido reportados en casos de cepas productoras de NDM y falsos positivos en casos de mecanismos mixtos de resistencia distintos a carbapenemasas [2]. En años recientes, muchos otros estudios fenotÃpicos han emergido y han mostrado claramente tasas de detección mas altas que el test modificado de Hodge. Entre ellos tenemos el método modificado de inactivación de carbapenem y las pruebas cromogénicas (Carba NP, Rapidec Carba NP, Rapid Cab Blue, etc.). Estas pruebas han mostrado una sensibilidad y especificidad de 88-99% y 99-100% respectivamente [3]. Una clara ventaja de estos métodos es su rapidez para dar resultados, el cual varÃa de 30 minutos a 2 horas [3].
Otro punto de discusión es la detección genotÃpica de KPC reportado por los autores. En su reporte, ellos señalan que la cepa de Klebsiella fue evaluada por el método convencional de reacción de cadena polimerasa, pero no mencionan el tipo de prueba genotÃpica utilizada y no proporcionaron información sobre el tipo de KPC o la secuencia genómica. Actualmente tenemos muchas pruebas para evaluación genotÃpica como el FilmArray BCID, Verigene BC-GN y el X-pert Carba-R. Otra prueba que ha ganado mucha popularidad es el next-generation sequencing. Este método secuencia todo el ADN cromosomal y extracromosomal, permitiendo la identificación de genes responsables de la producción de carbapenemasas, porinas y bombas de expulsión. Esta técnica también nos da datos acerca sobre la afinidad y transmisión de cepas. En el Perú hay información muy limitada acerca de la secuenciación de cepas de Klebsiella. El caso peruano publicado por Horna et al. revela la presencia de una cepa de Klebsiella productora de KPC-2 con una secuencia tipo (ST) 340 [4]. Otros reportes en Latinoamérica han documentado el ST 258 como el clon predominante. Yo creo que el reporte de Quispe Pari et al. es una excelente iniciativa que dará lugar a más estudios claramente necesarios en Perú para determinar la epidemiologÃa de cepas resistentes.
Declaración de Conflicto de Intereses
El autor declara que no tiene conflicto de intereses.
Referencias
1. Quispe Pari JF, Ingaruca Rojas JO, Castro Mucha AM, Castro Ortega ML, Ccoicca Hinojosa FJ, Montalvo Otivo R, Prieto Pozo AA, Salvador Sagüez FLD. Carbapenemase producing Klebsiella pneumoniae in Peru: a case report and antimicrobial resistance discussion. Medwave. 2018 Apr 3; 18(2):e7191. DOI: 10.5867/medwave.2018.02.7191. Spanish, English. PMID:29652297
2. Anderson KF, Lonsway DR, Rasheed JK, Biddle J, Jensen B, McDougal LK, Carey RB, Thompson A, Stocker S, Limbago B, Patel JB. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J Clin Microbiol. 2007 Aug; 45(8):2723-5. DOI:10.1128/JCM.00015-07. PMID:17581941
3. Tamma PD, Opene BN, Gluck A, Chambers KK, Carroll KC, Simner PJ. Comparison of 11 Phenotypic Assays for Accurate Detection of Carbapenemase-Producing Enterobacteriaceae. J Clin Microbiol. 2017 Apr; 55(4):1046-1055. DOI:10.1128/JCM.02338-16. PMID:28077701
4. Horna G, Velasquez J, Fernández N, Tamariz J, Ruiz J. Characterisation of the first KPC-2-producing Klebsiella pneumoniae ST340 from Peru. J Glob Antimicrob Resist. 2017 Jun; 9:36-40. DOI: 10.1371/journal.pone.0154092. ECollection 2016.PMID:27104910.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 WHO’s first global report on antibiotic resistance reveals serious, worldwide threat to public health. Who.int. 2018 [on line]. | Link |
WHO’s first global report on antibiotic resistance reveals serious, worldwide threat to public health. Who.int. 2018 [on line]. | Link | Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis. 2011 Jul 1;53(1):60-7. | CrossRef | PubMed |
Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis. 2011 Jul 1;53(1):60-7. | CrossRef | PubMed | Garro G. Enterobacterias resistentes a carbapenems, un desafío para la atención hospitalaria. Boletín Epidemiológico Ministerio de Salud del Perú. 2014;23:667-668. | Link |
Garro G. Enterobacterias resistentes a carbapenems, un desafío para la atención hospitalaria. Boletín Epidemiológico Ministerio de Salud del Perú. 2014;23:667-668. | Link | Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev. 2007 Jul;20(3):440-58, table of contents. | PubMed |
Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev. 2007 Jul;20(3):440-58, table of contents. | PubMed | Bush K, Jacoby GA. Updated functional classification of beta-lactamases.Antimicrob Agents Chemother. 2010 Mar;54(3):969-76. | CrossRef | PubMed |
Bush K, Jacoby GA. Updated functional classification of beta-lactamases.Antimicrob Agents Chemother. 2010 Mar;54(3):969-76. | CrossRef | PubMed | Velásquez J, Hernández R, Pamo O, Candiotti M, Pinedo Y, Sacsaquispe R et al. Klebsiella pneumoniae resistente a los carbapenemes. Primer caso de carbapenemasa tipo KPC en Perú. Revista de la Sociedad Peruana de Medicina Interna. 2013;26(4):192-196. | Link |
Velásquez J, Hernández R, Pamo O, Candiotti M, Pinedo Y, Sacsaquispe R et al. Klebsiella pneumoniae resistente a los carbapenemes. Primer caso de carbapenemasa tipo KPC en Perú. Revista de la Sociedad Peruana de Medicina Interna. 2013;26(4):192-196. | Link | Cifuentes M, García P, San Martín P, Silva F, Zúñiga J, Reyes S, et al. [First isolation of KPC in Chile: from Italy to a public hospital in Santiago]. Rev Chilena Infectol. 2012 Apr;29(2):224-8. | CrossRef | PubMed |
Cifuentes M, García P, San Martín P, Silva F, Zúñiga J, Reyes S, et al. [First isolation of KPC in Chile: from Italy to a public hospital in Santiago]. Rev Chilena Infectol. 2012 Apr;29(2):224-8. | CrossRef | PubMed | Jiao Y, Qin Y, Liu J, Li Q, Dong Y, Shang Y, et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection/colonization and predictors of mortality: a retrospective study. Pathog Glob Health. 2015 Mar;109(2):68-74. | CrossRef | PubMed |
Jiao Y, Qin Y, Liu J, Li Q, Dong Y, Shang Y, et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection/colonization and predictors of mortality: a retrospective study. Pathog Glob Health. 2015 Mar;109(2):68-74. | CrossRef | PubMed | Lin MY, Lyles-Banks RD, Lolans K, Hines DW, Spear JB, Petrak R, et al. The importance of long-term acute care hospitals in the regional epidemiology of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. lin Infect Dis. 2013 Nov;57(9):1246-52. | CrossRef | PubMed |
Lin MY, Lyles-Banks RD, Lolans K, Hines DW, Spear JB, Petrak R, et al. The importance of long-term acute care hospitals in the regional epidemiology of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. lin Infect Dis. 2013 Nov;57(9):1246-52. | CrossRef | PubMed | Ministerio de Salud del Perú. Protocolo para la detección de KPC en Enterobacterias. Lima: Centro Nacional de Salud Pública; 2014:1-8. | Link |
Ministerio de Salud del Perú. Protocolo para la detección de KPC en Enterobacterias. Lima: Centro Nacional de Salud Pública; 2014:1-8. | Link | Hayden MK, Lin MY, Lolans K, Weiner S, Blom D, Moore NM, et al. Prevention of colonization and infection by Klebsiella pneumoniae carbapenemase-producing enterobacteriaceae in long-term acute-care hospitals. Clin Infect Dis. 2015 Apr 15;60(8):1153-61. | CrossRef | PubMed |
Hayden MK, Lin MY, Lolans K, Weiner S, Blom D, Moore NM, et al. Prevention of colonization and infection by Klebsiella pneumoniae carbapenemase-producing enterobacteriaceae in long-term acute-care hospitals. Clin Infect Dis. 2015 Apr 15;60(8):1153-61. | CrossRef | PubMed | Guidance for Control of Infections with Carbapenem-Resistant or Carbapenemase-Producing Enterobacteriaceae in Acute Care Facilities. Cdc.gov. 2018 [on line]. | Link |
Guidance for Control of Infections with Carbapenem-Resistant or Carbapenemase-Producing Enterobacteriaceae in Acute Care Facilities. Cdc.gov. 2018 [on line]. | Link | Haverkate MR, Weiner S, Lolans K, Moore NM, Weinstein RA, Bonten MJ, et al. Duration of Colonization With Klebsiella pneumoniae Carbapenemase-Producing Bacteria at Long-Term Acute Care Hospitals in Chicago, Illinois. Open Forum Infect Dis. 2016 Aug 30;3(4):ofw178. | PubMed |
Haverkate MR, Weiner S, Lolans K, Moore NM, Weinstein RA, Bonten MJ, et al. Duration of Colonization With Klebsiella pneumoniae Carbapenemase-Producing Bacteria at Long-Term Acute Care Hospitals in Chicago, Illinois. Open Forum Infect Dis. 2016 Aug 30;3(4):ofw178. | PubMed | Lemmenmeier E, Kohler P, Bruderer T, Goldenberger D, Kleger GR, Schlegel M. First documented outbreak of KPC-2-producing Klebsiella pneumoniae in Switzerland: infection control measures and clinical management. Infection. 2014 Jun;42(3):529-34. | CrossRef | PubMed |
Lemmenmeier E, Kohler P, Bruderer T, Goldenberger D, Kleger GR, Schlegel M. First documented outbreak of KPC-2-producing Klebsiella pneumoniae in Switzerland: infection control measures and clinical management. Infection. 2014 Jun;42(3):529-34. | CrossRef | PubMed | Kyle JM, Stollings JL, White KD, Noto MJ, Wheeler AP. Fosfomycin for multidrug treatment of Klebsiella pneumoniae carbapenemase bacteremia. Ann Pharmacother. 2015 Mar;49(3):366-7. | CrossRef | PubMed |
Kyle JM, Stollings JL, White KD, Noto MJ, Wheeler AP. Fosfomycin for multidrug treatment of Klebsiella pneumoniae carbapenemase bacteremia. Ann Pharmacother. 2015 Mar;49(3):366-7. | CrossRef | PubMed | Simkins J, Fan J, Camargo JF, Aragon L, Frederick C. Intravenous Fosfomycin Treatment for Carbapenem-Resistant Klebsiella pneumoniae in the United States. Ann Pharmacother. 2015 Oct;49(10):1177-8. | CrossRef | PubMed |
Simkins J, Fan J, Camargo JF, Aragon L, Frederick C. Intravenous Fosfomycin Treatment for Carbapenem-Resistant Klebsiella pneumoniae in the United States. Ann Pharmacother. 2015 Oct;49(10):1177-8. | CrossRef | PubMed |