 Para Descargar PDF debe Abrir sesión.
Para Descargar PDF debe Abrir sesión.
Palabras clave: granulomatous prostatitis, BCG, intravesical BCG immunotherapy, active immunotherapy
Bacille Calmette-Guerin intravesical treatment is the most effective treatment for reducing the recurrence of non-muscle-invasive urothelial carcinomas. This treatment can sometimes have side effects and serious complications. Granulomatous prostatitis is a common histological finding but it rarely has a clinical presentation. We report a case of a 75-year-old, type 2 diabetic, male patient who was diagnosed with urothelial in situ carcinoma, for which he began treatment with Bacille Calmette-Guerin instillations. Five years later the patient presented nocturia, pollakiuria, severe urgency, and intense and recurrent perineal pain associated with marked elevation of prostatic specific antigen. A prostatic biopsy was performed that showed a moderate to severe granulomatous prostatitis related to bacille Calmette-Guerin. The patient received full antituberculosis combination drugs with a favorable clinical response.
Since 1973, intravesical bacillus Calmette-Guerin (BCG) immunotherapy has been used as a bladder cancer treatment for superficial urothelial carcinoma and carcinoma in situ (CIS). [1] Intravesical BCG slows tumor progression, decreases the need for cystectomy, and increases global survival rates [2],[3].
However, numerous complications have been described in association with BCG. The most frequent are of little clinical relevance and include bladder irritations (e.g. dysuria and urinary frequency/urgency) due to an inflammatory reaction, hematuria, self-contained spiking fevers, and, in some cases, cystitis. More severe complications described in the literature include urosepsis, pneumonitis, hepatic granulomas, pyelonephritis, hypersensitive reactions to BCG, intramedullary granulomas, reactive arthritis, rhabdomyolysis, and cutaneous metastatic infection [4],[5],[6],[7].
Among the urology-related complications, one common histopathological diagnosis in patients that have received BCG therapy is granulomatous prostatitis. However, symptomatic presentation of this condition is infrequent, with few reports in the existing literature [8],[9] and the late presentation of granulomatous prostatitis represents a clinical challenge in differential diagnosis with the pathology prostatic neoplasia [10],[11].
A 75-year-old male patient with a clinical history of diabetes mellitus type 2 was admitted in 2004 after presenting macroscopic hematuria for three weeks together with increased voiding frequency and severe urgency. A complete hematuria workup was performed. This included, among other exams, an elimination-phase computed tomography that showed a small bladder with rigid walls, as well as urine cytology congruent with urothelial neoplastic cells. Cystoscopy examination, performed under anesthesia, revealed diffuse hyperemic injuries, while multiple random biopsy analyses confirmed CIS.
Intravesical BCG therapy was administered following a standard regimen, with a 6-week induction course of weekly 30 mg doses. This treatment was poorly tolerated and induced dysuria and severe urgency, due to which the therapy was suspended at week 4. An outpatient cystoscopy and randomized cold biopsy were performed, with histopathological diagnosis indicating nonspecific chronic cystitis.
The therapy regimen was modified to intravesical doxorubicin administered weekly for six weeks at a 50 mg dose. This regimen was completed by the patient without presenting side-effects.
Five years after the instillations, the patient was admitted presenting symptoms of increased voiding frequency and severe urgency, nocturia, and intense, recurrent perineal pain. A pelvic ultrasound showed a prostate with heterogeneous echogenicity, an approximate volume of 38.6 cc, and 127 cc of a post-voiding residue. Urine cytology was requested, which resulted negative for neoplastic cells.
The patient presented temporal evolution for the prostate-specific antigen, which increased over time (Figure 1). Additionally, digital rectal examination results were non-suspicious. A transrectal ultrasound guided prostate biopsy was performed, taking two samples per sextant, resulting in a total of 12 samples. The biopsy results revealed chronic moderate to severe granulomatous prostatitis related to BCG (Figure 2).
An anti-tuberculosis drug treatment was prescribed following a first-line two-month regimen (50 doses) with isoniazid (300 mg), rifampicin (600 mg), and ethambutol (1,200 mg). Pyrazinamide was not prescribed as this drug does not affect Mycobacterium bovis. After the first-line regimen, a posterior tri-weekly regimen with isoniazid (300 mg) and rifampicin (600 mg) was used until week 16 of treatment (48 doses). The patient presented asymptomatic evolution, with an International Prostate Symptom Score of 8 and Quality of Life score of 3.
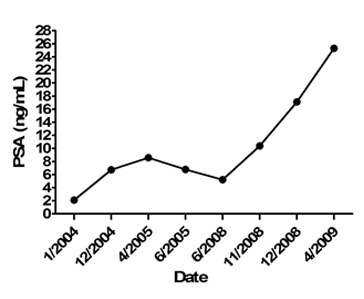
Figure 1. Temporal evolution of the prostate-specific antigen (PSA).
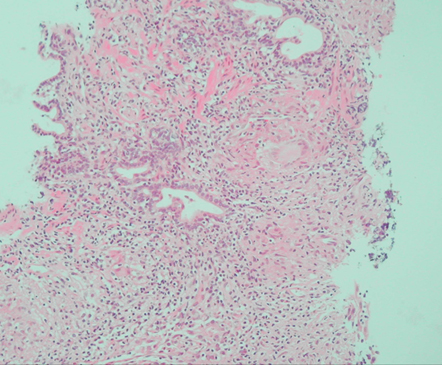
In the glandular stroma, a well-developed granuloma with a centralized Langhans giant cell was identified with an epithelioid crown and peripheral lymphocytes. Other granulomas were observed in the inferior half of the image.
Figure 2. Hematoxylin and eosin stain (x 200 magnification) of a prostate puncture biopsy.
The presence of granulomatous prostatitis in patients treated with intravesical BCG is a frequent histopathological finding. While the clinical repercussions of this condition are debated in asymptomatic patients, Lamm and cols. observed that granulomatous prostatitis was frequently diagnosed in routine biopsies that were generally asymptomatic and did not require specific treatment [7]. In turn, La Fontaine and cols. recorded this type of histopathological lesion in 9 out of 12 cystoprostatectomy patients that received prior BCG therapy [12]. Similarly, other studies report a 15% diagnosis rate in biopsies [13].
According to Orihuela and cols., granulomatous prostatitis diagnoses can reach up to 51% in patients presenting prostate induration during the first six months post- BCG instillation [14]. When a prostatic nodule appears a posteriori, prostatic neoplasia should be ruled out, secondary to evaluation of the tumor vesical itself or the appearance of a primary prostate tumor.
The incidence of symptomatic granulomatous prostatitis as a complication of intravesical BCG instillation in the treatment of superficial bladder tumors and CIS is unknown. However, it is estimated that between 0.9 and 1.3% of patients present prostate induration, elevated prostate-specific antigens, or clinical prostatitis [4],[15],[16].
As with granulomatous prostatitis arising from other etiologies, the clinical palpation of a nodule or prostate induration is frequently indistinguishable from carcinoma during a digital rectal exam [17], These symptoms are commonly caused by an increase in the prostate-specific antigen, which, on average, increases by 4.2 ng/mL (0.9-9.7 ng/mL) [18]. From a radiological perspective, there is no specific image of granulomatous prostatitis, and in many cases, no lesion is observed in test images. In cases where prostatic nodules are palpated during physical examination, a transrectal ultrasound is the most applied test for identifying pathology. The most predominant finding through ultrasound examination is a peripheral hypoechoic nodule located in the periphery or in the transition zone where this type of nodule cannot be differentiated from other processes, such as prostate cancer [19].
The role of multiparametric magnetic resonance imaging (MRI) in diagnosing granulomatous prostatitis has generated recent controversy. To increase diagnostic certainty, radiologists recommend using a 3 Tesla endorectal coil MRI to improve multiparametric images. Despite using state-of-the-art equipment, confirming this pathology through multiparametric MRI continues to be a challenge [11].
Kawada and cols. observed an early and prolonged reinforcement of a suspicious ring-shaped lesion using multiparametric MRI with a gadolinium contrast, describing this finding as a pathognomonic image for granulomatous prostatitis [20]. In the currently described case, clinical presentation occurred five years after instillations, a situation that, together with a marked increase in the prostate-specific antigen, obligated the use of a transrectal prostate biopsy as the diagnostic exam.
In histopathological terms, the lesion is characterized by the presence of discrete caseificant or non-caseificant granulomas without eosinophilic tissue [17]. This characteristic differentiates granulomatous prostatitis from non-specific granulomatous prostatitis, allergic prostatitis, and postoperative granulomas. Furthermore, acid-alcohol-resistant bacilli can be observed, particularly within caseificant granulomas. Differential diagnosis should be established with other types of granulomatous prostatitis as well as with prostate cancer and prostatic stromal invasion of CIS of the bladder, which have clinically similar symptoms and ultrasounds [21],[22]. The management of patients with clinical symptoms (e.g. dysuria, fever, irritating voiding symptoms) also consists in suspending instillations with BCG and use of complete first-line anti-tuberculosis drug treatment [4], excluding pyrazinamide as M. bovis lacks the pyrazinamidase enzyme.
While granulomatous prostatitis is rare from a clinical perspective, this condition is a frequent histological finding in patients treated with intravesical BCG instillations. In symptomatic patients or those with elevated prostate-specific antigen levels six months after the last instillation, granulomatous prostatitis should be differentially diagnosed. However, prostatic adenocarcinoma should be systematically excluded using ultrasound-guided biopsies in all patients, particularly considering the reported clinical and radiological similarities between granulomatous prostatitis and prostate cancer.
From the editor
The authors originally submitted this article in Spanish and subsequently translated it into English. The Journal has not copyedited this version.
Ethical aspects
The authors declare that, because it does not identify the patient in published images, no patient consent was requested for this publication.
Conflicts of interest
The authors completed the ICMJE declaration of conflicts of interest, translated into Spanish by Medwave, and declare they have not received funding for the completion of the report; have no financial relationships with organizations that may have interests in the article published in the last three years; and have no other relationships or activities that could influence the published article. Forms can be requested by contacting the responsible author or editorial direction of the Journal.
Funding
The authors declare that no external funding sources.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Bacille Calmette-Guerin intravesical treatment is the most effective treatment for reducing the recurrence of non-muscle-invasive urothelial carcinomas. This treatment can sometimes have side effects and serious complications. Granulomatous prostatitis is a common histological finding but it rarely has a clinical presentation. We report a case of a 75-year-old, type 2 diabetic, male patient who was diagnosed with urothelial in situ carcinoma, for which he began treatment with Bacille Calmette-Guerin instillations. Five years later the patient presented nocturia, pollakiuria, severe urgency, and intense and recurrent perineal pain associated with marked elevation of prostatic specific antigen. A prostatic biopsy was performed that showed a moderate to severe granulomatous prostatitis related to bacille Calmette-Guerin. The patient received full antituberculosis combination drugs with a favorable clinical response.
 Autores:
Octavio Castillo[1,2], Lorena Villasenín-Parrado[3], Vincenzo Borgna[4], Iván Gallegos[5], Virginia Martínez[6]
Autores:
Octavio Castillo[1,2], Lorena Villasenín-Parrado[3], Vincenzo Borgna[4], Iván Gallegos[5], Virginia Martínez[6]

Citación: Castillo O, Villasenín-Parrado L, Borgna V, Gallegos I, Martínez V. Late-onset granulomatous prostatitis following intravesical bacille Calmette-Guerin therapy: case report. Medwave 2016 Jun;16(5):e6473 doi: 10.5867/medwave.2016.05.6473
Fecha de envío: 10/3/2016
Fecha de aceptación: 8/6/2016
Fecha de publicación: 20/6/2016
Origen: no solicitado
Tipo de revisión: con revisión por cuatro pares revisores externos, a doble ciego

Nos complace que usted tenga interés en comentar uno de nuestros artículos. Su comentario será publicado inmediatamente. No obstante, Medwave se reserva el derecho a eliminarlo posteriormente si la dirección editorial considera que su comentario es: ofensivo en algún sentido, irrelevante, trivial, contiene errores de lenguaje, contiene arengas políticas, obedece a fines comerciales, contiene datos de alguna persona en particular, o sugiere cambios en el manejo de pacientes que no hayan sido publicados previamente en alguna revista con revisión por pares.
Aún no hay comentarios en este artículo.
Para comentar debe iniciar sesión
 Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
Medwave publica las vistas HTML y descargas PDF por artículo, junto con otras métricas de redes sociales.
 Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976 Aug;116(2):180-3. | PubMed |
Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976 Aug;116(2):180-3. | PubMed | Clark PE, Agarwal N, Biagioli MC, Eisenberger MA, Greenberg RE, Herr HW, et al. Bladder cancer. J Natl Compr Canc Netw. 2013 Apr 1;11(4):446-75. | PubMed |
Clark PE, Agarwal N, Biagioli MC, Eisenberger MA, Greenberg RE, Herr HW, et al. Bladder cancer. J Natl Compr Canc Netw. 2013 Apr 1;11(4):446-75. | PubMed | Burger M, Oosterlinck W, Konety B, Chang S, Gudjonsson S, Pruthi R, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: Non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2013 Jan;63(1):36-44. | CrossRef | PubMed |
Burger M, Oosterlinck W, Konety B, Chang S, Gudjonsson S, Pruthi R, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: Non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2013 Jan;63(1):36-44. | CrossRef | PubMed | Lamm DL, van der Meijden PM, Morales A, Brosman SA, Catalona WJ, Herr HW,et al. Incidence and treatment of complications of bacillus Calmette-Guerin intravesical therapy in superficial bladder cancer. J Urol. 1992 Mar;147(3):596-600. | PubMed |
Lamm DL, van der Meijden PM, Morales A, Brosman SA, Catalona WJ, Herr HW,et al. Incidence and treatment of complications of bacillus Calmette-Guerin intravesical therapy in superficial bladder cancer. J Urol. 1992 Mar;147(3):596-600. | PubMed | Palayew M, Briedis D, Libman M, Michel RP, Levy RD. Disseminated infection after intravesical BCG immunotherapy. Detection of organisms in pulmonary tissue. Chest. 1993 Jul;104(1):307-9. | PubMed |
Palayew M, Briedis D, Libman M, Michel RP, Levy RD. Disseminated infection after intravesical BCG immunotherapy. Detection of organisms in pulmonary tissue. Chest. 1993 Jul;104(1):307-9. | PubMed | Queiro R, Ballina J, Weruaga A, Fernández JA, Riestra JL, Torre JC, et al. Psoriatic arthropathy after BCG immunotherapy for bladder carcinoma. Br J Rheumatol. 1995 Nov;34(11):1097. | PubMed |
Queiro R, Ballina J, Weruaga A, Fernández JA, Riestra JL, Torre JC, et al. Psoriatic arthropathy after BCG immunotherapy for bladder carcinoma. Br J Rheumatol. 1995 Nov;34(11):1097. | PubMed | Lamm DL, Stogdill VD, Stogdill BJ, Crispen RG. Complications of bacillus Calmette-Guerin immunotherapy in 1,278 patients with bladder cancer. J Urol. 1986 Feb;135(2):272-4. | PubMed |
Lamm DL, Stogdill VD, Stogdill BJ, Crispen RG. Complications of bacillus Calmette-Guerin immunotherapy in 1,278 patients with bladder cancer. J Urol. 1986 Feb;135(2):272-4. | PubMed | Colombo R, Consonni P, Nava L, Guazzoni G, Montorsi F, Centemero A, et al. [Chronic granulomatous prostatitis: echographic features in patients submitted to immunoprophylaxis/therapy with BCG]. Arch Ital Urol Nefrol Androl. 1991 Jun;63 Suppl 2:147-9.
| PubMed |
Colombo R, Consonni P, Nava L, Guazzoni G, Montorsi F, Centemero A, et al. [Chronic granulomatous prostatitis: echographic features in patients submitted to immunoprophylaxis/therapy with BCG]. Arch Ital Urol Nefrol Androl. 1991 Jun;63 Suppl 2:147-9.
| PubMed | Noor MA, Biyabani SR, Burney IA, Talatia J. Symptomatic granulomatous prostatitis following bacillus Calmette-Guerin immunotherapy for bladder cancer. J Pak Med Assoc. 2002 Dec;52(12):578-80. | PubMed |
Noor MA, Biyabani SR, Burney IA, Talatia J. Symptomatic granulomatous prostatitis following bacillus Calmette-Guerin immunotherapy for bladder cancer. J Pak Med Assoc. 2002 Dec;52(12):578-80. | PubMed | Kobelev AA. [Chronic granulomatous prostatitis and cancer of the prostate]. Urol Nefrol (Mosk). 1974;39(4):54-5. | PubMed |
Kobelev AA. [Chronic granulomatous prostatitis and cancer of the prostate]. Urol Nefrol (Mosk). 1974;39(4):54-5. | PubMed | Suditu N, Negru D. Bacillus Calmette-Guérin therapy-associated granulomatous prostatitis mimicking prostate cancer on MRI: A case report and literature review. Mol Clin Oncol. 2015 Jan;3(1):249-251. | PubMed |
Suditu N, Negru D. Bacillus Calmette-Guérin therapy-associated granulomatous prostatitis mimicking prostate cancer on MRI: A case report and literature review. Mol Clin Oncol. 2015 Jan;3(1):249-251. | PubMed | LaFontaine PD, Middleman BR, Graham SD Jr, Sanders WH. Incidence of granulomatous prostatitis and acid-fast bacilli after intravesical BCG therapy. Urology. 1997 Mar;49(3):363-6. | PubMed |
LaFontaine PD, Middleman BR, Graham SD Jr, Sanders WH. Incidence of granulomatous prostatitis and acid-fast bacilli after intravesical BCG therapy. Urology. 1997 Mar;49(3):363-6. | PubMed | Balasar M, Doğan M, Kandemir A, Taskapu HH, Cicekci F, Toy H, Gurbuz R. Investigation of granulomatous prostatitis incidence following intravesical BCG therapy. Int J Clin Exp Med. 2014 Jun 15;7(6):1554-7. | PubMed |
Balasar M, Doğan M, Kandemir A, Taskapu HH, Cicekci F, Toy H, Gurbuz R. Investigation of granulomatous prostatitis incidence following intravesical BCG therapy. Int J Clin Exp Med. 2014 Jun 15;7(6):1554-7. | PubMed | Orihuela E, Herr HW, Pinsky CM, Whitmore WF Jr. Toxicity of intravesical BCG and its management in patients with superficial bladder tumors. Cancer. 1987 Aug 1;60(3):326-33. | PubMed |
Orihuela E, Herr HW, Pinsky CM, Whitmore WF Jr. Toxicity of intravesical BCG and its management in patients with superficial bladder tumors. Cancer. 1987 Aug 1;60(3):326-33. | PubMed | Leibovici D, Zisman A, Chen-Levyi Z, Cypele H, Siegel YI, Faitelovich S, et al. Elevated prostate specific antigen serum levels after intravesical instillation of bacillus Calmette-Guerin. J Urol. 2000 Nov;164(5):1546-9. | PubMed |
Leibovici D, Zisman A, Chen-Levyi Z, Cypele H, Siegel YI, Faitelovich S, et al. Elevated prostate specific antigen serum levels after intravesical instillation of bacillus Calmette-Guerin. J Urol. 2000 Nov;164(5):1546-9. | PubMed | Rifkin MD, Tessler FN, Tublin ME, Ross JS. US case of the day. Granulomatous prostatitis resulting from BCG therapy. Radiographics. 1998 Nov-Dec;18(6):1605-7. | PubMed |
Rifkin MD, Tessler FN, Tublin ME, Ross JS. US case of the day. Granulomatous prostatitis resulting from BCG therapy. Radiographics. 1998 Nov-Dec;18(6):1605-7. | PubMed | Oates RD, Stilmant MM, Freedlund MC, Siroky MB. Granulomatous prostatitis following bacillus Calmette-Guerin immunotherapy of bladder cancer. J Urol. 1988 Oct;140(4):751-4. | PubMed |
Oates RD, Stilmant MM, Freedlund MC, Siroky MB. Granulomatous prostatitis following bacillus Calmette-Guerin immunotherapy of bladder cancer. J Urol. 1988 Oct;140(4):751-4. | PubMed | Oppenheimer JR, Kahane H, Epstein JI. Granulomatous prostatitis on needle biopsy. Arch Pathol Lab Med. 1997 Jul;121(7):724-9. | PubMed |
Oppenheimer JR, Kahane H, Epstein JI. Granulomatous prostatitis on needle biopsy. Arch Pathol Lab Med. 1997 Jul;121(7):724-9. | PubMed | Miyashita H, Troncoso P, Babaian RJ. BCG-induced granulomatous prostatitis: a comparative ultrasound and pathologic study. Urology. 1992 Apr;39(4):364-7. | PubMed |
Miyashita H, Troncoso P, Babaian RJ. BCG-induced granulomatous prostatitis: a comparative ultrasound and pathologic study. Urology. 1992 Apr;39(4):364-7. | PubMed | Kawada H, Kanematsu M, Goshima S, Kondo H, Watanabe H, Noda Y, et al. Multiphase contrast-enhanced magnetic resonance imaging features of Bacillus Calmette-Guérin-induced granulomatous prostatitis in five patients. Korean J Radiol. 2015 Mar-Apr;16(2):342-8. | CrossRef | PubMed |
Kawada H, Kanematsu M, Goshima S, Kondo H, Watanabe H, Noda Y, et al. Multiphase contrast-enhanced magnetic resonance imaging features of Bacillus Calmette-Guérin-induced granulomatous prostatitis in five patients. Korean J Radiol. 2015 Mar-Apr;16(2):342-8. | CrossRef | PubMed | Okusa H, Irie A, Chin I, Baba S, Mitomi H, Shitara T. [Cases of bacillus Calmette-Guerin-induced granulomatous prostatitis and prostatic stromal invasion of the bladder carcinoma in situ, showing similar clinical findings]. Hinyokika Kiyo. 2003 Sep;49(9):555-7. | PubMed |
Okusa H, Irie A, Chin I, Baba S, Mitomi H, Shitara T. [Cases of bacillus Calmette-Guerin-induced granulomatous prostatitis and prostatic stromal invasion of the bladder carcinoma in situ, showing similar clinical findings]. Hinyokika Kiyo. 2003 Sep;49(9):555-7. | PubMed |