Abstract
INTRODUCTION
It is common for terminally ill patients to have a reduced fluid intake, which often results in a need for more medical support. However, it is not clear if this measure has a real clinical impact.
METHODS
To answer this question we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others. We extracted data from the systematic reviews, reanalyzed data from primary studies and generated a summary of findings table using the GRADE approach.
RESULTS AND CONCLUSIONS
We identified four systematic reviews including 51 studies overall, from which three were randomized trials. We concluded the administration of parenteral hydration might make little or no difference in terms of survival and quality of life in terminally ill cancer patients, and that it is not clear whether it has any other benefit because the certainty of the evidence is very low.
Problem
Reduced fluid intake in patients with end-stage cancer is a frequent event. It can be caused by different factors, such as complication of the disease (i.e obstruction due to neoplasia), or other symptoms like nausea or generalized weakness. Therefore, it is common for these patients to develop clinical signs of dehydration.
It is unknown whether parenteral hydration helps prolong life or reduce symptoms in these situation, or if it carries more risks than benefits such as local complications at the puncture site (e.g. erythema, edema, pain), or at systemic level (e.g. edema, congestive heart failure).
Methods
To answer the question, we used Epistemonikos, the largest database of systematic reviews in health, which is maintained by screening multiple information sources, including MEDLINE, EMBASE, Cochrane, among others, to identify systematic reviews and their included primary studies. We extracted data from the identified reviews and reanalyzed data from primary studies included in those reviews. With this information, we generated a structured summary denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos) using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies when it is possible, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found four systematic reviews [1],[2],[3],[4] that included 51 |
|
What types of patients were included* |
All trials included patients with terminal cancer, clinical dehydration and reduced fluid intake. Two trials [5],[6] included patients older than 18 years and one trial [7] did not specify it. |
|
What types of interventions were included* |
Two trials [5],[6] evaluated parenteral hydration with saline (1 liter) SC versus placebo (defined as saline 100 cc SC). One trial [7] evaluated parenteral hydration with 5% dextrose + 140 mEq/L NaCl SC versus no intervention. |
|
What types of outcomes |
The trials measured multiple outcomes, but the identified systematic reviews grouped them as follows: clinical signs of dehydration, thirst, delirium, survival, quality of life and complications. |
* The information about primary studies is extracted from the systematic reviews identified, unless otherwise specified.
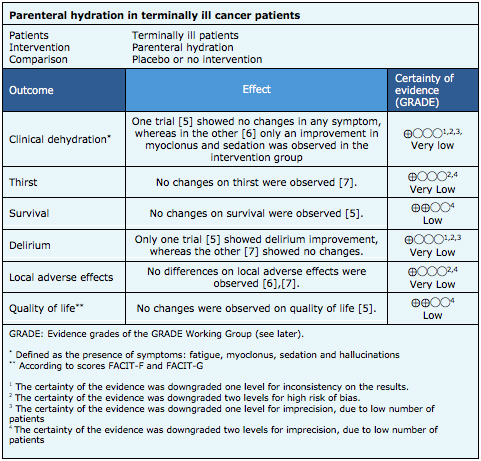
Summary of Findings
The information on the effects of parenteral hydration is based on three randomized trials [5],[6],[7] that included 230 patients.
Two trials [5],[6] reported the outcome clinical dehydration (defined as the following symptoms: fatigue, myoclonus, sedation, hallucinations) (180 patients). One trial [7] reported thirst (50 patients). Two trials [5],[7] (179 patients) evaluated incidence of delirium (according to Memorial Delirium Assessment Scale or MDAS), one trial [5] (129 patients) reported survival (at 17 days) and quality of life (FACIT-F and FACIT-G scores). Two trials [6],[7] (101 patients) measured local adverse effects of the puncture site (pain, edema).
The summary of findings is the following:
- It is not clear if parenteral hydration improves clinical dehydration in terminally ill cancer patients because the certainty of the evidence is very low.
- It is not clear if parenteral hydration improves thirst in terminally ill cancer patients because the certainty of the evidence is very low.
- Parenteral hydration might have little or no effect on survival of terminally ill cancer patients, but the certainty of the evidence is low.
- It is not clear if parenteral hydration prevents delirium in terminally ill cancer patients because the certainty of the evidence is very low
- It is not clear if parenteral hydration leads to greater local adverse effects in terminally ill cancer patients because the certainty of the evidence is very low
- Parenteral hydration may make little or no difference in the quality of life of terminally ill cancer patients, but the certainty of the evidence is low.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| Resource considerations |
|
| What would patients and their doctors think about this intervention |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
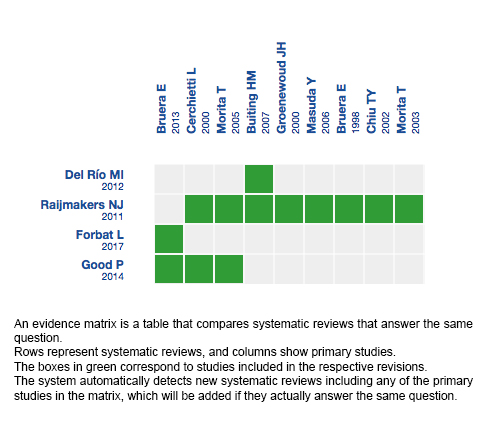
Follow the link to access the interactive version: Parenteral hydration in terminally ill patients
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier.
After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
This article is part of the Epistemonikos Evidence Synthesis project. It is elaborated with a pre-established methodology, following rigorous methodological standards and internal peer review process. Each of these articles corresponds to a summary, denominated FRISBEE (Friendly Summary of Body of Evidence using Epistemonikos), whose main objective is to synthesize the body of evidence for a specific question, with a friendly format to clinical professionals. Its main resources are based on the evidence matrix of Epistemonikos and analysis of results using GRADE methodology. Further details of the methods for developing this FRISBEE are described here (http://dx.doi.org/10.5867/medwave.2014.06.5997)
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
Potential conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

INTRODUCCIÓN
Es común que los pacientes en estado terminal tengan una reducida ingesta de líquidos, lo que muchas veces repercute en una necesidad de mayor apoyo médico. Sin embargo, no está claro si esta medida tiene un impacto clínico real.
MÉTODOS
Para responder esta pregunta utilizamos Epistemonikos, la mayor base de datos de revisiones sistemáticas en salud, la cual es mantenida mediante búsquedas en múltiples fuentes de información, incluyendo MEDLINE, EMBASE, Cochrane, entre otras. Extrajimos los datos desde las revisiones identificadas, reanalizamos los datos de los estudios primarios y preparamos una tabla de resumen de los resultados utilizando el método GRADE.
RESULTADOS Y CONCLUSIONES
Concluimos que la administración de hidratación parenteral podría hacer poca o ninguna diferencia en la sobrevida y en la calidad de vida de los pacientes oncológicos terminales, y que no está claro si tiene algún otro beneficio porque la certeza de la evidencia es muy baja.
 Authors:
José Canihuante[1,2], Pedro Pérez[2,3]
Authors:
José Canihuante[1,2], Pedro Pérez[2,3]
Affiliation:
[1] Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
[2] Proyecto Epistemonikos, Santiago, Chile
[3] Departamento de Medicina Interna, Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
E-mail: peperezc@gmail.com
Author address:
[1] Centro Evidencia UC Pontificia Universidad Católica de Chile Centro de Innovación UC Anacleto Angelini Avda.Vicuña Mackenna 4860 Macul Santiago Chile

Citation: Canihuante J, Pérez P. Is parenteral hydration beneficial in terminally ill cancer patients?. Medwave 2018 Ene-Feb;18(1):e7149 doi: 10.5867/medwave.2018.01.7149
Submission date: 23/11/2017
Acceptance date: 20/12/2017
Publication date: 12/2/2018
Origin: This article is a product of the Evidence Synthesis Project of Epistemonikos Fundation, in collaboration with Medwave for its publication.
Type of review: Non-blinded peer review by members of the methodological team of Epistemonikos Evidence Synthesis Project.

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Raijmakers NJ, van Zuylen L, Costantini M, Caraceni A, Clark J, Lundquist G, Voltz R, Ellershaw JE, van der Heide A; OPCARE9. Artificial nutrition and hydration in the last week of life in cancer patients. A systematic literatura review of practices and effects. Ann Oncol. 2011 Jul;22(7):1478-86. | CrossRef | PubMed |
- Good P, Richard R, Syrmis W, Jenkins-Marsh S, Stephens J. Medically assisted hydration for adult palliative care patients. Cochrane Database Syst Rev. 2014 Apr 23;(4):CD006273. | CrossRef | PubMed |
- Del Río MI, Shand B, Bonati P, Palma A, Maldonado A, Taboada P, Nervi F. Hydration and nutrition at the end of life: a systematic review of emotional impact, perceptions, and decision-making among patients, family, and health care staff. Psychooncology. 2012 Sep;21(9):913-21. | CrossRef | PubMed |
- Forbat L, Kunicki N, Chapman M, Lovell C. How and why are subcutaneous fluids administered in an advanced illness population: a systematic review. J Clin Nurs. 2017 May;26(9-10):1204-1216. | CrossRef | PubMed |
- Bruera E, Hui D, Dalal S, Torres-Vigil I, Trumble J, Roosth J, Krauter S, Strickland C, Unger K, Palmer JL, Allo J, Frisbee-Hume S, Tarleton K. Parenteral hydration in patients with advanced cancer: a multicenter, double-blind, placebo-controlled randomized trial. J Clin Oncol. 2013 Jan 1;31(1):111-8. | CrossRef | PubMed | PMC |
- Bruera E, Sala R, Rico MA, Moyano J, Centeno C, Willey J, Palmer JL. Effects of parenteral hydration in terminally ill cancer patients: a preliminary study. J Clin Oncol. 2005 Apr 1;23(10):2366-71. | PubMed |
- Cerchietti L, Navigante A, Sauri A, Palazzo F. Hypodermoclysis for control of dehydration in terminal-stage cancer. Int J Palliat Nurs. 2000 Sep;6(8):370-4. | PubMed |
- Bosshard G, Nilstun T, Bilsen J, Norup M, Miccinesi G, van Delden JJ, Faisst K, van der Heide A; European End-of-Life Consortium. Forgoing treatment at the end of life in 6 European countries. Arch Intern Med. 2005 Feb 28;165(4):401-7. | PubMed |
- Bruera E, Belzile M, Watanabe S, Fainsinger RL. Volume of hydration in terminal cancer patients. Support Care Cancer. 1996 Mar;4(2):147-50. | PubMed |
- Bruera E, Pruvost M, Schoeller T, Montejo G, Watanabe S. Proctoclysis for hydration of terminally ill cancer patients. J Pain Symptom Manage. 1998 Apr;15(4):216-9. | PubMed |
- Buiting HM, van Delden JJ, Rietjens JA, Onwuteaka-Philipsen BD, Bilsen J, Fischer S, Löfmark R, Miccinesi G, Norup M, van der Heide A; EURELD-Consortium. Forgoing artificial nutrition or hydration in patients nearing death in six European countries. J Pain Symptom Manage. 2007 Sep;34(3):305-14. Epub 2007 Jul 2. | PubMed |
- Byron E, Gastmans C, Diercks de Casterlé B. Decisionmaking about artificial feeding in end-of-life care: literatura review. J Adv Nurs 2008;63:2–14.
- Chiu TY, Hu WY, Chuang RB, Chen CY. Nutrition and hydration for terminal cancer patients in Taiwan. Support Care Cancer. 2002 Nov;10(8):630-6. Epub 2002 Sep 18. | PubMed |
- Chiu TY, Hu WY, Chuang RB, Cheng YR, Chen CY, Wakai S. Terminal cancer patients' wishes and influencing factors toward the provision of artificial nutrition and hydration in Taiwan. J Pain Symptom Manage. 2004 Mar;27(3):206-14. | PubMed |
- Claisse L, Grosshans C & Passadori Y (2005) The use of hypodermoclysis in palliative care. European Journal of Palliative Care 12, 243–246.
- Dasgupta M, Binns MA, Rochon PA. Subcutaneous fluid infusion in a long-term care setting. J Am Geriatr Soc. 2000 Jul;48(7):795-9. | PubMed |
- Fainsinger RL, MacEachern T, Miller MJ, Bruera E, Spachynski K, Kuehn N, Hanson J. The use of hypodermoclysis for rehydration in terminally ill cancer patients. J Pain Symptom Manage. 1994 Jul;9(5):298-302. | PubMed |
- Fins JJ, Miller FG, Acres CA, Bacchetta MD, Huzzard LL, Rapkin BD. End-of-life decision-making in the hospital: current practice and future prospects. J Pain Symptom Manage. 1999 Jan;17(1):6-15. | PubMed |
- Gonçalves JF, Alvarenga M, Silva A. The last forty-eight hours of life in a Portuguese palliative care unit: does it differ from elsewhere? J Palliat Med. 2003 Dec;6(6):895-900. | PubMed |
- Groenewoud JH, van der Heide A, Kester JG, de Graaff CL, van der Wal G, van der Maas PJ. A nationwide study of decisions to forego life-prolonging treatment in Dutch medical practice. Arch Intern Med. 2000 Feb 14;160(3):357-63. | PubMed |
- Hanson LC, Garrett JM, Lewis C, Phifer N, Jackman A, Carey TS. Physicians' expectations of benefit from tube feeding. J Palliat Med. 2008 Oct;11(8):1130-4. | CrossRef | PubMed | PMC |
- Hawkins C. Anorexia and anxiety in advanced malignancy: the relative problem. J Hum Nutr Diet 2000;13:113–117.
- Holden CM. Anorexia in the terminally ill cancer patient: the emotional impact on the patient and the family. Hosp J. 1991;7(3):73-84. | PubMed |
- Lanuke K, Fainsinger RL. Hydration management in palliative care settings--a survey of experts. J Palliat Care. 2003 Winter;19(4):278-9. | PubMed |
- Lanuke K, Fainsinger RL, DeMoissac D. Hydration management at the end of life. J Palliat Med. 2004 Apr;7(2):257-63. | PubMed |
- Ke LS, Chiu TY, Lo SS, Hu WY. Knowledge, attitudes, and behavioral intentions of nurses toward providing artificial nutrition and hydration for terminal cáncer patients in Taiwan. Cancer Nurs. 2008 Jan-Feb;31(1):67-76. | CrossRef | PubMed |
- Masuda Y, Noguchi H, Kuzuya M, Inoue A, Hirakawa Y, Iguchi A, Uemura K. Comparison of medical treatments for the dying in a hospice and a geriatric hospital in Japan. J Palliat Med. 2006 Feb;9(1):152-60. | PubMed |
- McClement SE, Degner LF, Harlos MS. Family beliefs regarding the nutritional care of a terminally ill relative: a qualitative study. J Palliat Med. 2003 Oct;6(5):737-48. | PubMed |
- McClement SE, Degner LF, Harlos M. Family responses to declining intake and weight loss in a terminally ill relative. Part 1: fighting back. J Palliat Care. 2004 Summer;20(2):93-100. | PubMed |
- Meares CJ. Primary caregiver perceptions of intake cessation in patients who are terminally Ill. Oncol Nurs Forum. 1997 Nov-Dec;24(10):1751-7. | PubMed |
- Mercadante S, Ferrera P, Girelli D, Casuccio A. Patients' and relatives' perceptions about intravenous and subcutaneous hydration. J Pain Symptom Manage. 2005 Oct;30(4):354-8. | PubMed |
- Miyashita M, Morita T, Shima Y, Kimura R, Takahashi M, Adachi I. Nurse views of the adequacy of decision making and nurse distress regarding artificial hydration for terminally ill cancer patients: a nationwide survey. Am J Hosp Palliat Care. 2007 Dec-2008 Jan;24(6):463-9. Epub 2007 Jun 29. | PubMed |
- Morita T, Hyodo I, Yoshimi T, Ikenaga M, Tamura Y, Yoshizawa A, Shimada A, Akechi T, Miyashita M, Adachi I; Japan Palliative Oncology Study Group. Association between hydration volume and symptoms in terminally ill cáncer patients with abdominal malignancies. Ann Oncol. 2005 Apr;16(4):640-7. Epub 2005 Jan 31. | PubMed |
- Morita T, Hyodo I, Yoshimi T, Ikenaga M, Tamura Y, Yoshizawa A, Shimada A, Akechi T, Miyashita M, Adachi I; Japan Palliative Oncology Study Group. Artificial hydration therapy, laboratory findings, and fluid balance in terminally ill patients with abdominal malignancies. J Pain Symptom Manage. 2006 Feb;31(2):130-9. | PubMed |
- Morita T, Tei Y, Inoue S. Agitated terminal delirium and association with partial opioid substitution and hydration. J Palliat Med. 2003 Aug;6(4):557-63. | PubMed |
- Morita T, Shima Y, Adachi I; Japan Palliative Oncology Study Group. Attitudes of Japanese physicians toward terminal dehydration: a nationwide survey. J Clin Oncol. 2002 Dec 15;20(24):4699-704. | PubMed |
- Morita T, Tsunoda J, Inoue S, Chihara S. Perceptions and decision-making on rehydration of terminally ill cancer patients and family members. Am J Hosp Palliat Care. 1999 May-Jun;16(3):509-16. | PubMed |
- Musgrave CF, Bartal N, Opstad J. Intravenous hydration for terminal patients: what are the attitudes of Israeli terminal patients, their families, and their health professionals? J Pain Symptom Manage. 1996 Jul;12(1):47-51. | PubMed |
- Oh DY, Kim JH, Lee SH, Kim DW, Im SA, Kim TY, Heo DS, Bang YJ, Kim NK. Artificial nutrition and hydration in terminal cancer patients: the real and the ideal. Support Care Cancer. 2007 Jun;15(6):631-6. Epub 2006 Nov 11. | PubMed |
- Oi-Ling K, Man-Wah DT, Kam-Hung DN. Symptom distress as rated by advanced cancer patients, caregivers and physicians in the last week of life. Palliat Med. 2005 Apr;19(3):228-33. | PubMed |
- Orrevall Y, Tishelman C, Herrington MK, Permert J. The path from oral nutrition to home parenteral nutrition: a qualitative interview study of the experiences of advanced cancer patients and their families. Clin Nutr. 2004Dec;23(6):1280-7. | PubMed |
- Parkash R, Burge F. The family's perspective on issues of hydration in terminal care. J Palliat Care. 1997 Winter;13(4):23-7. | PubMed |
- Roeline H, Pasman W, Mei The A, Onwuteaka-Philipsen B, Ribbe M, Van der Wal G. Participants in the decisión making on artificial nutrition and hydration to demented nursing home patients: a qualitative study. J Aging Stud 2004;18:321–335.
- Poole K, Froggatt K. Loss of weight and loss of appetite in advanced cancer: a problem for the patient, the carer, or the health professional? Palliat Med. 2002 Nov;16(6):499-506. | PubMed |
- Sato K, Miyashita M, Morita T, Sanjo M, Shima Y, Uchitomi Y. Quality of end-of-life treatment for cancer patients in general wards and the palliative care unit at a regional cancer center in Japan: a retrospective chart review. Support Care Cancer. 2008 Feb;16(2):113-22. Epub 2007 Oct 5. | PubMed |
- Shega JW, Hougham GW, Cox-Hayley D, Sachs GA, Stocking CB. Advanced dementia and feeding tubes: do physician factors contribute to state variation? J Am Geriatr Soc. 2004 Jul;52(7):1217-8. | PubMed |
- Shega JW, Hougham GW, Stocking CB, Cox-Hayley D, Sachs GA. Barriers to limiting the practice of feeding tube placement in advanced dementia. J Palliat Med. 2003 Dec;6(6):885-93. | PubMed |
- Shragge JE, Wismer WV, Olson KL, Baracos VE. Shifting to conscious control: psychosocial and dietary management of anorexia by patients with advanced cancer. Palliat Med. 2007 Apr;21(3):227-33. | PubMed |
- Sprung CL, Maia P, Bulow HH, Ricou B, Armaganidis A, Baras M, Wennberg E, Reinhart K, Cohen SL, Fries DR, Nakos G, Thijs LG; Ethicus Study Group. The importance of religious affiliation and culture on end-of-life decisions in European intensive care units. Intensive Care Med. 2007 Oct;33(10):1732-9. Erratum in: Intensive Care Med. 2007 Oct;33(10):1859. | PubMed |
- Strasser F, Binswanger J, Cerny T, Kesselring A. Fighting a losing battle: eating-related distress of men with advanced cancer and their female partners. A mixed-methods study. Palliat Med. 2007 Mar;21(2):129-37. | PubMed |
- van der Riet P, Good P, Higgins I, Sneesby L. Palliative care professionals' perceptions of nutrition and hydration at the end of life. Int J Palliat Nurs. 2008 Mar;14(3):145-51. | PubMed |
- van Wigcheren PT, Onwuteaka-Philipsen BD, Pasman HR, Ooms ME, Ribbe MW, van der Wal G. Starting artificial nutrition and hydration in patients with dementia in The Netherlands: frequencies, patient characteristics and decision-making process. Aging Clin Exp Res. 2007 Feb;19(1):26-33. | PubMed |
- Viola RA. Studying Fluid Status and the Dying: The Challenge of Clinical Research in Palliative Care [MSc thesis]. Canada: University of Ottawa, 1997.
- Waller A, Hershkowitz M, Adunsky A. The effect of intravenous fluid infusion on blood and urine parameters of hydration and on state of consciousness in terminal cancer patients. Am J Hosp Palliat Care. 1994 Nov-Dec;11(6):22-7. | PubMed |
- Worobec G, Brown MK. Hypodermoclysis therapy. In a chronic care hospital setting. J Gerontol Nurs. 1997 Jun;23(6):23-8. | PubMed |
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Palliative Care 2017 [cited November 12, 2017].
 Raijmakers NJ, van Zuylen L, Costantini M, Caraceni A, Clark J, Lundquist G, Voltz R, Ellershaw JE, van der Heide A; OPCARE9. Artificial nutrition and hydration in the last week of life in cancer patients. A systematic literatura review of practices and effects. Ann Oncol. 2011 Jul;22(7):1478-86. | CrossRef | PubMed |
Raijmakers NJ, van Zuylen L, Costantini M, Caraceni A, Clark J, Lundquist G, Voltz R, Ellershaw JE, van der Heide A; OPCARE9. Artificial nutrition and hydration in the last week of life in cancer patients. A systematic literatura review of practices and effects. Ann Oncol. 2011 Jul;22(7):1478-86. | CrossRef | PubMed | Good P, Richard R, Syrmis W, Jenkins-Marsh S, Stephens J. Medically assisted hydration for adult palliative care patients. Cochrane Database Syst Rev. 2014 Apr 23;(4):CD006273. | CrossRef | PubMed |
Good P, Richard R, Syrmis W, Jenkins-Marsh S, Stephens J. Medically assisted hydration for adult palliative care patients. Cochrane Database Syst Rev. 2014 Apr 23;(4):CD006273. | CrossRef | PubMed | Del Río MI, Shand B, Bonati P, Palma A, Maldonado A, Taboada P, Nervi F. Hydration and nutrition at the end of life: a systematic review of emotional impact, perceptions, and decision-making among patients, family, and health care staff. Psychooncology. 2012 Sep;21(9):913-21. | CrossRef | PubMed |
Del Río MI, Shand B, Bonati P, Palma A, Maldonado A, Taboada P, Nervi F. Hydration and nutrition at the end of life: a systematic review of emotional impact, perceptions, and decision-making among patients, family, and health care staff. Psychooncology. 2012 Sep;21(9):913-21. | CrossRef | PubMed | Forbat L, Kunicki N, Chapman M, Lovell C. How and why are subcutaneous fluids administered in an advanced illness population: a systematic review. J Clin Nurs. 2017 May;26(9-10):1204-1216. | CrossRef | PubMed |
Forbat L, Kunicki N, Chapman M, Lovell C. How and why are subcutaneous fluids administered in an advanced illness population: a systematic review. J Clin Nurs. 2017 May;26(9-10):1204-1216. | CrossRef | PubMed | Bruera E, Hui D, Dalal S, Torres-Vigil I, Trumble J, Roosth J, Krauter S, Strickland C, Unger K, Palmer JL, Allo J, Frisbee-Hume S, Tarleton K. Parenteral hydration in patients with advanced cancer: a multicenter, double-blind, placebo-controlled randomized trial. J Clin Oncol. 2013 Jan 1;31(1):111-8. | CrossRef | PubMed | PMC |
Bruera E, Hui D, Dalal S, Torres-Vigil I, Trumble J, Roosth J, Krauter S, Strickland C, Unger K, Palmer JL, Allo J, Frisbee-Hume S, Tarleton K. Parenteral hydration in patients with advanced cancer: a multicenter, double-blind, placebo-controlled randomized trial. J Clin Oncol. 2013 Jan 1;31(1):111-8. | CrossRef | PubMed | PMC | Bruera E, Sala R, Rico MA, Moyano J, Centeno C, Willey J, Palmer JL. Effects of parenteral hydration in terminally ill cancer patients: a preliminary study. J Clin Oncol. 2005 Apr 1;23(10):2366-71. | PubMed |
Bruera E, Sala R, Rico MA, Moyano J, Centeno C, Willey J, Palmer JL. Effects of parenteral hydration in terminally ill cancer patients: a preliminary study. J Clin Oncol. 2005 Apr 1;23(10):2366-71. | PubMed | Cerchietti L, Navigante A, Sauri A, Palazzo F. Hypodermoclysis for control of dehydration in terminal-stage cancer. Int J Palliat Nurs. 2000 Sep;6(8):370-4. | PubMed |
Cerchietti L, Navigante A, Sauri A, Palazzo F. Hypodermoclysis for control of dehydration in terminal-stage cancer. Int J Palliat Nurs. 2000 Sep;6(8):370-4. | PubMed | Bosshard G, Nilstun T, Bilsen J, Norup M, Miccinesi G, van Delden JJ, Faisst K, van der Heide A; European End-of-Life Consortium. Forgoing treatment at the end of life in 6 European countries. Arch Intern Med. 2005 Feb 28;165(4):401-7. | PubMed |
Bosshard G, Nilstun T, Bilsen J, Norup M, Miccinesi G, van Delden JJ, Faisst K, van der Heide A; European End-of-Life Consortium. Forgoing treatment at the end of life in 6 European countries. Arch Intern Med. 2005 Feb 28;165(4):401-7. | PubMed | Bruera E, Belzile M, Watanabe S, Fainsinger RL. Volume of hydration in terminal cancer patients. Support Care Cancer. 1996 Mar;4(2):147-50. | PubMed |
Bruera E, Belzile M, Watanabe S, Fainsinger RL. Volume of hydration in terminal cancer patients. Support Care Cancer. 1996 Mar;4(2):147-50. | PubMed | Bruera E, Pruvost M, Schoeller T, Montejo G, Watanabe S. Proctoclysis for hydration of terminally ill cancer patients. J Pain Symptom Manage. 1998 Apr;15(4):216-9. | PubMed |
Bruera E, Pruvost M, Schoeller T, Montejo G, Watanabe S. Proctoclysis for hydration of terminally ill cancer patients. J Pain Symptom Manage. 1998 Apr;15(4):216-9. | PubMed | Buiting HM, van Delden JJ, Rietjens JA, Onwuteaka-Philipsen BD, Bilsen J, Fischer S, Löfmark R, Miccinesi G, Norup M, van der Heide A; EURELD-Consortium. Forgoing artificial nutrition or hydration in patients nearing death in six European countries. J Pain Symptom Manage. 2007 Sep;34(3):305-14. Epub 2007 Jul 2. | PubMed |
Buiting HM, van Delden JJ, Rietjens JA, Onwuteaka-Philipsen BD, Bilsen J, Fischer S, Löfmark R, Miccinesi G, Norup M, van der Heide A; EURELD-Consortium. Forgoing artificial nutrition or hydration in patients nearing death in six European countries. J Pain Symptom Manage. 2007 Sep;34(3):305-14. Epub 2007 Jul 2. | PubMed | Byron E, Gastmans C, Diercks de Casterlé B. Decisionmaking about artificial feeding in end-of-life care: literatura review. J Adv Nurs 2008;63:2–14.
Byron E, Gastmans C, Diercks de Casterlé B. Decisionmaking about artificial feeding in end-of-life care: literatura review. J Adv Nurs 2008;63:2–14.  Chiu TY, Hu WY, Chuang RB, Chen CY. Nutrition and hydration for terminal cancer patients in Taiwan. Support Care Cancer. 2002 Nov;10(8):630-6. Epub 2002 Sep 18. | PubMed |
Chiu TY, Hu WY, Chuang RB, Chen CY. Nutrition and hydration for terminal cancer patients in Taiwan. Support Care Cancer. 2002 Nov;10(8):630-6. Epub 2002 Sep 18. | PubMed | Chiu TY, Hu WY, Chuang RB, Cheng YR, Chen CY, Wakai S. Terminal cancer patients' wishes and influencing factors toward the provision of artificial nutrition and hydration in Taiwan. J Pain Symptom Manage. 2004 Mar;27(3):206-14. | PubMed |
Chiu TY, Hu WY, Chuang RB, Cheng YR, Chen CY, Wakai S. Terminal cancer patients' wishes and influencing factors toward the provision of artificial nutrition and hydration in Taiwan. J Pain Symptom Manage. 2004 Mar;27(3):206-14. | PubMed | Claisse L, Grosshans C & Passadori Y (2005) The use of hypodermoclysis in palliative care. European Journal of Palliative Care 12, 243–246.
Claisse L, Grosshans C & Passadori Y (2005) The use of hypodermoclysis in palliative care. European Journal of Palliative Care 12, 243–246.  Dasgupta M, Binns MA, Rochon PA. Subcutaneous fluid infusion in a long-term care setting. J Am Geriatr Soc. 2000 Jul;48(7):795-9. | PubMed |
Dasgupta M, Binns MA, Rochon PA. Subcutaneous fluid infusion in a long-term care setting. J Am Geriatr Soc. 2000 Jul;48(7):795-9. | PubMed | Fainsinger RL, MacEachern T, Miller MJ, Bruera E, Spachynski K, Kuehn N, Hanson J. The use of hypodermoclysis for rehydration in terminally ill cancer patients. J Pain Symptom Manage. 1994 Jul;9(5):298-302. | PubMed |
Fainsinger RL, MacEachern T, Miller MJ, Bruera E, Spachynski K, Kuehn N, Hanson J. The use of hypodermoclysis for rehydration in terminally ill cancer patients. J Pain Symptom Manage. 1994 Jul;9(5):298-302. | PubMed | Fins JJ, Miller FG, Acres CA, Bacchetta MD, Huzzard LL, Rapkin BD. End-of-life decision-making in the hospital: current practice and future prospects. J Pain Symptom Manage. 1999 Jan;17(1):6-15. | PubMed |
Fins JJ, Miller FG, Acres CA, Bacchetta MD, Huzzard LL, Rapkin BD. End-of-life decision-making in the hospital: current practice and future prospects. J Pain Symptom Manage. 1999 Jan;17(1):6-15. | PubMed | Gonçalves JF, Alvarenga M, Silva A. The last forty-eight hours of life in a Portuguese palliative care unit: does it differ from elsewhere? J Palliat Med. 2003 Dec;6(6):895-900. | PubMed |
Gonçalves JF, Alvarenga M, Silva A. The last forty-eight hours of life in a Portuguese palliative care unit: does it differ from elsewhere? J Palliat Med. 2003 Dec;6(6):895-900. | PubMed | Groenewoud JH, van der Heide A, Kester JG, de Graaff CL, van der Wal G, van der Maas PJ. A nationwide study of decisions to forego life-prolonging treatment in Dutch medical practice. Arch Intern Med. 2000 Feb 14;160(3):357-63. | PubMed |
Groenewoud JH, van der Heide A, Kester JG, de Graaff CL, van der Wal G, van der Maas PJ. A nationwide study of decisions to forego life-prolonging treatment in Dutch medical practice. Arch Intern Med. 2000 Feb 14;160(3):357-63. | PubMed | Hanson LC, Garrett JM, Lewis C, Phifer N, Jackman A, Carey TS. Physicians' expectations of benefit from tube feeding. J Palliat Med. 2008 Oct;11(8):1130-4. | CrossRef | PubMed | PMC |
Hanson LC, Garrett JM, Lewis C, Phifer N, Jackman A, Carey TS. Physicians' expectations of benefit from tube feeding. J Palliat Med. 2008 Oct;11(8):1130-4. | CrossRef | PubMed | PMC | Hawkins C. Anorexia and anxiety in advanced malignancy: the relative problem. J Hum Nutr Diet 2000;13:113–117.
Hawkins C. Anorexia and anxiety in advanced malignancy: the relative problem. J Hum Nutr Diet 2000;13:113–117.  Holden CM. Anorexia in the terminally ill cancer patient: the emotional impact on the patient and the family. Hosp J. 1991;7(3):73-84. | PubMed |
Holden CM. Anorexia in the terminally ill cancer patient: the emotional impact on the patient and the family. Hosp J. 1991;7(3):73-84. | PubMed | Lanuke K, Fainsinger RL. Hydration management in palliative care settings--a survey of experts. J Palliat Care. 2003 Winter;19(4):278-9. | PubMed |
Lanuke K, Fainsinger RL. Hydration management in palliative care settings--a survey of experts. J Palliat Care. 2003 Winter;19(4):278-9. | PubMed | Lanuke K, Fainsinger RL, DeMoissac D. Hydration management at the end of life. J Palliat Med. 2004 Apr;7(2):257-63. | PubMed |
Lanuke K, Fainsinger RL, DeMoissac D. Hydration management at the end of life. J Palliat Med. 2004 Apr;7(2):257-63. | PubMed | Ke LS, Chiu TY, Lo SS, Hu WY. Knowledge, attitudes, and behavioral intentions of nurses toward providing artificial nutrition and hydration for terminal cáncer patients in Taiwan. Cancer Nurs. 2008 Jan-Feb;31(1):67-76. | CrossRef | PubMed |
Ke LS, Chiu TY, Lo SS, Hu WY. Knowledge, attitudes, and behavioral intentions of nurses toward providing artificial nutrition and hydration for terminal cáncer patients in Taiwan. Cancer Nurs. 2008 Jan-Feb;31(1):67-76. | CrossRef | PubMed | Masuda Y, Noguchi H, Kuzuya M, Inoue A, Hirakawa Y, Iguchi A, Uemura K. Comparison of medical treatments for the dying in a hospice and a geriatric hospital in Japan. J Palliat Med. 2006 Feb;9(1):152-60. | PubMed |
Masuda Y, Noguchi H, Kuzuya M, Inoue A, Hirakawa Y, Iguchi A, Uemura K. Comparison of medical treatments for the dying in a hospice and a geriatric hospital in Japan. J Palliat Med. 2006 Feb;9(1):152-60. | PubMed | McClement SE, Degner LF, Harlos MS. Family beliefs regarding the nutritional care of a terminally ill relative: a qualitative study. J Palliat Med. 2003 Oct;6(5):737-48. | PubMed |
McClement SE, Degner LF, Harlos MS. Family beliefs regarding the nutritional care of a terminally ill relative: a qualitative study. J Palliat Med. 2003 Oct;6(5):737-48. | PubMed | McClement SE, Degner LF, Harlos M. Family responses to declining intake and weight loss in a terminally ill relative. Part 1: fighting back. J Palliat Care. 2004 Summer;20(2):93-100. | PubMed |
McClement SE, Degner LF, Harlos M. Family responses to declining intake and weight loss in a terminally ill relative. Part 1: fighting back. J Palliat Care. 2004 Summer;20(2):93-100. | PubMed | Meares CJ. Primary caregiver perceptions of intake cessation in patients who are terminally Ill. Oncol Nurs Forum. 1997 Nov-Dec;24(10):1751-7. | PubMed |
Meares CJ. Primary caregiver perceptions of intake cessation in patients who are terminally Ill. Oncol Nurs Forum. 1997 Nov-Dec;24(10):1751-7. | PubMed | Mercadante S, Ferrera P, Girelli D, Casuccio A. Patients' and relatives' perceptions about intravenous and subcutaneous hydration. J Pain Symptom Manage. 2005 Oct;30(4):354-8. | PubMed |
Mercadante S, Ferrera P, Girelli D, Casuccio A. Patients' and relatives' perceptions about intravenous and subcutaneous hydration. J Pain Symptom Manage. 2005 Oct;30(4):354-8. | PubMed | Miyashita M, Morita T, Shima Y, Kimura R, Takahashi M, Adachi I. Nurse views of the adequacy of decision making and nurse distress regarding artificial hydration for terminally ill cancer patients: a nationwide survey. Am J Hosp Palliat Care. 2007 Dec-2008 Jan;24(6):463-9. Epub 2007 Jun 29. | PubMed |
Miyashita M, Morita T, Shima Y, Kimura R, Takahashi M, Adachi I. Nurse views of the adequacy of decision making and nurse distress regarding artificial hydration for terminally ill cancer patients: a nationwide survey. Am J Hosp Palliat Care. 2007 Dec-2008 Jan;24(6):463-9. Epub 2007 Jun 29. | PubMed | Morita T, Hyodo I, Yoshimi T, Ikenaga M, Tamura Y, Yoshizawa A, Shimada A, Akechi T, Miyashita M, Adachi I; Japan Palliative Oncology Study Group. Association between hydration volume and symptoms in terminally ill cáncer patients with abdominal malignancies. Ann Oncol. 2005 Apr;16(4):640-7. Epub 2005 Jan 31. | PubMed |
Morita T, Hyodo I, Yoshimi T, Ikenaga M, Tamura Y, Yoshizawa A, Shimada A, Akechi T, Miyashita M, Adachi I; Japan Palliative Oncology Study Group. Association between hydration volume and symptoms in terminally ill cáncer patients with abdominal malignancies. Ann Oncol. 2005 Apr;16(4):640-7. Epub 2005 Jan 31. | PubMed | Morita T, Hyodo I, Yoshimi T, Ikenaga M, Tamura Y, Yoshizawa A, Shimada A, Akechi T, Miyashita M, Adachi I; Japan Palliative Oncology Study Group. Artificial hydration therapy, laboratory findings, and fluid balance in terminally ill patients with abdominal malignancies. J Pain Symptom Manage. 2006 Feb;31(2):130-9. | PubMed |
Morita T, Hyodo I, Yoshimi T, Ikenaga M, Tamura Y, Yoshizawa A, Shimada A, Akechi T, Miyashita M, Adachi I; Japan Palliative Oncology Study Group. Artificial hydration therapy, laboratory findings, and fluid balance in terminally ill patients with abdominal malignancies. J Pain Symptom Manage. 2006 Feb;31(2):130-9. | PubMed | Morita T, Tei Y, Inoue S. Agitated terminal delirium and association with partial opioid substitution and hydration. J Palliat Med. 2003 Aug;6(4):557-63. | PubMed |
Morita T, Tei Y, Inoue S. Agitated terminal delirium and association with partial opioid substitution and hydration. J Palliat Med. 2003 Aug;6(4):557-63. | PubMed | Morita T, Shima Y, Adachi I; Japan Palliative Oncology Study Group. Attitudes of Japanese physicians toward terminal dehydration: a nationwide survey. J Clin Oncol. 2002 Dec 15;20(24):4699-704. | PubMed |
Morita T, Shima Y, Adachi I; Japan Palliative Oncology Study Group. Attitudes of Japanese physicians toward terminal dehydration: a nationwide survey. J Clin Oncol. 2002 Dec 15;20(24):4699-704. | PubMed | Morita T, Tsunoda J, Inoue S, Chihara S. Perceptions and decision-making on rehydration of terminally ill cancer patients and family members. Am J Hosp Palliat Care. 1999 May-Jun;16(3):509-16. | PubMed |
Morita T, Tsunoda J, Inoue S, Chihara S. Perceptions and decision-making on rehydration of terminally ill cancer patients and family members. Am J Hosp Palliat Care. 1999 May-Jun;16(3):509-16. | PubMed | Musgrave CF, Bartal N, Opstad J. Intravenous hydration for terminal patients: what are the attitudes of Israeli terminal patients, their families, and their health professionals? J Pain Symptom Manage. 1996 Jul;12(1):47-51. | PubMed |
Musgrave CF, Bartal N, Opstad J. Intravenous hydration for terminal patients: what are the attitudes of Israeli terminal patients, their families, and their health professionals? J Pain Symptom Manage. 1996 Jul;12(1):47-51. | PubMed | Oh DY, Kim JH, Lee SH, Kim DW, Im SA, Kim TY, Heo DS, Bang YJ, Kim NK. Artificial nutrition and hydration in terminal cancer patients: the real and the ideal. Support Care Cancer. 2007 Jun;15(6):631-6. Epub 2006 Nov 11. | PubMed |
Oh DY, Kim JH, Lee SH, Kim DW, Im SA, Kim TY, Heo DS, Bang YJ, Kim NK. Artificial nutrition and hydration in terminal cancer patients: the real and the ideal. Support Care Cancer. 2007 Jun;15(6):631-6. Epub 2006 Nov 11. | PubMed | Oi-Ling K, Man-Wah DT, Kam-Hung DN. Symptom distress as rated by advanced cancer patients, caregivers and physicians in the last week of life. Palliat Med. 2005 Apr;19(3):228-33. | PubMed |
Oi-Ling K, Man-Wah DT, Kam-Hung DN. Symptom distress as rated by advanced cancer patients, caregivers and physicians in the last week of life. Palliat Med. 2005 Apr;19(3):228-33. | PubMed | Orrevall Y, Tishelman C, Herrington MK, Permert J. The path from oral nutrition to home parenteral nutrition: a qualitative interview study of the experiences of advanced cancer patients and their families. Clin Nutr. 2004Dec;23(6):1280-7. | PubMed |
Orrevall Y, Tishelman C, Herrington MK, Permert J. The path from oral nutrition to home parenteral nutrition: a qualitative interview study of the experiences of advanced cancer patients and their families. Clin Nutr. 2004Dec;23(6):1280-7. | PubMed | Parkash R, Burge F. The family's perspective on issues of hydration in terminal care. J Palliat Care. 1997 Winter;13(4):23-7. | PubMed |
Parkash R, Burge F. The family's perspective on issues of hydration in terminal care. J Palliat Care. 1997 Winter;13(4):23-7. | PubMed | Roeline H, Pasman W, Mei The A, Onwuteaka-Philipsen B, Ribbe M, Van der Wal G. Participants in the decisión making on artificial nutrition and hydration to demented nursing home patients: a qualitative study. J Aging Stud 2004;18:321–335.
Roeline H, Pasman W, Mei The A, Onwuteaka-Philipsen B, Ribbe M, Van der Wal G. Participants in the decisión making on artificial nutrition and hydration to demented nursing home patients: a qualitative study. J Aging Stud 2004;18:321–335.  Poole K, Froggatt K. Loss of weight and loss of appetite in advanced cancer: a problem for the patient, the carer, or the health professional? Palliat Med. 2002 Nov;16(6):499-506. | PubMed |
Poole K, Froggatt K. Loss of weight and loss of appetite in advanced cancer: a problem for the patient, the carer, or the health professional? Palliat Med. 2002 Nov;16(6):499-506. | PubMed | Sato K, Miyashita M, Morita T, Sanjo M, Shima Y, Uchitomi Y. Quality of end-of-life treatment for cancer patients in general wards and the palliative care unit at a regional cancer center in Japan: a retrospective chart review. Support Care Cancer. 2008 Feb;16(2):113-22. Epub 2007 Oct 5. | PubMed |
Sato K, Miyashita M, Morita T, Sanjo M, Shima Y, Uchitomi Y. Quality of end-of-life treatment for cancer patients in general wards and the palliative care unit at a regional cancer center in Japan: a retrospective chart review. Support Care Cancer. 2008 Feb;16(2):113-22. Epub 2007 Oct 5. | PubMed | Shega JW, Hougham GW, Cox-Hayley D, Sachs GA, Stocking CB. Advanced dementia and feeding tubes: do physician factors contribute to state variation? J Am Geriatr Soc. 2004 Jul;52(7):1217-8. | PubMed |
Shega JW, Hougham GW, Cox-Hayley D, Sachs GA, Stocking CB. Advanced dementia and feeding tubes: do physician factors contribute to state variation? J Am Geriatr Soc. 2004 Jul;52(7):1217-8. | PubMed | Shega JW, Hougham GW, Stocking CB, Cox-Hayley D, Sachs GA. Barriers to limiting the practice of feeding tube placement in advanced dementia. J Palliat Med. 2003 Dec;6(6):885-93. | PubMed |
Shega JW, Hougham GW, Stocking CB, Cox-Hayley D, Sachs GA. Barriers to limiting the practice of feeding tube placement in advanced dementia. J Palliat Med. 2003 Dec;6(6):885-93. | PubMed | Shragge JE, Wismer WV, Olson KL, Baracos VE. Shifting to conscious control: psychosocial and dietary management of anorexia by patients with advanced cancer. Palliat Med. 2007 Apr;21(3):227-33. | PubMed |
Shragge JE, Wismer WV, Olson KL, Baracos VE. Shifting to conscious control: psychosocial and dietary management of anorexia by patients with advanced cancer. Palliat Med. 2007 Apr;21(3):227-33. | PubMed | Sprung CL, Maia P, Bulow HH, Ricou B, Armaganidis A, Baras M, Wennberg E, Reinhart K, Cohen SL, Fries DR, Nakos G, Thijs LG; Ethicus Study Group. The importance of religious affiliation and culture on end-of-life decisions in European intensive care units. Intensive Care Med. 2007 Oct;33(10):1732-9. Erratum in: Intensive Care Med. 2007 Oct;33(10):1859. | PubMed |
Sprung CL, Maia P, Bulow HH, Ricou B, Armaganidis A, Baras M, Wennberg E, Reinhart K, Cohen SL, Fries DR, Nakos G, Thijs LG; Ethicus Study Group. The importance of religious affiliation and culture on end-of-life decisions in European intensive care units. Intensive Care Med. 2007 Oct;33(10):1732-9. Erratum in: Intensive Care Med. 2007 Oct;33(10):1859. | PubMed | Strasser F, Binswanger J, Cerny T, Kesselring A. Fighting a losing battle: eating-related distress of men with advanced cancer and their female partners. A mixed-methods study. Palliat Med. 2007 Mar;21(2):129-37. | PubMed |
Strasser F, Binswanger J, Cerny T, Kesselring A. Fighting a losing battle: eating-related distress of men with advanced cancer and their female partners. A mixed-methods study. Palliat Med. 2007 Mar;21(2):129-37. | PubMed | van der Riet P, Good P, Higgins I, Sneesby L. Palliative care professionals' perceptions of nutrition and hydration at the end of life. Int J Palliat Nurs. 2008 Mar;14(3):145-51. | PubMed |
van der Riet P, Good P, Higgins I, Sneesby L. Palliative care professionals' perceptions of nutrition and hydration at the end of life. Int J Palliat Nurs. 2008 Mar;14(3):145-51. | PubMed | van Wigcheren PT, Onwuteaka-Philipsen BD, Pasman HR, Ooms ME, Ribbe MW, van der Wal G. Starting artificial nutrition and hydration in patients with dementia in The Netherlands: frequencies, patient characteristics and decision-making process. Aging Clin Exp Res. 2007 Feb;19(1):26-33. | PubMed |
van Wigcheren PT, Onwuteaka-Philipsen BD, Pasman HR, Ooms ME, Ribbe MW, van der Wal G. Starting artificial nutrition and hydration in patients with dementia in The Netherlands: frequencies, patient characteristics and decision-making process. Aging Clin Exp Res. 2007 Feb;19(1):26-33. | PubMed | Viola RA. Studying Fluid Status and the Dying: The Challenge of Clinical Research in Palliative Care [MSc thesis]. Canada: University of Ottawa, 1997.
Viola RA. Studying Fluid Status and the Dying: The Challenge of Clinical Research in Palliative Care [MSc thesis]. Canada: University of Ottawa, 1997.  Waller A, Hershkowitz M, Adunsky A. The effect of intravenous fluid infusion on blood and urine parameters of hydration and on state of consciousness in terminal cancer patients. Am J Hosp Palliat Care. 1994 Nov-Dec;11(6):22-7. | PubMed |
Waller A, Hershkowitz M, Adunsky A. The effect of intravenous fluid infusion on blood and urine parameters of hydration and on state of consciousness in terminal cancer patients. Am J Hosp Palliat Care. 1994 Nov-Dec;11(6):22-7. | PubMed | Worobec G, Brown MK. Hypodermoclysis therapy. In a chronic care hospital setting. J Gerontol Nurs. 1997 Jun;23(6):23-8. | PubMed |
Worobec G, Brown MK. Hypodermoclysis therapy. In a chronic care hospital setting. J Gerontol Nurs. 1997 Jun;23(6):23-8. | PubMed | National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Palliative Care 2017 [cited November 12, 2017].
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Palliative Care 2017 [cited November 12, 2017]. Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








