Abstract
Idiopathic pulmonary fibrosis has an ominous prognosis and there are virtually no effective therapies. It has been suggested that pirfenidone, an antifibrotic agent, could change its course. Searching in Epistemonikos database, which is maintained by screening multiple databases, we identified 13 systematic reviews comprising nine trials addressing the question of this article, seven of which are randomized and whose results were analyzed in this summary. We combined the evidence using meta-analysis and generated a summary of findings following the GRADE approach. We concluded pirfenidone decreases disease progression and mortality in idiopathic pulmonary fibrosis. Although it is associated with frequent gastrointestinal and cutaneous adverse effects, these are generally not severe.
Problem
Idiopathic pulmonary fibrosis is a disease of low frequency and of uncertain etiology [1]. Its prognosis is similar to lung cancer and until the last decade no intervention had demonstrated survival benefits [2]. After diagnosis, survival decreases rapidly and several factors, including exacerbations, time to disease progression and deterioration in respiratory function are associated with poor prognosis [3],[4].
Pirfenidone [5-methyl-1-phenyl-2- [1H] -pyridone) is an oral antifibrotic, antioxidant and anti-inflammatory drug whose mechanism is based on acting as a scavenger of free hydroxyl radicals (OH-) and superoxide anions (O-), leading to decreased cytokine production (TNF-alpha, IFN-gamma, IL-1Beta, IL-6) and inhibiting fibroblast proliferation [5]. In 1999 the first published trial showed improvement in functional capacity, exacerbations and survival, after which other trials have been conducted [6],[7], which led to the FDA's approval in 2014 for its use in the treatment of idiopathic pulmonary fibrosis [8].
Methods
We used Epistemonikos database, which is maintained by screening multiple databases, to identify systematic reviews and their included primary studies. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found 13 systematic reviews reported in 14 references [2], [9],[10],[11],[12],[13],[14],[15],[16],[17],[18], [19],[20],[21] comprising nine primary studies, reported in 10 references [6],[22],[23],[24],[25],[26],[27],[28], [29],[30], including seven randomized controlled trials published in eight references [22],[23],[24],[25],[26],[27],[29],[30]. Two randomized trials [30],[31] were not considered in this summary because the study population did not correspond to that of the question under investigation. This table and the overall summary are based on the five randomized trials relevant to the question. |
|
What types of patients were included |
All trials included patients diagnosed with idiopathic pulmonary fibrosis according to the standards of the American Thoracic Society of 2011. Patients ranged from 20 to 80 years of age. One trial included patients with PaO2 ≥ 70 mmHg at rest and SpO2 ≤ 90% on exercise [25], two trials included patients diagnosed with pulmonary fibrosis in the past year [24],[25], while three others included them if the diagnosis had been made in the past four years [22],[23],[26],[27]. Three trials included patients with functional capacity between 50 and 90% [22],[23],[26],[27], three trials included patients with DLCO> 30% [22],[23],[26],[27], two trials included patients with walk test in six minutes higher or equal to 150 m [23],[26],[27] and one trial included patients with FEV1 / FVC> 70%. [22]. |
|
What types of interventions were included |
All trials used pirfenidone as monotherapy. Three trials used pirfenidone at doses of 2,400 mg daily [22],[23],[26],[27] and two trials used pirfenidone at doses of 1,800 mg daily [24],[25].Two trials administered concomitant treatment with prednisolone at doses of less than 10 mg daily [24],[25]. All trials compared against placebo. |
|
What types of outcomes |
The different systematic reviews identified grouped the outcomes as follows:
|
Summary of findings
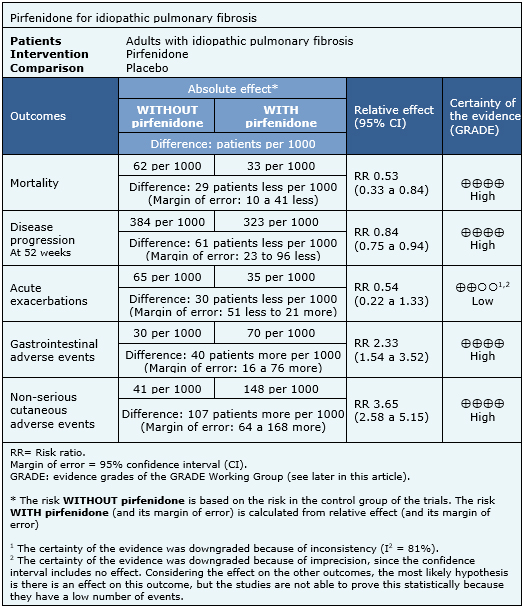
The information on the effects of pirfenidone is based on five randomized trials [22],[23],[24],[25],[26],[27] involving 1,567 patients. All trials reported mortality, two trials [24],[25] reported acute exacerbations and four trials [22],[23],[24],[26],[27] reported progression-free survival. The summary of findings is as follows:
- Pirfenidone decreases mortality in idiopathic pulmonary fibrosis. The certainty of the evidence is high.
- Pirfenidone decreases disease progression in idiopathic pulmonary fibrosis. The certainty of the evidence is high.
- Pirfenidone might reduce the risk of acute exacerbations, but the certainty of the evidence is low.
- Pirfenidone has frequent, but not severe, gastrointestinal side effects. The certainty of the evidence is high.
- Pirfenidone has frequent, but not severe, cutaneous adverse effects. The certainty of the evidence is high.
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
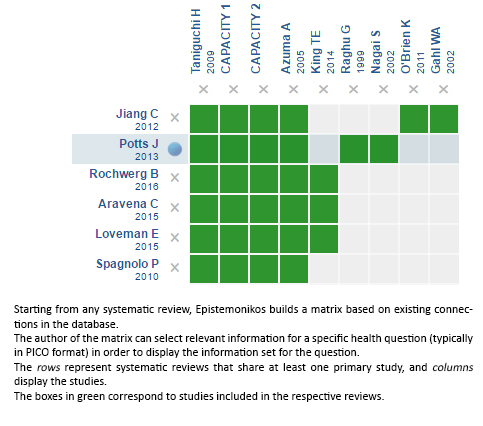
Follow the link to access the interactive version: Pirfenidone for idiopathic pulmonary fibrosis
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Se ha planteado que la pirfenidona, un agente antifibrótico, podría cambiar el curso de la fibrosis pulmonar idiopática, una enfermedad de pronóstico ominoso y para la cual prácticamente no existen terapias efectivas. Utilizando la base de datos Epistemonikos, la cual es mantenida mediante búsquedas en múltiples bases de datos, identificamos trece revisiones sistemáticas que en conjunto incluyen nueve estudios primarios, siete de los cuales son aleatorizados y cuyos resultados se analizaron en este resumen. Extrajimos los datos, realizamos un metanálisis y preparamos tablas de resumen de los resultados utilizando el método GRADE. Concluimos que la pirfenidona disminuye la progresión de la enfermedad y la mortalidad en la fibrosis pulmonar idiopática. Si bien se asocia a efectos adversos gastrointestinales y cutáneos frecuentes, estos en general no son severos.
 Authors:
Alejandro Jeldres[1,2], Gonzalo Labarca[2,3,4,5]
Authors:
Alejandro Jeldres[1,2], Gonzalo Labarca[2,3,4,5]
Affiliation:
[1] Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
[2] Proyecto Epistemonikos, Santiago, Chile
[3] Facultad de Medicina, Universidad San Sebastian, Concepción, Chile
[4] Departamento de Medicina Interna, Complejo Asistencial Dr. VÍctor RÍos Ruiz, Los Ángeles, Chile
[5] Programa de Salud Basada en Evidencia, Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
E-mail: glabarcat@gmail.com
Author address:
[1] Facultad de Medicina Pontificia Universidad Católica de Chile Diagonal Paraguay 476 Santiago Centro Chile

Citation: Jeldres A, Labarca G. Is pirfenidone effective for idiopathic pulmonary fibrosis?. Medwave 2017;17(Suppl1):e6844 doi: 10.5867/medwave.2017.6844
Publication date: 17/1/2017

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011 Mar 15;183(6):788-824 | CrossRef | PubMed |
- Spagnolo P, Del Giovane C, Luppi F, Cerri S, Balduzzi S, Walters EH, et al. Non-steroid agents for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev. 2010 Sep 8;(9):CD003134 | CrossRef | PubMed |
- Vancheri C, Failla M, Crimi N, Raghu G. Idiopathic pulmonary fibrosis: a disease with similarities and links to cancer biology. Eur Respir J. 2010 Mar;35(3):496-504 | CrossRef | PubMed |
- Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006 Oct 1;174(7):810-6 | PubMed |
- Margaritopoulos GA, Vasarmidi E, Antoniou KM. Pirfenidone in the treatment of idiopathic pulmonary fibrosis: an evidence-based review of its place in therapy. Core Evid. 2016 Jul 1;11:11-22 | CrossRef | PubMed |
- Raghu G, Johnson WC, Lockhart D, Mageto Y. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: results of a prospective, open-label Phase II study. Am J Respir Crit Care Med. 1999 Apr;159(4 Pt 1):1061-9 | PubMed |
- Nice.org.uk. [Online]. Available from: https://www.nice.org.uk/guidance/ta282/documents/idiopathic-pulmonary-fibrosis-pirfenidone-pre-meeting-briefing2 [Accessed 28 November 2016]. | Link |
- Fda.gov. [Online]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022535s000lbl.pdf. [Accessed 28 November 2016]. | Link |
- Loveman E, Copley VR, Scott DA, Colquitt JL, Clegg AJ, O'Reilly KM. Comparing new treatments for idiopathic pulmonary fibrosis--a network meta-analysis. BMC Pulm Med. 2015 Apr 18;15:37 | CrossRef | PubMed |
- Loveman E, Copley VR, Colquitt JL, Scott DA, Clegg AJ, Jones J, et al. The effectiveness and cost-effectiveness of treatments for idiopathic pulmonary fibrosis: systematic review, network meta-analysis and health economic evaluation. BMC Pharmacol Toxicol. 2014 Nov 19;15:63 | CrossRef | PubMed |
- Atkins CP, Loke YK, Wilson AM. Outcomes in idiopathic pulmonary fibrosis: a meta-analysis from placebo controlled trials. Respir Med. 2014 Feb;108(2):376-87 | CrossRef | PubMed |
- Loveman E, Copley VR, Colquitt J, Scott DA, Clegg A, Jones J, et al. The clinical effectiveness and cost-effectiveness of treatments for idiopathic pulmonary fibrosis: a systematic review and economic evaluation. Health Technol Assess. 2015 Mar;19(20):i-xxiv, 1-336 | CrossRef | PubMed |
- Aravena C, Labarca G, Venegas C, Arenas A, Rada G. Pirfenidone for Idiopathic Pulmonary Fibrosis: A Systematic Review and Meta-Analysis. PLoS One. 2015 Aug 26;10(8):e0136160 | CrossRef | PubMed |
- Canestaro WJ, Forrester SH, Raghu G, Ho L, Devine BE. Drug Treatment of Idiopathic Pulmonary Fibrosis: Systematic Review and Network Meta-Analysis. Chest. 2016 Mar;149(3):756-66 | CrossRef | PubMed |
- Bajwah S, Ross JR, Peacock JL, Higginson IJ, Wells AU, Patel AS, et al. Interventions to improve symptoms and quality of life of patients with fibrotic interstitial lung disease: a systematic review of the literature. Thorax. 2013 Sep;68(9):867-79 | CrossRef | PubMed |
- Rochwerg B, Neupane B, Zhang Y, Garcia CC, Raghu G, Richeldi L, et al. Treatment of idiopathic pulmonary fibrosis: a network meta-analysis. BMC Med. 2016 Feb 3;14:18 | CrossRef | PubMed |
- Rogliani P, Calzetta L, Cavalli F, Matera MG, Cazzola M. Pirfenidone, nintedanib and N-acetylcysteine for the treatment of idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Pulm Pharmacol Ther. 2016 Oct;40:95-103 | CrossRef | PubMed |
- Cooper K, Mendes D, Picot, J, Loveman E. Pirfenidone for the treatment of idiopathic pulmonary fibrosis: A Single Technology Appraisal. SHTAC, 2012. Available in http://www.nets.nihr.ac.uk/__data/assets/pdf_file/0007/82591/ERGReport-09-135-01.pdf | Link |
- Potts J, Yogaratnam D. Pirfenidone: a novel agent for the treatment of idiopathic pulmonary fibrosis. Ann Pharmacother. 2013 Mar;47(3):361-7 | CrossRef | PubMed |
- Institute for Quality and Efficiency in Health Care. Pirfenidone: Benefit assessment according to § 35a Social Code Book V; extract of dossier assessment; Commission No. A11-18 [online]. 12.1.2011 | Link |
- Jiang C, Huang H, Liu J, Wang Y, Lu Z, Xu Z. Adverse events of pirfenidone for the treatment of pulmonary fibrosis: a meta-analysis of randomized controlled trials. PLoS One. 2012;7(10):e47024 | CrossRef | PubMed |
- King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014 May 29;370(22):2083-92 | CrossRef | PubMed |
- Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011 May 21;377(9779):1760-9 | CrossRef | PubMed |
- Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010 Apr;35(4):821-9 | CrossRef | PubMed |
- Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005 May 1;171(9):1040-7 | PubMed |
- CAPACITY 1. Intermune. A randomized, double-blind, placebo-controlled, phase 3 study of the safety and efficacy of pirfenidone in patients with idiopathic pulmonary fibrosis; study PIPF-006; clinical study report [unpublished]. 2009
- CAPACITY 2. Intermune. A randomized, double-blind, placebo-controlled, phase 3, three-arm study of the safety and efficacy of pirfenidone in patients with idiopathic pulmonary fibrosis: study PIPF-004; clinical study report [unpublished]. 2009.
- Nagai S, Hamada K, Shigematsu M, Taniyama M, Yamauchi S, Izumi T. Open- label compassionate use one year-treatment with pirfenidone to patients with chronic pulmonary fibrosis. Intern Med. 2002 Dec;41(12):1118-23 | PubMed |
- O'Brien K, Troendle J, Gochuico BR, Markello TC, Salas J, Cardona H, et al. Pirfenidone for the treatment of Hermansky-Pudlak syndrome pulmonary fibrosis. Mol Genet Metab. 2011 Jun;103(2):128-34 | CrossRef | PubMed |
- Gahl WA, Brantly M, Troendle J, Avila NA, Padua A, Montalvo C, et al. Effect of pirfenidone on the pulmonary fibrosis of Hermansky-Pudlak syndrome. Mol Genet Metab. 2002 Jul;76(3):234-42 | PubMed |
- Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al; American Thoracic Society.; European Respiratory society.; Japanese Respiratory Society.; Latin American Thoracic Association.. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med. 2015 Jul 15;192(2):e3-19 | CrossRef | PubMed |
 Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011 Mar 15;183(6):788-824 | CrossRef | PubMed |
Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011 Mar 15;183(6):788-824 | CrossRef | PubMed | Spagnolo P, Del Giovane C, Luppi F, Cerri S, Balduzzi S, Walters EH, et al. Non-steroid agents for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev. 2010 Sep 8;(9):CD003134 | CrossRef | PubMed |
Spagnolo P, Del Giovane C, Luppi F, Cerri S, Balduzzi S, Walters EH, et al. Non-steroid agents for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev. 2010 Sep 8;(9):CD003134 | CrossRef | PubMed | Vancheri C, Failla M, Crimi N, Raghu G. Idiopathic pulmonary fibrosis: a disease with similarities and links to cancer biology. Eur Respir J. 2010 Mar;35(3):496-504 | CrossRef | PubMed |
Vancheri C, Failla M, Crimi N, Raghu G. Idiopathic pulmonary fibrosis: a disease with similarities and links to cancer biology. Eur Respir J. 2010 Mar;35(3):496-504 | CrossRef | PubMed | Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006 Oct 1;174(7):810-6 | PubMed |
Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006 Oct 1;174(7):810-6 | PubMed | Margaritopoulos GA, Vasarmidi E, Antoniou KM. Pirfenidone in the treatment of idiopathic pulmonary fibrosis: an evidence-based review of its place in therapy. Core Evid. 2016 Jul 1;11:11-22 | CrossRef | PubMed |
Margaritopoulos GA, Vasarmidi E, Antoniou KM. Pirfenidone in the treatment of idiopathic pulmonary fibrosis: an evidence-based review of its place in therapy. Core Evid. 2016 Jul 1;11:11-22 | CrossRef | PubMed | Raghu G, Johnson WC, Lockhart D, Mageto Y. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: results of a prospective, open-label Phase II study. Am J Respir Crit Care Med. 1999 Apr;159(4 Pt 1):1061-9 | PubMed |
Raghu G, Johnson WC, Lockhart D, Mageto Y. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: results of a prospective, open-label Phase II study. Am J Respir Crit Care Med. 1999 Apr;159(4 Pt 1):1061-9 | PubMed | Nice.org.uk. [Online]. Available from: https://www.nice.org.uk/guidance/ta282/documents/idiopathic-pulmonary-fibrosis-pirfenidone-pre-meeting-briefing2 [Accessed 28 November 2016]. | Link |
Nice.org.uk. [Online]. Available from: https://www.nice.org.uk/guidance/ta282/documents/idiopathic-pulmonary-fibrosis-pirfenidone-pre-meeting-briefing2 [Accessed 28 November 2016]. | Link | Fda.gov. [Online]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022535s000lbl.pdf. [Accessed 28 November 2016]. | Link |
Fda.gov. [Online]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022535s000lbl.pdf. [Accessed 28 November 2016]. | Link | Loveman E, Copley VR, Scott DA, Colquitt JL, Clegg AJ, O'Reilly KM. Comparing new treatments for idiopathic pulmonary fibrosis--a network meta-analysis. BMC Pulm Med. 2015 Apr 18;15:37 | CrossRef | PubMed |
Loveman E, Copley VR, Scott DA, Colquitt JL, Clegg AJ, O'Reilly KM. Comparing new treatments for idiopathic pulmonary fibrosis--a network meta-analysis. BMC Pulm Med. 2015 Apr 18;15:37 | CrossRef | PubMed | Loveman E, Copley VR, Colquitt JL, Scott DA, Clegg AJ, Jones J, et al. The effectiveness and cost-effectiveness of treatments for idiopathic pulmonary fibrosis: systematic review, network meta-analysis and health economic evaluation. BMC Pharmacol Toxicol. 2014 Nov 19;15:63 | CrossRef | PubMed |
Loveman E, Copley VR, Colquitt JL, Scott DA, Clegg AJ, Jones J, et al. The effectiveness and cost-effectiveness of treatments for idiopathic pulmonary fibrosis: systematic review, network meta-analysis and health economic evaluation. BMC Pharmacol Toxicol. 2014 Nov 19;15:63 | CrossRef | PubMed | Atkins CP, Loke YK, Wilson AM. Outcomes in idiopathic pulmonary fibrosis: a meta-analysis from placebo controlled trials. Respir Med. 2014 Feb;108(2):376-87 | CrossRef | PubMed |
Atkins CP, Loke YK, Wilson AM. Outcomes in idiopathic pulmonary fibrosis: a meta-analysis from placebo controlled trials. Respir Med. 2014 Feb;108(2):376-87 | CrossRef | PubMed | Loveman E, Copley VR, Colquitt J, Scott DA, Clegg A, Jones J, et al. The clinical effectiveness and cost-effectiveness of treatments for idiopathic pulmonary fibrosis: a systematic review and economic evaluation. Health Technol Assess. 2015 Mar;19(20):i-xxiv, 1-336 | CrossRef | PubMed |
Loveman E, Copley VR, Colquitt J, Scott DA, Clegg A, Jones J, et al. The clinical effectiveness and cost-effectiveness of treatments for idiopathic pulmonary fibrosis: a systematic review and economic evaluation. Health Technol Assess. 2015 Mar;19(20):i-xxiv, 1-336 | CrossRef | PubMed | Aravena C, Labarca G, Venegas C, Arenas A, Rada G. Pirfenidone for Idiopathic Pulmonary Fibrosis: A Systematic Review and Meta-Analysis. PLoS One. 2015 Aug 26;10(8):e0136160 | CrossRef | PubMed |
Aravena C, Labarca G, Venegas C, Arenas A, Rada G. Pirfenidone for Idiopathic Pulmonary Fibrosis: A Systematic Review and Meta-Analysis. PLoS One. 2015 Aug 26;10(8):e0136160 | CrossRef | PubMed | Canestaro WJ, Forrester SH, Raghu G, Ho L, Devine BE. Drug Treatment of Idiopathic Pulmonary Fibrosis: Systematic Review and Network Meta-Analysis. Chest. 2016 Mar;149(3):756-66 | CrossRef | PubMed |
Canestaro WJ, Forrester SH, Raghu G, Ho L, Devine BE. Drug Treatment of Idiopathic Pulmonary Fibrosis: Systematic Review and Network Meta-Analysis. Chest. 2016 Mar;149(3):756-66 | CrossRef | PubMed | Bajwah S, Ross JR, Peacock JL, Higginson IJ, Wells AU, Patel AS, et al. Interventions to improve symptoms and quality of life of patients with fibrotic interstitial lung disease: a systematic review of the literature. Thorax. 2013 Sep;68(9):867-79 | CrossRef | PubMed |
Bajwah S, Ross JR, Peacock JL, Higginson IJ, Wells AU, Patel AS, et al. Interventions to improve symptoms and quality of life of patients with fibrotic interstitial lung disease: a systematic review of the literature. Thorax. 2013 Sep;68(9):867-79 | CrossRef | PubMed | Rochwerg B, Neupane B, Zhang Y, Garcia CC, Raghu G, Richeldi L, et al. Treatment of idiopathic pulmonary fibrosis: a network meta-analysis. BMC Med. 2016 Feb 3;14:18 | CrossRef | PubMed |
Rochwerg B, Neupane B, Zhang Y, Garcia CC, Raghu G, Richeldi L, et al. Treatment of idiopathic pulmonary fibrosis: a network meta-analysis. BMC Med. 2016 Feb 3;14:18 | CrossRef | PubMed | Rogliani P, Calzetta L, Cavalli F, Matera MG, Cazzola M. Pirfenidone, nintedanib and N-acetylcysteine for the treatment of idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Pulm Pharmacol Ther. 2016 Oct;40:95-103 | CrossRef | PubMed |
Rogliani P, Calzetta L, Cavalli F, Matera MG, Cazzola M. Pirfenidone, nintedanib and N-acetylcysteine for the treatment of idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Pulm Pharmacol Ther. 2016 Oct;40:95-103 | CrossRef | PubMed | Cooper K, Mendes D, Picot, J, Loveman E. Pirfenidone for the treatment of idiopathic pulmonary fibrosis: A Single Technology Appraisal. SHTAC, 2012. Available in http://www.nets.nihr.ac.uk/__data/assets/pdf_file/0007/82591/ERGReport-09-135-01.pdf | Link |
Cooper K, Mendes D, Picot, J, Loveman E. Pirfenidone for the treatment of idiopathic pulmonary fibrosis: A Single Technology Appraisal. SHTAC, 2012. Available in http://www.nets.nihr.ac.uk/__data/assets/pdf_file/0007/82591/ERGReport-09-135-01.pdf | Link | Potts J, Yogaratnam D. Pirfenidone: a novel agent for the treatment of idiopathic pulmonary fibrosis. Ann Pharmacother. 2013 Mar;47(3):361-7 | CrossRef | PubMed |
Potts J, Yogaratnam D. Pirfenidone: a novel agent for the treatment of idiopathic pulmonary fibrosis. Ann Pharmacother. 2013 Mar;47(3):361-7 | CrossRef | PubMed | Institute for Quality and Efficiency in Health Care. Pirfenidone: Benefit assessment according to § 35a Social Code Book V; extract of dossier assessment; Commission No. A11-18 [online]. 12.1.2011 | Link |
Institute for Quality and Efficiency in Health Care. Pirfenidone: Benefit assessment according to § 35a Social Code Book V; extract of dossier assessment; Commission No. A11-18 [online]. 12.1.2011 | Link | Jiang C, Huang H, Liu J, Wang Y, Lu Z, Xu Z. Adverse events of pirfenidone for the treatment of pulmonary fibrosis: a meta-analysis of randomized controlled trials. PLoS One. 2012;7(10):e47024 | CrossRef | PubMed |
Jiang C, Huang H, Liu J, Wang Y, Lu Z, Xu Z. Adverse events of pirfenidone for the treatment of pulmonary fibrosis: a meta-analysis of randomized controlled trials. PLoS One. 2012;7(10):e47024 | CrossRef | PubMed | King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014 May 29;370(22):2083-92 | CrossRef | PubMed |
King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014 May 29;370(22):2083-92 | CrossRef | PubMed | Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011 May 21;377(9779):1760-9 | CrossRef | PubMed |
Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011 May 21;377(9779):1760-9 | CrossRef | PubMed | Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010 Apr;35(4):821-9 | CrossRef | PubMed |
Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010 Apr;35(4):821-9 | CrossRef | PubMed | Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005 May 1;171(9):1040-7 | PubMed |
Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005 May 1;171(9):1040-7 | PubMed | CAPACITY 1. Intermune. A randomized, double-blind, placebo-controlled, phase 3 study of the safety and efficacy of pirfenidone in patients with idiopathic pulmonary fibrosis; study PIPF-006; clinical study report [unpublished]. 2009
CAPACITY 1. Intermune. A randomized, double-blind, placebo-controlled, phase 3 study of the safety and efficacy of pirfenidone in patients with idiopathic pulmonary fibrosis; study PIPF-006; clinical study report [unpublished]. 2009  CAPACITY 2. Intermune. A randomized, double-blind, placebo-controlled, phase 3, three-arm study of the safety and efficacy of pirfenidone in patients with idiopathic pulmonary fibrosis: study PIPF-004; clinical study report [unpublished]. 2009.
CAPACITY 2. Intermune. A randomized, double-blind, placebo-controlled, phase 3, three-arm study of the safety and efficacy of pirfenidone in patients with idiopathic pulmonary fibrosis: study PIPF-004; clinical study report [unpublished]. 2009.  Nagai S, Hamada K, Shigematsu M, Taniyama M, Yamauchi S, Izumi T. Open- label compassionate use one year-treatment with pirfenidone to patients with chronic pulmonary fibrosis. Intern Med. 2002 Dec;41(12):1118-23 | PubMed |
Nagai S, Hamada K, Shigematsu M, Taniyama M, Yamauchi S, Izumi T. Open- label compassionate use one year-treatment with pirfenidone to patients with chronic pulmonary fibrosis. Intern Med. 2002 Dec;41(12):1118-23 | PubMed | O'Brien K, Troendle J, Gochuico BR, Markello TC, Salas J, Cardona H, et al. Pirfenidone for the treatment of Hermansky-Pudlak syndrome pulmonary fibrosis. Mol Genet Metab. 2011 Jun;103(2):128-34 | CrossRef | PubMed |
O'Brien K, Troendle J, Gochuico BR, Markello TC, Salas J, Cardona H, et al. Pirfenidone for the treatment of Hermansky-Pudlak syndrome pulmonary fibrosis. Mol Genet Metab. 2011 Jun;103(2):128-34 | CrossRef | PubMed | Gahl WA, Brantly M, Troendle J, Avila NA, Padua A, Montalvo C, et al. Effect of pirfenidone on the pulmonary fibrosis of Hermansky-Pudlak syndrome. Mol Genet Metab. 2002 Jul;76(3):234-42 | PubMed |
Gahl WA, Brantly M, Troendle J, Avila NA, Padua A, Montalvo C, et al. Effect of pirfenidone on the pulmonary fibrosis of Hermansky-Pudlak syndrome. Mol Genet Metab. 2002 Jul;76(3):234-42 | PubMed | Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al; American Thoracic Society.; European Respiratory society.; Japanese Respiratory Society.; Latin American Thoracic Association.. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med. 2015 Jul 15;192(2):e3-19 | CrossRef | PubMed |
Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al; American Thoracic Society.; European Respiratory society.; Japanese Respiratory Society.; Latin American Thoracic Association.. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med. 2015 Jul 15;192(2):e3-19 | CrossRef | PubMed |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








