Abstract
During an episode of sepsis, systemic inflammatory response phenomenon triggers a series of procoagulant mechanisms. It has been suggested that the use of activated protein C could play a role in the management of this pathology, but there is no consensus. Searching in Epistemonikos database, which is maintained by screening multiple databases, we identified seven systematic reviews covering 35 primary studies addressing the question of this article, including six randomized trials. We extracted data, combined the evidence using meta-analysis and generated a summary of findings table following the GRADE approach. We concluded activated protein C in sepsis probably does not decrease the mortality rate and increases the rate of hemorrhagic events.
Problem
Sepsis continues leading the causes of morbidity and mortality in intensive care units. Its incidence has been increasing, with greater complications and with more resistant infectious agents. While there has been some tendency to decreased mortality through some interventions, effective therapeutic tools remain limited.
Human protein C is a vitamin K dependent glycoprotein, structurally similar to other proteins that affect blood clotting, such as prothrombin, Factor VII, Factor IX and Factor X. It is thought that it would play an important role by regulating the anticoagulation, inflammation, cell death and maintaining the permeability of the walls of blood vessels.
The pharmaceutical company that produced this drug withdrew it from the market in 2011.
Methods
We used Epistemonikos database, which is maintained by screening multiple databases, to identify systematic reviews and their included primary studies. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), meta-analysis of the total of studies, a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found seven systematic reviews [1],[2],[3],[4],[5],[6],[7], including 35 primary studies [8],[9],[10],[11], This table and the summary in general are based on the latter. |
|
What types of patients were included |
Regarding the types of patients, it should be noted that the six trials were carried out after 1991, the year that the first consensus definition for sepsis and septic shock was performed, so that the definition and graduation of the severity of sepsis is homogenous between trials. |
|
What types of interventions were included |
In four of six trials, the type of activated protein C used was drotrecogin alpha-activated [8],[11],[12],[13] and in two was activated human C protein (rhAPC) [9],[10]. The route of administration was intravenous in all trials. Regarding the dose, five used a standard dose of 24 μg/kg/hour for a total of 96 hours [8],[10],[11],[12],[13]. One trial used variable doses and different time than 96 hours [9]. All trials compared against placebo or standard treatment. |
|
What types of outcomes |
The different systematic reviews identified grouped the outcomes as follows:
|
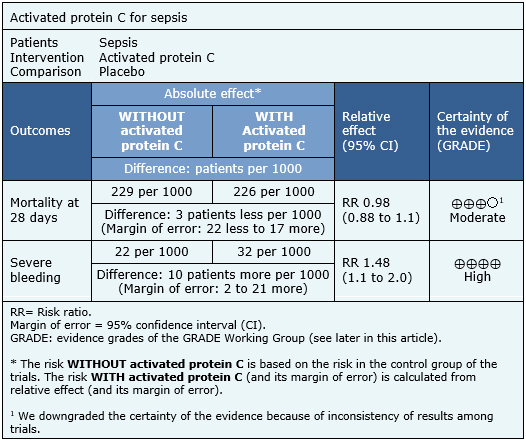
Summary of findings
The information on the effects of activated protein C on sepsis is based on six randomized trials including 6,781 patients. All trials measured the outcome mortality at 28 days and severe bleeding.
The summary of findings is as follows:
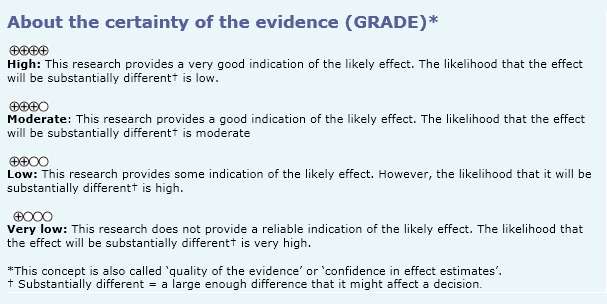
- The use of activated protein C in patients with sepsis probably does not decrease the mortality rate. The certainty of the evidence is moderate.
- The use of activated protein C in patients with sepsis increases the rate of severe bleeding during hospitalization. The certainty of the evidence is high.
Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
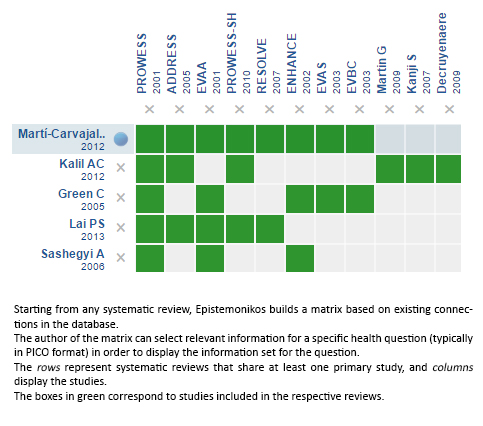
Follow the link to access the interactive version: Recombinant activated protein C for the treatment of sepsis
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

Durante un episodio de sepsis, el fenómeno de respuesta inflamatoria sistémica desencadena una serie de mecanismos procoagulantes. Se ha planteado que el uso de proteína C activada podría tener un rol en el manejo de esta patología, pero no existe consenso al respecto. Utilizando la base de datos Epistemonikos, la cual es mantenida mediante búsquedas en múltiples bases de datos, identificamos siete revisiones sistemáticas que en conjunto incluyen 35 estudios primarios, de los cuales seis corresponden a estudios aleatorizados. Realizamos un metanálisis y tablas de resumen de los resultados utilizando el método GRADE. Concluimos que el uso de proteína C activada en cuadros sépticos probablemente no disminuye la tasa de mortalidad y aumenta la tasa de eventos hemorrágicos.
 Authors:
Juan Alvarado[1,2], Ricardo Castro[2,3]
Authors:
Juan Alvarado[1,2], Ricardo Castro[2,3]
Affiliation:
[1] Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
[2] Proyecto Epistemonikos, Santiago, Chile
[3] Departamento de Medicina Intensiva, Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
E-mail: rcastro@med.puc.cl
Author address:
[1] Facultad de Medicina Pontificia Universidad Católica de Chile Lira 63 Santiago Centro Chile

Citation: Alvarado J, Castro R. What is the role of recombinant activated protein C in the management of sepsis?. Medwave 2016; 16(Suppl5):e6801 doi: 10.5867/medwave.2016.6801
Publication date: 20/12/2016

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Costa V, Brophy JM. Drotrecogin alfa (activated) in severe sepsis: a systematic review and new cost-effectiveness analysis. BMC Anesthesiol. 2007 Jun 25;7:5 | PubMed |
- Green C, Dinnes J, Takeda A, Shepherd J, Hartwell D, Cave C, Payne E, Cuthbertson BH. Clinical effectiveness and cost-effectiveness of drotrecogin alfa (activated) (Xigris) for the treatment of severe sepsis in adults: a systematic review and economic evaluation. Health Technol Assess. 2005 Mar;9(11):1-126, iii-iv | PubMed |
- Kalil AC, LaRosa SP. Effectiveness and safety of drotrecogin alfa (activated) for severe sepsis: a meta-analysis and metaregression. Lancet Infect Dis. 2012 Sep;12(9):678-86 | CrossRef | PubMed |
- Lai PS, Matteau A, Iddriss A, Hawes JC, Ranieri V, Thompson BT. An updated meta-analysis to understand the variable efficacy of drotrecogin alfa (activated) in severe sepsis and septic shock. Minerva Anestesiol. 2013 Jan;79(1):33-43. | PubMed |
- Martí-Carvajal AJ, Solà I, Gluud C, Lathyris D, Cardona AF. Human recombinant protein C for severe sepsis and septic shock in adult and paediatric patients. Cochrane Database Syst Rev. 2012 Dec 12;12:CD004388 | CrossRef | PubMed |
- Sashegyi A, Trzaskoma BL, Nelson DR, Williams MD, Macias W. International INtegrated Database for the Evaluation of severe sePsis and drotrecogin alfa (activated) THerapy: component trials and statistical methods for INDEPTH. Curr Med Res Opin. 2006 May;22(5):1001-12 | PubMed |
- Wiedermann CJ, Kaneider NC. A meta-analysis of controlled trials of recombinant human activated protein C therapy in patients with sepsis. BMC Emerg Med. 2005 Oct 14;5:7 | PubMed |
- Abraham E, Laterre PF, Garg R, Levy H, Talwar D, Trzaskoma BL, François B, Guy JS, Brückmann M, Rea-Neto A, Rossaint R, Perrotin D, Sablotzki A, Arkins N, Utterback BG, Macias WL; Administration of Drotrecogin Alfa (Activated) in Early Stage Severe Sepsis (ADDRESS) Study Group.. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. N Engl J Med. 2005 Sep 29;353(13):1332-41 | PubMed |
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ Jr; Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group.. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001 Mar 8;344(10):699-709 | PubMed |
- Bernard GR, Ely EW, Wright TJ, Fraiz J, Stasek JE Jr, Russell JA, Mayers I, Rosenfeld BA, Morris PE, Yan SB, Helterbrand JD. Safety and dose relationship of recombinant human activated protein C for coagulopathy in severe sepsis. Crit Care Med. 2001 Nov;29(11):2051-9 | PubMed |
- Dhainaut JF, Antonelli M, Wright P, Desachy A, Reignier J, Lavoue S, Charpentier J, Belger M, Cobas-Meyer M, Maier C, Mignini MA, Janes J. Extended drotrecogin alfa (activated) treatment in patients with prolonged septic shock. Intensive Care Med. 2009 Jul;35(7):1187-95 | CrossRef | PubMed |
- Nadel S, Goldstein B, Williams MD, Dalton H, Peters M, Macias WL, Abd-Allah SA, Levy H, Angle R, Wang D, Sundin DP, Giroir B; REsearching severe Sepsis and Organ dysfunction in children: a gLobal perspective (RESOLVE) study group. Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet. 2007 Mar 10;369(9564):836-43 | PubMed |
- PROWESS SHOCK Steering Committee., Thompson BT, Ranieri VM, Finfer S, Barie PS, Dhainaut JF, Douglas IS, Gårdlund B, Marshall JC, Rhodes A. Statistical analysis plan of PROWESS SHOCK study. Intensive Care Med. 2010 Nov;36(11):1972-3. | CrossRef | PubMed |
- Martin G, Brunkhorst FM, Janes JM, Reinhart K, Sundin DP, Garnett K, Beale R. The international PROGRESS registry of patients with severe sepsis: drotrecogin alfa (activated) use and patient outcomes. Crit Care. 2009;13(3):R103 | CrossRef | PubMed |
- Lindenauer PK, Rothberg MB, Nathanson BH, Pekow PS, Steingrub JS. Activated protein C and hospital mortality in septic shock: a propensity-matched analysis. Crit Care Med. 2010 Apr;38(4):1101-7 | CrossRef | PubMed |
- Kanji S, Perreault MM, Chant C, Williamson D, Burry L. Evaluating the use of Drotrecogin alfa (activated) in adult severe sepsis: a Canadian multicenter observational study. Intensive Care Med. 2007 Mar;33(3):517-23 | PubMed |
- Decruyenaere J, De Backer D, Spapen H, Laterre PF, Raemaekers J, Rogiers P, Trine H, Sartral M, Haentjens T, Wagner T. 90-day follow-up of patients treated with Drotrecogin Alfa (activated) for severe sepsis: a Belgian open label study. Acta Clin Belg. 2009 Jan-Feb;64(1):16-22 | PubMed |
- Hecksher CA, Lacerda HR, Maciel MA. Characteristics and outcomes of patients treated with drotrecogin alpha and other interventions of the "Surviving Sepsis" campaign in clinical practice. Rev Bras Ter Intensiva. 2008 Jun;20(2):135-43. | PubMed |
- Gentry CA, Gross KB, Sud B, Drevets DA. Adverse outcomes associated with the use of drotrecogin alfa (activated) in patients with severe sepsis and baseline bleeding precautions. Crit Care Med. 2009 Jan;37(1):19-25 | CrossRef | PubMed |
- Bertolini G, Rossi C, Anghileri A, Livigni S, Addis A, Poole D. Use of Drotrecogin alfa (activated) in Italian intensive care units: the results of a nationwide survey. Intensive Care Med. 2007 Mar;33(3):426-34 | PubMed |
- Anger KE, Degrado JR, Greenwood BC, Cohen SA, Szumita PM. Evaluation of recombinant activated protein C for severe sepsis at a tertiary academic medical center. Therapeutics and clinical risk management. 2013;9:277-84. | Link |
- Bollinger J, Coley K, Rea R, Saul M. Outcomes in patients with recombinant human activated protein c at an academic medical center versus the ENHANCE US trial. Crit Care Med 2006; 34: A111. | Link |
- Wheeler A, Steingrub J, Schmidt GA, Sanchez P, Jacobi J, Linde-Zwirble W, Bates B, Qualy RL, Woodward B, Zeckel M. A retrospective observational study of drotrecogin alfa (activated) in adults with severe sepsis: comparison with a controlled clinical trial. Crit Care Med. 2008 Jan;36(1):14-23 | PubMed |
- Spriet I, Meersseman W, Wilmer A, Meyfroidt G, Casteels M, Willems L. Evaluation of drotrecogin alpha use in a Belgian university hospital. Pharm World Sci. 2006 Oct;28(5):290-5 | PubMed |
- Rowan KM, Welch CA, North E, Harrison DA. Drotrecogin alfa (activated): real-life use and outcomes for the UK. Crit Care. 2008;12(2):R58 | CrossRef | PubMed |
- Higgins TL, Steingrub JS, Tereso GJ, Tidswell MA, McGee WT. Drotrecogin alfa (activated) in sepsis: initial experience with patient selection, cost, and clinical outcomes. J Intensive Care Med. 2005 Nov-Dec;20(6):339-45 | PubMed |
- Ridley S, Lwin A, Wyncoll D, Lippett S, Watson D, Gunning K, Higgins D. Drotrecogin alfa (activated): diffusion from clinical trials to clinical practice. Eur J Anaesthesiol. 2008 Mar;25(3):211-6 | PubMed |
- Sadaka F, O'Brien J, Migneron M, Stortz J, Vanston A, Taylor RW. Activated protein C in septic shock: a propensity-matched analysis. Crit Care. 2011;15(2):R89 | CrossRef | PubMed |
- Ferrer R, Artigas A, Suarez D, Palencia E, Levy MM, Arenzana A, Pérez XL, Sirvent JM; Edusepsis Study Group.. Effectiveness of treatments for severe sepsis: a prospective, multicenter, observational study. Am J Respir Crit Care Med. 2009 Nov 1;180(9):861-6 | CrossRef | PubMed |
- Kübler A, Mayzner-Zawadzka E, Durek G, Gaszynski W, Karpel E, Mikaszewska-Sokolewicz M, Majak P. Results of severe sepsis treatment program using recombinant human activated protein C in Poland. Med Sci Monit. 2006 Mar;12(3):CR107-12 | PubMed |
- Maurice A, Seguin P, Aguillon D, Chanavaz C, Mallédant Y. [Activated protein C treatment: experience about 23 patients in the operative period]. Ann Fr Anesth Reanim. 2005 Apr;24(4):343-6 | PubMed |
- Dhainaut JF, Payet S, Vallet B, França LR, Annane D, Bollaert PE, Le Tulzo Y, Runge I, Malledant Y, Guidet B, Le Lay K, Launois R; PREMISS Study Group. Cost-effectiveness of activated protein C in real-life clinical practice. Crit Care. 2007;11(5):R99 | PubMed |
- Vincent JL, Bernard GR, Beale R, Doig C, Putensen C, Dhainaut JF, Artigas A, Fumagalli R, Macias W, Wright T, Wong K, Sundin DP, Turlo MA, Janes J. Drotrecogin alfa (activated) treatment in severe sepsis from the global open-label trial ENHANCE: further evidence for survival and safety and implications for early treatment. Crit Care Med. 2005 Oct;33(10):2266-77 | PubMed |
- Vincent JL, Laterre PF, Decruyenaere J, Spapen H, Raemaekers J, Damas F, Rogiers P, Sartral M, Haentjens T, Nelson D, Janes J. A registry of patients treated with drotrecogin alfa (activated) in Belgian intensive care units an observational study. Acta Clin Belg. 2008 Jan-Feb;63(1):25-30 | PubMed |
- Ernst FR, Johnston JA, Pulgar S, He J, Ball DE, Young JK, Cooper LM. Timing of drotrecogin alfa (activated) initiation in treatment of severe sepsis: a database cohort study of hospital mortality, length of stay, and costs. Curr Med Res Opin. 2007 Jan;23(1):235-44 | PubMed |
- Eli Lilly. An open label study of drotrecogin alfa (activated) in adult patients with severe sepsis and multiple organ dysfunctions, a phase IV protocol. 2006; Summary ID 7159: 1–6. | Link |
- Macchiavello L, Ellis G, Bowden S, Smithies M. A large, single-centre UK registry of drotrecogin alfa-activated use. Crit Care 2007; 11 (suppl 2): 58. | Link |
- Steingrub JS, Cheatham ML, Woodward B, Wang HT, Effron MB; XEUS Investigators.. A prospective, observational study of Xigris Use in the United States (XEUS). J Crit Care. 2010 Dec;25(4):660.e9-16 | CrossRef | PubMed |
- Bernard GR, Vincent JL, Laterre PF, et al, and the Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group. Effi cacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 2001; 344: 699–709. | CrossRef | PubMed | Link |
- Bernard GR, Macias WL, Joyce DE,Williams MD, Bailey J, Vincent JL. Safety assessment of drotrecogin alfa (activated) in the treatment of adult patients with severe sepsis. Critical Care 2003;7(2):155–63. | PubMed |
- Bernard G, Margolis B, Shanies H, Ely E, Wheeler A, Levy H. Efficacy and safety of References 66 drotrecogin alfa (activated) in the treatment of adult patients with severe sepsis: report from a single open-label trial in the United States 2002. | Link |
- Eli Lilly. Xigris NICE submission: drotrecogin alfa (activated) for severe sepsis. Basingstoke: Eli Lilly; 2003. | Link |
- Dr. R. Phillip Dellinger; Dr. Mitchell M.Levy; Dr. Andrew Rhodes; Dr. Djillali Annane et al. Campaña para sobrevivir a la sepsis: recomendaciones internacionales para el tratamiento de sepsis grave y shock séptico, 2012: Crit Care Med 2013; Febrero de 2013 Volumen 41. | CrossRef | Link |
 Costa V, Brophy JM. Drotrecogin alfa (activated) in severe sepsis: a systematic review and new cost-effectiveness analysis. BMC Anesthesiol. 2007 Jun 25;7:5 | PubMed |
Costa V, Brophy JM. Drotrecogin alfa (activated) in severe sepsis: a systematic review and new cost-effectiveness analysis. BMC Anesthesiol. 2007 Jun 25;7:5 | PubMed | Green C, Dinnes J, Takeda A, Shepherd J, Hartwell D, Cave C, Payne E, Cuthbertson BH. Clinical effectiveness and cost-effectiveness of drotrecogin alfa (activated) (Xigris) for the treatment of severe sepsis in adults: a systematic review and economic evaluation. Health Technol Assess. 2005 Mar;9(11):1-126, iii-iv | PubMed |
Green C, Dinnes J, Takeda A, Shepherd J, Hartwell D, Cave C, Payne E, Cuthbertson BH. Clinical effectiveness and cost-effectiveness of drotrecogin alfa (activated) (Xigris) for the treatment of severe sepsis in adults: a systematic review and economic evaluation. Health Technol Assess. 2005 Mar;9(11):1-126, iii-iv | PubMed | Kalil AC, LaRosa SP. Effectiveness and safety of drotrecogin alfa (activated) for severe sepsis: a meta-analysis and metaregression. Lancet Infect Dis. 2012 Sep;12(9):678-86 | CrossRef | PubMed |
Kalil AC, LaRosa SP. Effectiveness and safety of drotrecogin alfa (activated) for severe sepsis: a meta-analysis and metaregression. Lancet Infect Dis. 2012 Sep;12(9):678-86 | CrossRef | PubMed | Lai PS, Matteau A, Iddriss A, Hawes JC, Ranieri V, Thompson BT. An updated meta-analysis to understand the variable efficacy of drotrecogin alfa (activated) in severe sepsis and septic shock. Minerva Anestesiol. 2013 Jan;79(1):33-43. | PubMed |
Lai PS, Matteau A, Iddriss A, Hawes JC, Ranieri V, Thompson BT. An updated meta-analysis to understand the variable efficacy of drotrecogin alfa (activated) in severe sepsis and septic shock. Minerva Anestesiol. 2013 Jan;79(1):33-43. | PubMed | Martí-Carvajal AJ, Solà I, Gluud C, Lathyris D, Cardona AF. Human recombinant protein C for severe sepsis and septic shock in adult and paediatric patients. Cochrane Database Syst Rev. 2012 Dec 12;12:CD004388 | CrossRef | PubMed |
Martí-Carvajal AJ, Solà I, Gluud C, Lathyris D, Cardona AF. Human recombinant protein C for severe sepsis and septic shock in adult and paediatric patients. Cochrane Database Syst Rev. 2012 Dec 12;12:CD004388 | CrossRef | PubMed | Sashegyi A, Trzaskoma BL, Nelson DR, Williams MD, Macias W. International INtegrated Database for the Evaluation of severe sePsis and drotrecogin alfa (activated) THerapy: component trials and statistical methods for INDEPTH. Curr Med Res Opin. 2006 May;22(5):1001-12 | PubMed |
Sashegyi A, Trzaskoma BL, Nelson DR, Williams MD, Macias W. International INtegrated Database for the Evaluation of severe sePsis and drotrecogin alfa (activated) THerapy: component trials and statistical methods for INDEPTH. Curr Med Res Opin. 2006 May;22(5):1001-12 | PubMed | Wiedermann CJ, Kaneider NC. A meta-analysis of controlled trials of recombinant human activated protein C therapy in patients with sepsis. BMC Emerg Med. 2005 Oct 14;5:7 | PubMed |
Wiedermann CJ, Kaneider NC. A meta-analysis of controlled trials of recombinant human activated protein C therapy in patients with sepsis. BMC Emerg Med. 2005 Oct 14;5:7 | PubMed | Abraham E, Laterre PF, Garg R, Levy H, Talwar D, Trzaskoma BL, François B, Guy JS, Brückmann M, Rea-Neto A, Rossaint R, Perrotin D, Sablotzki A, Arkins N, Utterback BG, Macias WL; Administration of Drotrecogin Alfa (Activated) in Early Stage Severe Sepsis (ADDRESS) Study Group.. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. N Engl J Med. 2005 Sep 29;353(13):1332-41 | PubMed |
Abraham E, Laterre PF, Garg R, Levy H, Talwar D, Trzaskoma BL, François B, Guy JS, Brückmann M, Rea-Neto A, Rossaint R, Perrotin D, Sablotzki A, Arkins N, Utterback BG, Macias WL; Administration of Drotrecogin Alfa (Activated) in Early Stage Severe Sepsis (ADDRESS) Study Group.. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. N Engl J Med. 2005 Sep 29;353(13):1332-41 | PubMed | Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ Jr; Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group.. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001 Mar 8;344(10):699-709 | PubMed |
Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ Jr; Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group.. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001 Mar 8;344(10):699-709 | PubMed | Bernard GR, Ely EW, Wright TJ, Fraiz J, Stasek JE Jr, Russell JA, Mayers I, Rosenfeld BA, Morris PE, Yan SB, Helterbrand JD. Safety and dose relationship of recombinant human activated protein C for coagulopathy in severe sepsis. Crit Care Med. 2001 Nov;29(11):2051-9 | PubMed |
Bernard GR, Ely EW, Wright TJ, Fraiz J, Stasek JE Jr, Russell JA, Mayers I, Rosenfeld BA, Morris PE, Yan SB, Helterbrand JD. Safety and dose relationship of recombinant human activated protein C for coagulopathy in severe sepsis. Crit Care Med. 2001 Nov;29(11):2051-9 | PubMed | Dhainaut JF, Antonelli M, Wright P, Desachy A, Reignier J, Lavoue S, Charpentier J, Belger M, Cobas-Meyer M, Maier C, Mignini MA, Janes J. Extended drotrecogin alfa (activated) treatment in patients with prolonged septic shock. Intensive Care Med. 2009 Jul;35(7):1187-95 | CrossRef | PubMed |
Dhainaut JF, Antonelli M, Wright P, Desachy A, Reignier J, Lavoue S, Charpentier J, Belger M, Cobas-Meyer M, Maier C, Mignini MA, Janes J. Extended drotrecogin alfa (activated) treatment in patients with prolonged septic shock. Intensive Care Med. 2009 Jul;35(7):1187-95 | CrossRef | PubMed | Nadel S, Goldstein B, Williams MD, Dalton H, Peters M, Macias WL, Abd-Allah SA, Levy H, Angle R, Wang D, Sundin DP, Giroir B; REsearching severe Sepsis and Organ dysfunction in children: a gLobal perspective (RESOLVE) study group. Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet. 2007 Mar 10;369(9564):836-43 | PubMed |
Nadel S, Goldstein B, Williams MD, Dalton H, Peters M, Macias WL, Abd-Allah SA, Levy H, Angle R, Wang D, Sundin DP, Giroir B; REsearching severe Sepsis and Organ dysfunction in children: a gLobal perspective (RESOLVE) study group. Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet. 2007 Mar 10;369(9564):836-43 | PubMed | PROWESS SHOCK Steering Committee., Thompson BT, Ranieri VM, Finfer S, Barie PS, Dhainaut JF, Douglas IS, Gårdlund B, Marshall JC, Rhodes A. Statistical analysis plan of PROWESS SHOCK study. Intensive Care Med. 2010 Nov;36(11):1972-3. | CrossRef | PubMed |
PROWESS SHOCK Steering Committee., Thompson BT, Ranieri VM, Finfer S, Barie PS, Dhainaut JF, Douglas IS, Gårdlund B, Marshall JC, Rhodes A. Statistical analysis plan of PROWESS SHOCK study. Intensive Care Med. 2010 Nov;36(11):1972-3. | CrossRef | PubMed | Martin G, Brunkhorst FM, Janes JM, Reinhart K, Sundin DP, Garnett K, Beale R. The international PROGRESS registry of patients with severe sepsis: drotrecogin alfa (activated) use and patient outcomes. Crit Care. 2009;13(3):R103 | CrossRef | PubMed |
Martin G, Brunkhorst FM, Janes JM, Reinhart K, Sundin DP, Garnett K, Beale R. The international PROGRESS registry of patients with severe sepsis: drotrecogin alfa (activated) use and patient outcomes. Crit Care. 2009;13(3):R103 | CrossRef | PubMed | Lindenauer PK, Rothberg MB, Nathanson BH, Pekow PS, Steingrub JS. Activated protein C and hospital mortality in septic shock: a propensity-matched analysis. Crit Care Med. 2010 Apr;38(4):1101-7 | CrossRef | PubMed |
Lindenauer PK, Rothberg MB, Nathanson BH, Pekow PS, Steingrub JS. Activated protein C and hospital mortality in septic shock: a propensity-matched analysis. Crit Care Med. 2010 Apr;38(4):1101-7 | CrossRef | PubMed | Kanji S, Perreault MM, Chant C, Williamson D, Burry L. Evaluating the use of Drotrecogin alfa (activated) in adult severe sepsis: a Canadian multicenter observational study. Intensive Care Med. 2007 Mar;33(3):517-23 | PubMed |
Kanji S, Perreault MM, Chant C, Williamson D, Burry L. Evaluating the use of Drotrecogin alfa (activated) in adult severe sepsis: a Canadian multicenter observational study. Intensive Care Med. 2007 Mar;33(3):517-23 | PubMed | Decruyenaere J, De Backer D, Spapen H, Laterre PF, Raemaekers J, Rogiers P, Trine H, Sartral M, Haentjens T, Wagner T. 90-day follow-up of patients treated with Drotrecogin Alfa (activated) for severe sepsis: a Belgian open label study. Acta Clin Belg. 2009 Jan-Feb;64(1):16-22 | PubMed |
Decruyenaere J, De Backer D, Spapen H, Laterre PF, Raemaekers J, Rogiers P, Trine H, Sartral M, Haentjens T, Wagner T. 90-day follow-up of patients treated with Drotrecogin Alfa (activated) for severe sepsis: a Belgian open label study. Acta Clin Belg. 2009 Jan-Feb;64(1):16-22 | PubMed | Hecksher CA, Lacerda HR, Maciel MA. Characteristics and outcomes of patients treated with drotrecogin alpha and other interventions of the "Surviving Sepsis" campaign in clinical practice. Rev Bras Ter Intensiva. 2008 Jun;20(2):135-43. | PubMed |
Hecksher CA, Lacerda HR, Maciel MA. Characteristics and outcomes of patients treated with drotrecogin alpha and other interventions of the "Surviving Sepsis" campaign in clinical practice. Rev Bras Ter Intensiva. 2008 Jun;20(2):135-43. | PubMed | Gentry CA, Gross KB, Sud B, Drevets DA. Adverse outcomes associated with the use of drotrecogin alfa (activated) in patients with severe sepsis and baseline bleeding precautions. Crit Care Med. 2009 Jan;37(1):19-25 | CrossRef | PubMed |
Gentry CA, Gross KB, Sud B, Drevets DA. Adverse outcomes associated with the use of drotrecogin alfa (activated) in patients with severe sepsis and baseline bleeding precautions. Crit Care Med. 2009 Jan;37(1):19-25 | CrossRef | PubMed | Bertolini G, Rossi C, Anghileri A, Livigni S, Addis A, Poole D. Use of Drotrecogin alfa (activated) in Italian intensive care units: the results of a nationwide survey. Intensive Care Med. 2007 Mar;33(3):426-34 | PubMed |
Bertolini G, Rossi C, Anghileri A, Livigni S, Addis A, Poole D. Use of Drotrecogin alfa (activated) in Italian intensive care units: the results of a nationwide survey. Intensive Care Med. 2007 Mar;33(3):426-34 | PubMed | Anger KE, Degrado JR, Greenwood BC, Cohen SA, Szumita PM. Evaluation of recombinant activated protein C for severe sepsis at a tertiary academic medical center. Therapeutics and clinical risk management. 2013;9:277-84. | Link |
Anger KE, Degrado JR, Greenwood BC, Cohen SA, Szumita PM. Evaluation of recombinant activated protein C for severe sepsis at a tertiary academic medical center. Therapeutics and clinical risk management. 2013;9:277-84. | Link | Bollinger J, Coley K, Rea R, Saul M. Outcomes in patients with recombinant human activated protein c at an academic medical center versus the ENHANCE US trial. Crit Care Med 2006; 34: A111. | Link |
Bollinger J, Coley K, Rea R, Saul M. Outcomes in patients with recombinant human activated protein c at an academic medical center versus the ENHANCE US trial. Crit Care Med 2006; 34: A111. | Link | Wheeler A, Steingrub J, Schmidt GA, Sanchez P, Jacobi J, Linde-Zwirble W, Bates B, Qualy RL, Woodward B, Zeckel M. A retrospective observational study of drotrecogin alfa (activated) in adults with severe sepsis: comparison with a controlled clinical trial. Crit Care Med. 2008 Jan;36(1):14-23 | PubMed |
Wheeler A, Steingrub J, Schmidt GA, Sanchez P, Jacobi J, Linde-Zwirble W, Bates B, Qualy RL, Woodward B, Zeckel M. A retrospective observational study of drotrecogin alfa (activated) in adults with severe sepsis: comparison with a controlled clinical trial. Crit Care Med. 2008 Jan;36(1):14-23 | PubMed | Spriet I, Meersseman W, Wilmer A, Meyfroidt G, Casteels M, Willems L. Evaluation of drotrecogin alpha use in a Belgian university hospital. Pharm World Sci. 2006 Oct;28(5):290-5 | PubMed |
Spriet I, Meersseman W, Wilmer A, Meyfroidt G, Casteels M, Willems L. Evaluation of drotrecogin alpha use in a Belgian university hospital. Pharm World Sci. 2006 Oct;28(5):290-5 | PubMed | Rowan KM, Welch CA, North E, Harrison DA. Drotrecogin alfa (activated): real-life use and outcomes for the UK. Crit Care. 2008;12(2):R58 | CrossRef | PubMed |
Rowan KM, Welch CA, North E, Harrison DA. Drotrecogin alfa (activated): real-life use and outcomes for the UK. Crit Care. 2008;12(2):R58 | CrossRef | PubMed | Higgins TL, Steingrub JS, Tereso GJ, Tidswell MA, McGee WT. Drotrecogin alfa (activated) in sepsis: initial experience with patient selection, cost, and clinical outcomes. J Intensive Care Med. 2005 Nov-Dec;20(6):339-45 | PubMed |
Higgins TL, Steingrub JS, Tereso GJ, Tidswell MA, McGee WT. Drotrecogin alfa (activated) in sepsis: initial experience with patient selection, cost, and clinical outcomes. J Intensive Care Med. 2005 Nov-Dec;20(6):339-45 | PubMed | Ridley S, Lwin A, Wyncoll D, Lippett S, Watson D, Gunning K, Higgins D. Drotrecogin alfa (activated): diffusion from clinical trials to clinical practice. Eur J Anaesthesiol. 2008 Mar;25(3):211-6 | PubMed |
Ridley S, Lwin A, Wyncoll D, Lippett S, Watson D, Gunning K, Higgins D. Drotrecogin alfa (activated): diffusion from clinical trials to clinical practice. Eur J Anaesthesiol. 2008 Mar;25(3):211-6 | PubMed | Sadaka F, O'Brien J, Migneron M, Stortz J, Vanston A, Taylor RW. Activated protein C in septic shock: a propensity-matched analysis. Crit Care. 2011;15(2):R89 | CrossRef | PubMed |
Sadaka F, O'Brien J, Migneron M, Stortz J, Vanston A, Taylor RW. Activated protein C in septic shock: a propensity-matched analysis. Crit Care. 2011;15(2):R89 | CrossRef | PubMed | Ferrer R, Artigas A, Suarez D, Palencia E, Levy MM, Arenzana A, Pérez XL, Sirvent JM; Edusepsis Study Group.. Effectiveness of treatments for severe sepsis: a prospective, multicenter, observational study. Am J Respir Crit Care Med. 2009 Nov 1;180(9):861-6 | CrossRef | PubMed |
Ferrer R, Artigas A, Suarez D, Palencia E, Levy MM, Arenzana A, Pérez XL, Sirvent JM; Edusepsis Study Group.. Effectiveness of treatments for severe sepsis: a prospective, multicenter, observational study. Am J Respir Crit Care Med. 2009 Nov 1;180(9):861-6 | CrossRef | PubMed | Kübler A, Mayzner-Zawadzka E, Durek G, Gaszynski W, Karpel E, Mikaszewska-Sokolewicz M, Majak P. Results of severe sepsis treatment program using recombinant human activated protein C in Poland. Med Sci Monit. 2006 Mar;12(3):CR107-12 | PubMed |
Kübler A, Mayzner-Zawadzka E, Durek G, Gaszynski W, Karpel E, Mikaszewska-Sokolewicz M, Majak P. Results of severe sepsis treatment program using recombinant human activated protein C in Poland. Med Sci Monit. 2006 Mar;12(3):CR107-12 | PubMed | Maurice A, Seguin P, Aguillon D, Chanavaz C, Mallédant Y. [Activated protein C treatment: experience about 23 patients in the operative period]. Ann Fr Anesth Reanim. 2005 Apr;24(4):343-6 | PubMed |
Maurice A, Seguin P, Aguillon D, Chanavaz C, Mallédant Y. [Activated protein C treatment: experience about 23 patients in the operative period]. Ann Fr Anesth Reanim. 2005 Apr;24(4):343-6 | PubMed | Dhainaut JF, Payet S, Vallet B, França LR, Annane D, Bollaert PE, Le Tulzo Y, Runge I, Malledant Y, Guidet B, Le Lay K, Launois R; PREMISS Study Group. Cost-effectiveness of activated protein C in real-life clinical practice. Crit Care. 2007;11(5):R99 | PubMed |
Dhainaut JF, Payet S, Vallet B, França LR, Annane D, Bollaert PE, Le Tulzo Y, Runge I, Malledant Y, Guidet B, Le Lay K, Launois R; PREMISS Study Group. Cost-effectiveness of activated protein C in real-life clinical practice. Crit Care. 2007;11(5):R99 | PubMed | Vincent JL, Bernard GR, Beale R, Doig C, Putensen C, Dhainaut JF, Artigas A, Fumagalli R, Macias W, Wright T, Wong K, Sundin DP, Turlo MA, Janes J. Drotrecogin alfa (activated) treatment in severe sepsis from the global open-label trial ENHANCE: further evidence for survival and safety and implications for early treatment. Crit Care Med. 2005 Oct;33(10):2266-77 | PubMed |
Vincent JL, Bernard GR, Beale R, Doig C, Putensen C, Dhainaut JF, Artigas A, Fumagalli R, Macias W, Wright T, Wong K, Sundin DP, Turlo MA, Janes J. Drotrecogin alfa (activated) treatment in severe sepsis from the global open-label trial ENHANCE: further evidence for survival and safety and implications for early treatment. Crit Care Med. 2005 Oct;33(10):2266-77 | PubMed | Vincent JL, Laterre PF, Decruyenaere J, Spapen H, Raemaekers J, Damas F, Rogiers P, Sartral M, Haentjens T, Nelson D, Janes J. A registry of patients treated with drotrecogin alfa (activated) in Belgian intensive care units an observational study. Acta Clin Belg. 2008 Jan-Feb;63(1):25-30 | PubMed |
Vincent JL, Laterre PF, Decruyenaere J, Spapen H, Raemaekers J, Damas F, Rogiers P, Sartral M, Haentjens T, Nelson D, Janes J. A registry of patients treated with drotrecogin alfa (activated) in Belgian intensive care units an observational study. Acta Clin Belg. 2008 Jan-Feb;63(1):25-30 | PubMed | Ernst FR, Johnston JA, Pulgar S, He J, Ball DE, Young JK, Cooper LM. Timing of drotrecogin alfa (activated) initiation in treatment of severe sepsis: a database cohort study of hospital mortality, length of stay, and costs. Curr Med Res Opin. 2007 Jan;23(1):235-44 | PubMed |
Ernst FR, Johnston JA, Pulgar S, He J, Ball DE, Young JK, Cooper LM. Timing of drotrecogin alfa (activated) initiation in treatment of severe sepsis: a database cohort study of hospital mortality, length of stay, and costs. Curr Med Res Opin. 2007 Jan;23(1):235-44 | PubMed | Eli Lilly. An open label study of drotrecogin alfa (activated) in adult patients with severe sepsis and multiple organ dysfunctions, a phase IV protocol. 2006; Summary ID 7159: 1–6. | Link |
Eli Lilly. An open label study of drotrecogin alfa (activated) in adult patients with severe sepsis and multiple organ dysfunctions, a phase IV protocol. 2006; Summary ID 7159: 1–6. | Link | Macchiavello L, Ellis G, Bowden S, Smithies M. A large, single-centre UK registry of drotrecogin alfa-activated use. Crit Care 2007; 11 (suppl 2): 58. | Link |
Macchiavello L, Ellis G, Bowden S, Smithies M. A large, single-centre UK registry of drotrecogin alfa-activated use. Crit Care 2007; 11 (suppl 2): 58. | Link | Steingrub JS, Cheatham ML, Woodward B, Wang HT, Effron MB; XEUS Investigators.. A prospective, observational study of Xigris Use in the United States (XEUS). J Crit Care. 2010 Dec;25(4):660.e9-16 | CrossRef | PubMed |
Steingrub JS, Cheatham ML, Woodward B, Wang HT, Effron MB; XEUS Investigators.. A prospective, observational study of Xigris Use in the United States (XEUS). J Crit Care. 2010 Dec;25(4):660.e9-16 | CrossRef | PubMed | Bernard GR, Vincent JL, Laterre PF, et al, and the Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group. Effi cacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 2001; 344: 699–709. | CrossRef | PubMed | Link |
Bernard GR, Vincent JL, Laterre PF, et al, and the Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group. Effi cacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 2001; 344: 699–709. | CrossRef | PubMed | Link | Bernard GR, Macias WL, Joyce DE,Williams MD, Bailey J, Vincent JL. Safety assessment of drotrecogin alfa (activated) in the treatment of adult patients with severe sepsis. Critical Care 2003;7(2):155–63. | PubMed |
Bernard GR, Macias WL, Joyce DE,Williams MD, Bailey J, Vincent JL. Safety assessment of drotrecogin alfa (activated) in the treatment of adult patients with severe sepsis. Critical Care 2003;7(2):155–63. | PubMed | Bernard G, Margolis B, Shanies H, Ely E, Wheeler A, Levy H. Efficacy and safety of References 66 drotrecogin alfa (activated) in the treatment of adult patients with severe sepsis: report from a single open-label trial in the United States 2002. | Link |
Bernard G, Margolis B, Shanies H, Ely E, Wheeler A, Levy H. Efficacy and safety of References 66 drotrecogin alfa (activated) in the treatment of adult patients with severe sepsis: report from a single open-label trial in the United States 2002. | Link | Eli Lilly. Xigris NICE submission: drotrecogin alfa (activated) for severe sepsis. Basingstoke: Eli Lilly; 2003. | Link |
Eli Lilly. Xigris NICE submission: drotrecogin alfa (activated) for severe sepsis. Basingstoke: Eli Lilly; 2003. | Link |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








