Abstract
The treatment of polymyositis is based on corticosteroid therapy, with addition of azathioprine for non responsive cases or as an attempt to diminish corticosteroids requirements. However, there is no clear evidence of its benefit in controlling symptoms. Searching in Epistemonikos database, which is maintained by screening 30 databases, we identified only one systematic review including one pertinent randomized trial. We generated a summary of findings following the GRADE approach. We concluded there is uncertainty if azathioprine improves or not muscular strength in polymyositis because the certainty of the evidence is very low.
Problem
Corticosteroids constitute the standard treatment for dermatomyositis and polymyositis. Given their multiple short and long term side effects there is interest in other immunomodulators, such as azathioprine, especially for non-responsive cases or as an attempt to diminish corticosteroids requirements. However, there is no clear evidence of its benefit in controlling symptoms, and it also carry important side effects.
Methods
We used Epistemonikos database, which is maintained by screening more than 30 databases, to identify systematic reviews and their included primary studies. With this information we generated a structured summary using a pre-established format, which includes key messages, a summary of the body of evidence (presented as an evidence matrix in Epistemonikos), a summary of findings table following the GRADE approach and a table of other considerations for decision-making.
|
Key messages
|
About the body of evidence for this question
|
What is the evidence. |
We found one systematic review [1] including only one pertinent randomized controlled trial [2]. |
|
What types of patients were included |
The study included adults with polymyositis diagnosed with Bohan and Peter criteria and positive muscle biopsy Patients with previous corticosteroid or immunosuppresive therapy were excluded. |
|
What types of interventions were included |
Azathioprine 2mg/kg qd plus prednisone 60 mg qd, compared with prednisone 60 mg qd. |
|
What types of outcomes were measured |
Improvement in muscular strength evaluated in each muscular group (score from 0= no deficit, to -140= maximum deficit), serum creatine kinase, muscular biopsy (0= normal, to 36= maximum inflammation). |
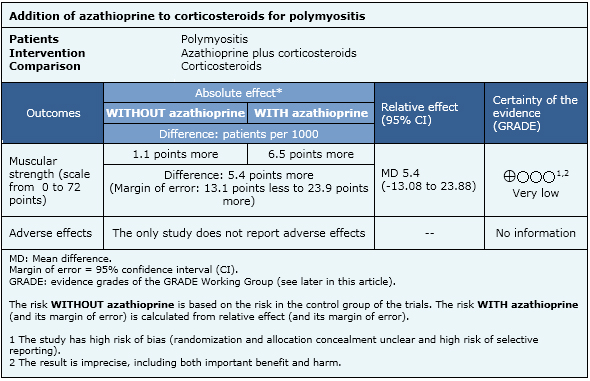
Summary of findings
The information on the effects of azathioprine is based on one randomized controlled trial [2] including 16 patients.
- There is uncertainty if azathioprine improves or not muscular strength in polymyositis. The certainty of the evidence is very low.

Other considerations for decision-making
|
To whom this evidence does and does not apply |
|
| About the outcomes included in this summary |
|
| Balance between benefits and risks, and certainty of the evidence |
|
| What would patients and their doctors think about this intervention |
|
| Resource considerations |
|
|
Differences between this summary and other sources |
|
| Could this evidence change in the future? |
|
How we conducted this summary
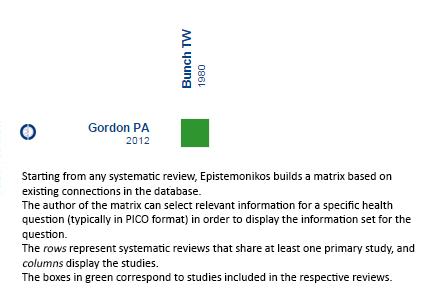
Using automated and collaborative means, we compiled all the relevant evidence for the question of interest and we present it as a matrix of evidence.
Matrix of evidence (static version)
Follow the link to access the interactive version Corticosteroids plus azathioprine versus corticosteroids alone for polymyositis
Notes
The upper portion of the matrix of evidence will display a warning of “new evidence” if new systematic reviews are published after the publication of this summary. Even though the project considers the periodical update of these summaries, users are invited to comment in Medwave or to contact the authors through email if they find new evidence and the summary should be updated earlier. After creating an account in Epistemonikos, users will be able to save the matrixes and to receive automated notifications any time new evidence potentially relevant for the question appears.
The details about the methods used to produce these summaries are described here http://dx.doi.org/10.5867/medwave.2014.06.5997.
Epistemonikos foundation is a non-for-profit organization aiming to bring information closer to health decision-makers with technology. Its main development is Epistemonikos database (www.epistemonikos.org).
These summaries follow a rigorous process of internal peer review.
Conflicts of interest
The authors do not have relevant interests to declare.

 Matrix of evidence (static version)
Matrix of evidence (static version)
 Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.
Esta obra de Medwave está bajo una licencia Creative Commons Atribución-NoComercial 3.0 Unported. Esta licencia permite el uso, distribución y reproducción del artículo en cualquier medio, siempre y cuando se otorgue el crédito correspondiente al autor del artículo y al medio en que se publica, en este caso, Medwave.

El tratamiento de la polimiositis se basa en el uso de corticoides, con adición de azatioprina en casos de difícil manejo o como medida para disminuir la dosis de corticoides, si bien no existe evidencia clara de su beneficio en el control de síntomas. Utilizando la base de datos Epistemonikos, la cual es mantenida mediante búsquedas en 30 bases de datos, identificamos 1 revisión sistemática que incluye sólo un estudio controlado aleatorizado pertinente. Realizamos una tabla de resumen de los resultados utilizando el método GRADE. Se concluye que existe incertidumbre sobre si agregar azatioprina mejora o no la fuerza muscular en la polimiositis porque la certeza de la evidencia es muy baja.
 Authors:
Cristina Meneses[1,2], Gabriel Rada[1,2,3,4,5,6]
Authors:
Cristina Meneses[1,2], Gabriel Rada[1,2,3,4,5,6]
Affiliation:
[1] Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
[2] Proyecto Epistemonikos
[3] Programa de Salud Basada en Evidencia, Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
[4] Departamento de Medicina Interna, Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
[5] GRADE working group
[6] The Cochrane Collaboration
E-mail: radagabriel@epistemonikos.org

Citation: Meneses C, Rada G. What are the effects of adding azathioprine to corticosteroids in polymyositis?. Medwave 2015 Jul;15(Suppl 1):e6179 doi: 10.5867/medwave.2015.6179
Publication date: 8/7/2015

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Gordon PA, Winer JB, Hoogendijk JE, Choy EH. Immunosuppressant and immunomodulatory treatment for dermatomyositis and polymyositis. Cochrane Database Syst Rev. 2012 Aug 5;8:CD003643. | CrossRef | PubMed |
- Bunch TW, Worthington JW, Combs JJ, Ilstrup DM, Engel AG. Azathioprine with prednisone for polymyositis. A controlled, clinical trial. Ann Intern Med. 1980 Mar;92(3):365-9. | PubMed |
 Gordon PA, Winer JB, Hoogendijk JE, Choy EH. Immunosuppressant and immunomodulatory treatment for dermatomyositis and polymyositis. Cochrane Database Syst Rev. 2012 Aug 5;8:CD003643. | CrossRef | PubMed |
Gordon PA, Winer JB, Hoogendijk JE, Choy EH. Immunosuppressant and immunomodulatory treatment for dermatomyositis and polymyositis. Cochrane Database Syst Rev. 2012 Aug 5;8:CD003643. | CrossRef | PubMed | Bunch TW, Worthington JW, Combs JJ, Ilstrup DM, Engel AG. Azathioprine with prednisone for polymyositis. A controlled, clinical trial. Ann Intern Med. 1980 Mar;92(3):365-9. | PubMed |
Bunch TW, Worthington JW, Combs JJ, Ilstrup DM, Engel AG. Azathioprine with prednisone for polymyositis. A controlled, clinical trial. Ann Intern Med. 1980 Mar;92(3):365-9. | PubMed |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis








