Key Words: Covid-19, Pandemics, Coronavirus-2019-nCoV, Coronavirus infection, Newborn, Infants
Abstract
Introduction
COVID-19 disease affects newborns, but its middle and long-term effects are still unclear.
Objective
To describe the clinical and epidemiological characteristics and follow-up of newborns infected with SARS-CoV-2.
Methods
An observational and descriptive study. We included newborns with SARS-CoV-2 positive RT-PCR born from SARS-CoV-2 seropositive mothers. Delivery and newborn care were provided at the 'Instituto Nacional Materno Perinatal' from Peru between June 1 and September 30, 2020. Perinatal information was collected from medical records. Remote follow-up and face-to-face evaluations gathered epidemiological and clinical information, in addition to serological and RT-PCR tests for SARS-CoV-2. Descriptive statistics were used for analysis.
Results
During the study period, 4733 neonates were born at the institution. We found that 1488 (31.4%) were born from seropositive for SARS-CoV-2 mothers. Finally, we included the 34 (2.3%) newborns with positive RT-PCR for SARS-CoV-2. Regarding the included newborns, 29.4% were delivered by cesarean section, 26.5% had low birth weight, 11.8% were preterm, 26.5% were hospitalized, and one died. Twenty-eight had a remote follow-up, and 18 also had a face-to-face follow-up. A total of 64.3% were exclusively breastfed, 28.6% were mixed breastfed, and 7.1% used a substitute formula. The face-to-face evaluation was performed between one and four months of chronological age. We found that 100% had negative control RT-PCR test for COVID-19, 38.9% had a negative serological test (IgM, IgG), and 61.1% positive IgG.
Conclusions
Neonatal SARS-CoV-2 infection is rare, and most infected infants are asymptomatic. Vaginal delivery, breastfeeding, and joint isolation did not related with complications during hospital care. Infants under remote and in-person follow-up showed favorable clinical evolution during the study period.
|
Main messages
|
Introduction
The SARS-CoV-2 virus pandemic has become the most severe public health emergency globally [1]. Coronavirus disease 2019 (COVID-19) is an infection caused by an emerging virus belonging to the RNA virus group, specifically a βcoronavirus, which is genetically similar to the SARS virus [2],[3]. The World Health Organization (WHO) declared it a pandemic on March 11, 2020. At present, the virus has spread to 213 nations [4],[5].
Early into the pandemic, there was no evidence supporting vertical transmission. Many studies evaluated several samples (i.e., amniotic fluid, umbilical cord blood, pharyngeal swabs in neonates, placental swabs, genital fluid, and breast milk samples) from infected mothers that were negative for the virus [6],[7],[8]. However, one year into the pandemic, evidence of intrauterine transmission was found [9]. Wang et al. confirmed intrauterine SARS-CoV-2 infection through positive RT-PCR detection or sequencing virus genes from the upper and lower respiratory tract, blood, and digestive tract [10].
In general, pediatric patients with COVID-19 experience a mild disease. However, severe symptoms of illness and vomiting in children under one age may warrant care [11]. Little is known about the sequelae of SARS-CoV-2 infection in children hospitalized with COVID-19, and short-term sequelae are rarely reported [12]. The infection of SARS-CoV-2 in newborns is rare despite having a mother with COVID-19 [13].
The literature presents inconclusive evidence on how COVID-19 infection behaves in the perinatal stage and its subsequent evolution [11]. There is substantial evidence of breastfeeding benefits over immunity, which reduces the risk of neonatal infection by other pathogens [14]. Moreover, there is evidence that breastmilk also transmits specific antibodies against coronaviruses that may protect newborns [15].
This study aimed to describe the clinical and epidemiological characteristics in the follow-up of newborns who presented SARS-CoV-2 infection at birth and were attended in a level III hospital in Peru.
Methods
Study design and setting
This is an observational, descriptive, and prospective study. The study was conducted at the 'Instituto Nacional Materno Perinatal' of Lima, Peru. This hospital is a third-level care facility, category III-2, and national reference center.
Due to the COVID-19 pandemic, all pregnant women were admitted through the emergency department, where they underwent a serological test for SARS-CoV-2. In addition, according to medical criteria, pharyngeal and nasal swabs were taken for analysis by real-time reverse transcription-polymerase chain reaction (RT-PCR), either due to epidemiological contact links or negative serological test but suspicious clinical symptoms.
When the pregnant woman had a positive serologic test (Immunoglobulin M, IgM or Immunoglobulin M and G, IgM/ IgG) or positive RT-PCR, she was attended in a separate delivery room or a one-person COVID-19 operation room. After immediate care, the mother and her baby were taken to a COVID-19 isolation room. Each room had a capacity for two mothers, where each bed had a separation of two meters between them, in addition to a mobile separator. If the mother had respiratory symptoms, she was placed in a one-person room. The mothers always wore mask and a face shield.
When the newborn required hospitalization, it was taken to the COVID-19 intensive or intermediate care ward, where it remained in a closed incubator. All neonates born from a seropositive woman or with positive RT-PCR for SARS-CoV-2 were tested for RT-PCR by nasopharyngeal swabbing within 12 to 24 hours of birth. Institutional protocols were followed to reduce the risk of horizontal transmission by implementing isolation areas, intermediate care, and neonatal intensive care units adapted for differentiated care.
Population and sample
The study included newborns with a positive RT-PCR test in respiratory secretion taken through nasopharyngeal swabbing of those mothers with positive serology for SARS-CoV-2. The study was conducted between June 1 and September 30, 2020. The inclusion criteria were:
a) Newborns whose mothers presented anti-SARS-CoV-2 antibodies at hospital admission.
b) Newborns from seropositive for SARS-CoV-2 mothers.
c) Newborns with positive RT-PCR for SARS-CoV-2. Exclusion criteria:
a) Medical history with incomplete maternal variables.
b) Medical history with incomplete neonatal variables.
All data available in the remote and face-to-face evaluations were gathered during follow-up. Infants in remote follow-up who did not attend face-to-face evaluations were not excluded.
Sars-coV-2 detection test
The newborns' biological samples were obtained from pharyngeal swabs and were processed at the 'Instituto Nacional Materno Perinatal' of Peru to identify the SARS-CoV-2 virus by RT-PCR test.
Serological tests were performed with the One Step Test Kit Covid-19. This method is validated by the European Community Commission and simultaneously detects immunoglobulins M and G through a rapid immunochromatographic assay. The RTPCR and serological tests were performed by qualified and trained professional personnel from the 'Instituto Nacional Materno Perinatal'. The health personnel recorded the results on a standard serological and RT-PCR test card from the Epidemiology and Environmental Health office of the same institute.
Variables
We recorded maternal data including age, respiratory symptoms (cough, sore throat, headache, fever, chills and/or nasal congestion) present at hospital admission and/or at the time of delivery, pregnancy complications and type of delivery (vaginal or cesarean section, cesarean section indication). The newborn data included type of hospital admission (intermediate care, intensive care, joint isolation), comorbidities, days of hospitalization, and condition at hospital discharge (dead or alive).
During follow-up, maternal medical history, contacts with COVID-19 infected individuals, infection from family members after returning home, post-discharge infant symptoms, type of feeding (exclusive breastfeeding, mixed breastfeeding, substitute feeding), and vaccinations received were recorded. Anthropometric data, clinical anamnesis, physical examination, RT-PCR test results, and serologic coronavirus test were recorded through a face-to-face evaluation.
Data collection
The rapid serological tests applied to pregnant women, RTPCR tests, and serological tests in newborns were obtained from the standardized test cards of the Epidemiology and Environmental Health office of the 'Instituto Nacional Materno Perinatal'. Data collection on perinatal variables was obtained from medical records. The first stage of the postdischarge follow-up was by telephone, where the newborn’s mother was contacted and invited to participate the face-to-face evaluation. In addition, necessary safeguards were taken to guarantee the anonymity of the participants by coding their identities. Informed consent for participation in this study was obtained from parents.
Statistical analysis
Contingency tables with representations of absolute frequencies and proportional frequencies by column were made. Data were processed in R statistical software version 4.0 and through R studio platform.
Ethics
The research was approved by the Institutional Research Ethics Committee of ‘Instituto Nacional Materno Perinatal’, as stated in the approval report No. 20-11693-1-CIEI/INMP. The corresponding institutional permission was obtained. Informed consent was given to newborns' mothers to include perinatal and post-hospital discharge follow-up data for research purposes and to always safeguard the identity of the participants.
Results
During the study period, 4733 births were attended at this center. Of all births, 1488 (31.4%) had mothers with positive serology for SARS CoV-2. Their newborns were tested by RT-PCR for COVID-19, and we found that 2.3% had a positive result. This latter group of newborns entered the study for perinatal data collection and post-discharge follow-up (Figure 1).
In all, 34 newborns with positive RT-PCR were included in the study. Regarding mother’s age, 18 (52.9%) had between 20 and 34 years, nine (26.5%) between 35 and 43 years, and seven
(20.6%) between 16 and 19 years. Twelve newborns (35.3%) had primiparous mothers.
Figure 1. Flow chart of newborns included in the study.
We found several maternal comorbidities, including preeclampsia, chorioamnionitis, obesity, anemia, oligohydramnios, polyhydramnios, bicornuate uterus, and scabies. Regarding mothers' COVID-19 infection, 10 (29.4%) had direct contact with someone with COVID-19, 27 (79.4%) had positive IgM/IgG, and three (3%) presented respiratory distress as a complication of COVID-19 infection. Twenty-four pregnant women (70.6%) completed gestation by vaginal delivery, and 10 (29.4%) had a cesarean section. The cesarean section was indicated for twin pregnancies, preeclampsia, chorioamnionitis, history of cesarean section, umbilical cord entanglement, bicornuate uterus, prolonged rupture of membranes, funicular dystocia, breech or transverse presentation, and congenital cerebral malformation. Twenty-five mothers (73.5%) were hospitalized for less than three days (Table 1).
cesarean section, umbilical cord entanglement, bicornuate uterus, prolonged rupture of membranes, funicular dystocia, breech or transverse presentation, and congenital cerebral malformation. Twenty-five mothers (73.5%) were hospitalized for less than three days (Table 1).
Among newborns with positive RT-PCR, 18 (52.9%) were female, nine (26.5%) had a low birth weight, four (11.8%) were preterm, and three (8.8%) were macrosomic. Regarding Apgar scores, six (17.6%) had less than seven at one minute and one (3%) at five minutes. Nine newborns (73.5%) had no morbidity and remained in joint isolation for less than 72 hours, and nine (26.5%) were hospitalized due to some pathology. Eleven newborns (32.3%) underwent metabolic screening, and 12 (35.3%) underwent hearing screening. Lastly, 33 newborns (97%) were discharged alive, and one died during hospitalization (Table 2).
Four out of the nine newborns requiring hospitalization due to morbidity were preterms. One of them was a male born from a twin pregnancy of 28 weeks, with a birth weight of 1025 grams with hyaline membrane disease, apnea, jaundice, sepsis, and systemic candidiasis, and remained hospitalized for 65 days. The second baby was male, had 35 weeks of gestation with a weight of 1680 grams, was dysmorphic, had a prenatal ultrasound with a gastrointestinal tract malformation, presented with hyaline membrane disease, thrombocytopenia, and died at 17 hours from birth. The third was preterm of 33 weeks of gestation, weighed 2112 grams, and was diagnosed with transient tachypnea, neonatal sepsis, jaundice, and thrombocytosis. He required hospitalization for seven days. The last preterm was a twin of 34 weeks of gestation, weighing 1366 grams at birth, and presented an Enterobacter spp. sepsis, meningoencephalitis, pneumonia, multifactorial shock, jaundice due to blood group
coagulation disorder, severe anemia and was hospitalized for 45 days.
Of the five other newborns requiring hospitalization, two were born by cesarean section due to fetal distress and congenital brain malformation, respectively. Three were male, one of them had low birth weight, and two had a congenital malformation of the central nervous system (encephalocele and congenital hydrocephalus), which underwent surgery. These latter two patients presented sepsis, one of them due to coagulasenegative staphylococcus, in addition to jaundice and anemia. One other patient presented neonatal sepsis, pneumonia, pulmonary hypertension, chronic lung disease, and anemia. These three infants required hospitalization for 48 to 160 days. The other two patients had hypoglycemia and polycythemia, requiring hospitalization for two to six days
A control RT-PCR test for SARS-CoV-2 was performed in the nine hospitalized newborns. Of these, eight had negative RTPCR for SARS CoV2 at five to ten days of life, and one remained positive on the 11th day and became negative on the 18th day.
Table 1. Maternal characteristics of newborns with COVID-19.
Table 2. Perinatal characteristics of newborns with COVID-19.
Two patients were hospitalized during the remote follow-up period, and three patients could not be reached despite multiple telephone calls. Therefore, 28 patients were reached by telephone. One newborn had a functioning ventriculoperitoneal drainage system. A family member diagnosed with COVID-19 was reported in three cases. A total of 64.3% were exclusively breastfed, 28.6% were mixed breastfed, and 7.1% used a substitute formula.
Within the first week of life, two of the not exclusively breastfed cases presented upper respiratory tract signs of infection, nasal congestion, and fever that reverted without requiring treatment, which may have been related to COVID-19 infection. Two other children receiving mixed feeding developed upper respiratory symptoms for more than 15 days postdischarge, also self-limited. The 18 exclusively breastfed patients had no morbidity during the follow-up period. All mothers reported their infants being asymptomatic at the time of telephone contact (Table 3).
All newborns who were followed remotely were invited for an on-site evaluation. Given mobilization difficulties, the risk of exposure to COVID-19, and because two newborns were in districts far from Lima, only 18 infants between one and four months of age attended the on-site evaluation. All attended newborns were asymptomatic, with normal physical examination and weight curve, and had height and head circumference within appropriate percentiles for their age. We found 83.3% of complete immunizations for their age, and exclusive breastfeeding in 61.1% of the infants. Regarding COVID-19 infection, all infants had a negative control RT-PCR test. The serologic test (IgM, IgG) was negative in 38.9% and IgG positive in 61.1%. In one infant, no serologic test was performed (Table 4).
Table 3. Characterization of newborns with COVID-19 infection at birth, through a remote follow-up.
Discussion
We found that COVID-19 infection in newborns was infrequent (2.3%) and that most newborns with SARS-CoV-2 infection did not present morbidity (73.5%). There was no evidence of perinatal complications related to the route of delivery, skinto-skin contact, breastfeeding, nor in the follow-up of infants who continued breastfeeding. The low prevalence of SARSCoV-2 infection in neonates is consistent with previous studies [13],[16]; however, the clinical presentation is variable [16],[17],[18].
Regarding mothers with positive serology for COVID-19 and their newborns with positive RT-PCR, SARS-CoV-2 infection was not the main indication for cesarean section. Cesarean section was present in 29.4% (10) of the cases, nine for obstetric reasons and one due to SARS-CoV-2 pneumonia. We found that 8.8% (3) had respiratory symptoms of COVID-19, two had upper tract respiratory symptomatology, and only one case of pneumonia. These results align with a multicenter study, which found 26% prevalence of cesarean sections, and that most of them had upper tract respiratory symptoms [19].
We found that 26.5% of newborns with COVID-19 infection presented morbidities, including respiratory symptoms, hematologic disorders, jaundice, sepsis, and congenital malformations. Systematic reviews have reported a higher frequency of morbidity in neonates with COVID-19. Raschetti et al. [17] reported that 55.1% of infected newborns presented symptoms during hospitalization. In another systematic review, Dhir et al. [18] found that 50% of infected neonates presented some symptomatology, being respiratory symptoms the most frequent (70%). The most frequent clinical features found in systematic reviews were respiratory symptoms, and less frequent were lethargy, gastrointestinal symptoms, fever, and neurological symptoms [17],[18]. Differences with our study may be due to the viral load and severity of the maternal condition [8]. Additionally, we found a higher prevalence of congenital malformations (8.8%) than the 1.9% found in a retrospective observational study conducted in the same institution in 2018 [19].
We found 20.6% low birth weight and 11.8% of prematurity, which aligns with a multicenter study that found that children of COVID-19 infected mothers presented a higher risk of preterm delivery [20]. Other studies indicate that infected newborns are mostly asymptomatic and that perinatal outcomes depend mainly on the morbidities associated with their condition, such as prematurity, malformations, among other perinatal infections [13],[21],[22].
Newborns in our study had breastfeeding, skin-to-skin contact, and the mothers followed a protocol of mask usage and hand hygiene measures. These practices are supported by guidelines given by the Spanish society of neonatology, United Nations Children’s Fund (UNICEF), WHO, Pan American Health Organization (PAHO), Iberoamerican Society of Neonatology (SIBEN), and most international organizations [1],[10],[23],[24]. Current evidence mentioned that most neonates born from mothers with COVID-19 that share joint accommodation and breastfeeding practices are not infected within the first month of life follow-up [19]. On the other hand, few reports of children of SARS-CoV-2 infected mothers breastfeeding their newborns report horizontal infection [25]. In our study, follow-up was performed between one and four months, and no complications were reported concerning breastfeeding.
Follow-up studies of newborns born from COVID-19 infected mothers are limited, even more so in newborns with infection confirmed by RT-PCR tests. In our study, 82.4% of newborns with COVID-19 infection identified by RT-PCR were followed up remotely, and 53% of them attended a face-to-face evaluation between one and four months of age. Among the limited evidence on follow-up studies, there are favorable short-term results in asymptomatic newborns who could continue cohousing with their mothers and received breastfeeding [26]. Similar results are confirmed by the present study, even months after hospital discharge.
Routine immunization, follow-up visits of newborns, and early identification of high-risk infants for developmental delay have been affected by the COVID 19 pandemic [27]. Despite limited health care access and pandemic conditions for COVID-19, 83.3% of infants completed immunization for age, and infants who attended face-to-face evaluation were in average growth and developmental condition, with no evidence of any pathology.
No infant presented a positive result in the RT-PCR test during the face-to-face evaluation between one and four months of age. However, 61.1% presented a positive IgG result, and 38.9% had a negative serological result for SARS-CoV-2. In all, a good prognosis was evidenced in infants who had SARS-CoV-2 infection at birth. These findings are in line with other reports, where infants with SARS-CoV-2 infection were asymptomatic or had mild symptoms and a good prognosis at short-term follow-up of less than one month [28]. However, evidence of long-term sequelae is limited [12].
Regarding limitations in the present study, we must consider the low number of participants due to the infrequency of COVID-19 detection in newborns. Despite the follow-up plan, we could not contact all participants. The present study was a prospective follow-up, with newborns entering the study chronologically according to SARS-CoV-2 detection. Therefore, we report results from participants within an age range of one to four months.
Conclusions
This study’s findings suggest that COVID-19 infection in newborns is infrequent (2.3%) and that most infected neonates are asymptomatic. Likewise, the low detection of SARS-CoV-2 suggests that vertical transmission and/or postpartum horizontal transmission considering protective measures is unlikely.
Associated morbidity in newborns is similar to the general neonatal population, with predominantly respiratory symptoms. Vaginal delivery, breastfeeding, and joint isolation in managing the mother-child binomial did not show COVID-19 complications.
Likewise, during a follow-up period, breastfeeding and mother and newborn contact did not report subsequent complications due to COVID-19. Remote follow-up and face-to-face evaluation allowed us to identify the clinical evolution of infants. Its implementation is recommended to contribute to greater adherence to children heath checks and the complete immunizations for the age.
Notes
Contributor roles
CDA, ETM, RPZ, RHP, YEV: conceptualization, data management, research, supervision, data submission, manuscript preparation (development of original draft), writing (revisions and edits), approval of the final version of the article. EMI, DM, MES, JDD, PAO: conceptualization, research, data presentation, writing (revisions and edits), approval of the final version of the article.
Acknowledgments
The authors express their gratitude to the mothers and children who participated in the follow-up. Also, thank the health personnel of the 'Instituto Nacional Materno Perinatal' of Peru.
Instituto Nacional Materno Perinatal: contributed with the clinical field and availability of human resources for this study. Universidad Nacional Federico Villareal: contributed to the availability of human resources during school hours for the present study. Universidad Privada del Norte: contributed with the availability of human resources during school hours for the present study. Universidad Ricardo Palma: contributed with the availability of human resources during school hours for the present study.
Competing interest
The authors completed the ICMJE conflict of interest declaration form and declared that they did not receive funds for this article; they have no financial relationships with organizations that could have an interest in the article published in the last three years and they have no other relationships or activities that could influence the publication of the article.
Funding
The authors declare that they did not receive financial support for this study.
Ethics
The study was conducted following the International Ethical Guidelines for Health-Related Research Involving Human Subjects, Fourth Edition. Geneva: Council for International Organizations of Medical Sciences (CIOMS); 2016. The study protocol was approved by the Institutional Ethics Committee of the 'Instituto Nacional Materno Perinatal' (INMP) of Peru. The protocol obtained permission for data collection under conditions of the current COVID-19 pandemic, and the absence of associated risks for patients. Data confidentiality was safeguarded through a process of anonymization of the database by identity coding.
Provenance and peer review
Not commissioned. Externally peer-reviewed by three reviewers, double-blind.
Language of submission
Spanish.

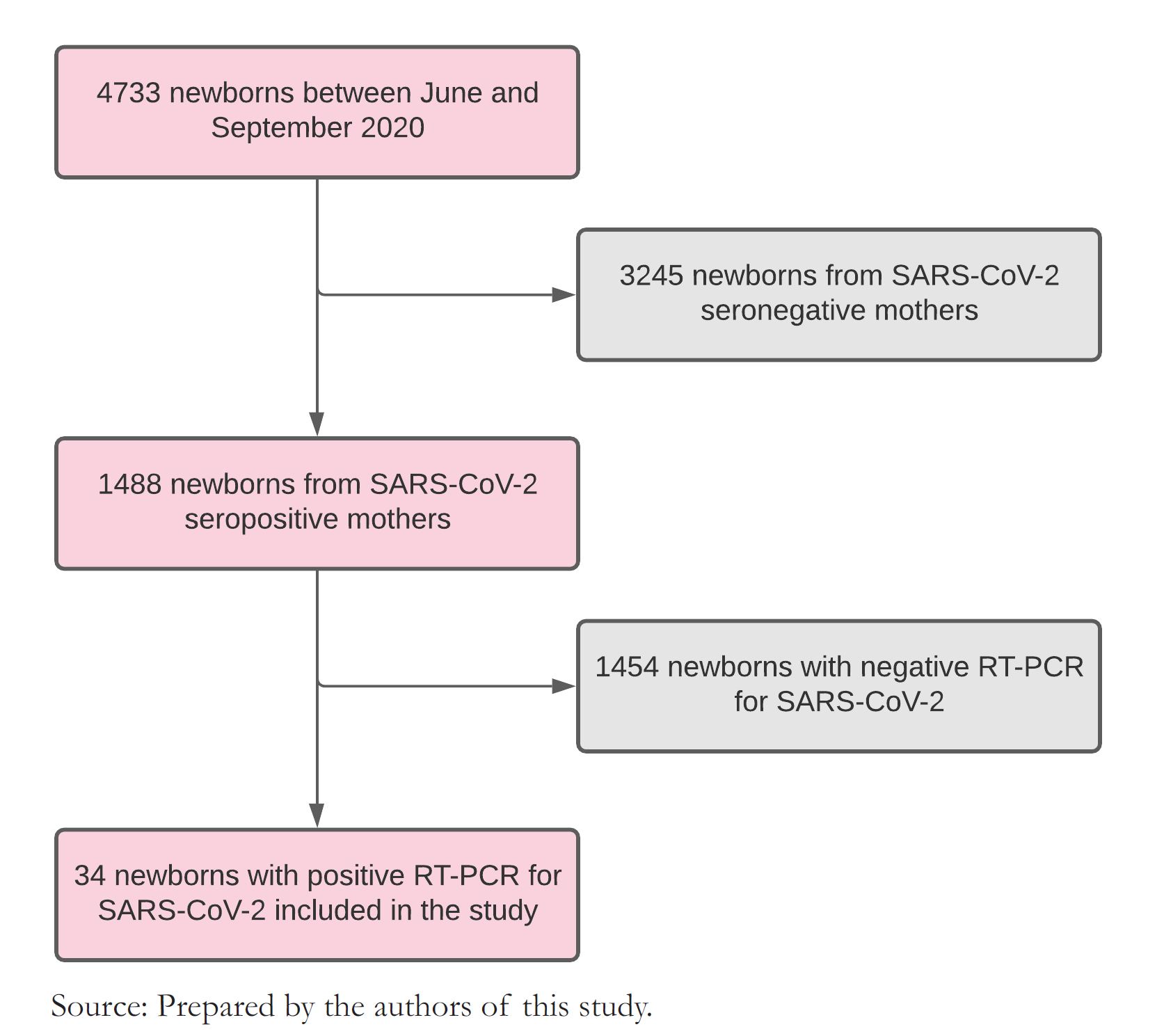 Figure 1. Flow chart of newborns included in the study.
Figure 1. Flow chart of newborns included in the study.

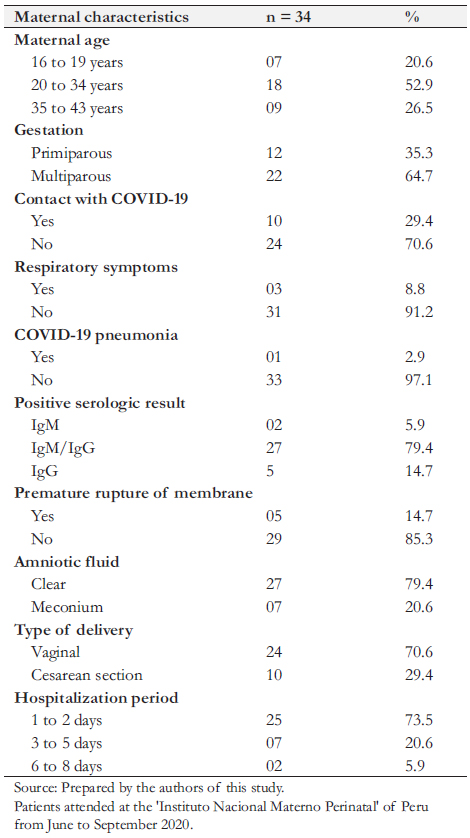 Table 1. Maternal characteristics of newborns with COVID-19.
Table 1. Maternal characteristics of newborns with COVID-19.

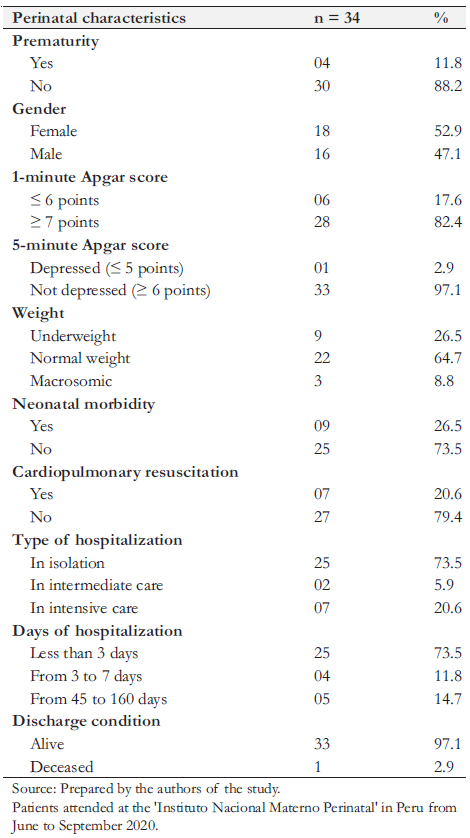 Table 2. Perinatal characteristics of newborns with COVID-19.
Table 2. Perinatal characteristics of newborns with COVID-19.

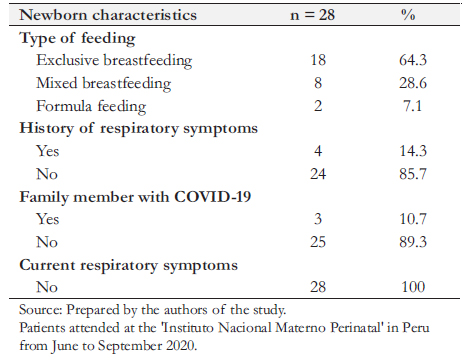 Table 3. Characterization of newborns with COVID-19 infection at birth, through a remote follow-up.
Table 3. Characterization of newborns with COVID-19 infection at birth, through a remote follow-up.

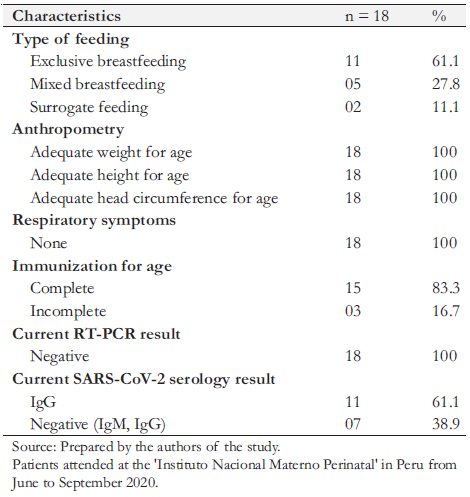 Table 4. Characterization of newborns with COVID-19 infection at birth, through a face-to- face follow-up.
Table 4. Characterization of newborns with COVID-19 infection at birth, through a face-to- face follow-up.

Introducción
La enfermedad por COVID-19 ha sido reportada en recién nacidos; sin embargo, aún no son claros sus efectos en el seguimiento de neonatos.
Objetivo
Describir las características clínicas, epidemiológicas y el seguimiento de recién nacidos infectados con SARS-CoV-2.
Métodos
Estudio observacional y descriptivo. Participaron recién nacidos que tuvieron PCR-TR positivo a SARS-CoV-2, hijos de madres seropositivas a SARS-CoV-2. La atención del parto y del recién nacido fueron en el Instituto Nacional Materno Perinatal de Perú, entre el 1 de junio y el 30 de septiembre de 2020. Se recogió información perinatal de registros médicos. Se realizó seguimiento remoto y evaluación presencial para descripción epidemiológica, clínica y resultados de pruebas serológicas y PCR-TR para SARS-CoV-2. En el análisis se usó estadística descriptiva.
Resultados
Durante el período de estudio nacieron 4733 recién nacidos. De estos niños, 1488 (31,4%) procedieron de gestantes seropositivas a SARS-CoV-2 y de ellos 34 (2,3%) tuvieron PCR-TR positivo a SARS-CoV-2. De los 34 recién nacidos 29,4% nació por cesárea, 26,5% tuvo bajo peso, 11,8% fue prematuro 26,5% tuvo indicación de hospitalización por patología y un neonato falleció. De los 34 neonatos, 28 tuvieron seguimiento remoto y de ellos 18 tuvieron además seguimiento presencial post alta. El 64,3% recibía lactancia materna exclusiva, 28,6% lactancia mixta y 7,1% usaba un sucedáneo. La evaluación presencial se realizó entre uno a cuatro meses de edad cronológica. El 100% tuvo prueba de PCR-TR de control para coronavirus negativa y 38,9% tuvo prueba serológica (IgM, IgG) negativa y 61,1% IgG positiva.
Conclusiones
La infección neonatal por SARS-CoV-2 es poco frecuente, la mayoría de infectados fueron asintomáticos. El parto vaginal, la lactancia materna y aislamiento conjunto no reportaron complicaciones en la evolución durante la atención hospitalaria. Los infantes en seguimiento remoto y presencial mostraron evolución clínica favorable durante el período de estudio.
 Authors:
Carmen Dávila-Aliaga[1,2], Elsa Torres-Marcos[1], Rafael Paucar-Zegarra[1], Rosmary Hinojosa-Pérez[1], Ylia Espinoza-Vivas[1], Elina Mendoza-Ibáñez[1], Diego Marín[1], Marcos Espínola-Sánchez[3,4], Jonathan De la Cruz-Dávila[1], Pedro Arango-Ochante[3,5]
Authors:
Carmen Dávila-Aliaga[1,2], Elsa Torres-Marcos[1], Rafael Paucar-Zegarra[1], Rosmary Hinojosa-Pérez[1], Ylia Espinoza-Vivas[1], Elina Mendoza-Ibáñez[1], Diego Marín[1], Marcos Espínola-Sánchez[3,4], Jonathan De la Cruz-Dávila[1], Pedro Arango-Ochante[3,5]
Affiliation:
[1] Departamento de Neonatología, Instituto Nacional Materno Perinatal, Lima, Perú
[2] Facultad de Medicina Humana, Universidad Nacional Federico Villarreal, Lima, Perú
[3] Unidad de Investigación, Instituto Nacional Materno Perinatal, Lima, Perú
[4] Facultad de ciencias de la salud, Universidad Privada del Norte, Lima, Perú
[5] Instituto de Investigaciones en Ciencia Biomédica, Universidad Ricardo Palma, Lima, Perú
E-mail: davilacarmen@hotmail.com

Citation: Dávila-Aliaga C, Torres-Marcos E, Paucar-Zegarra R, Hinojosa-Pérez R, Espinoza-Vivas Y, Mendoza-Ibáñez E, et al. Clinical and epidemiological characterization in the follow-up of newborns with COVID-19: a descriptive study. Medwave 2021;21(11):e002141 doi: 10.5867/medwave.2021.11.002141
Submission date: 10/6/2021
Acceptance date: 17/11/2021
Publication date: 15/12/2021
Origin: Not commissioned
Type of review: Externally peer-reviewed by three reviewers, double-blind

Comments (0)
We are pleased to have your comment on one of our articles. Your comment will be published as soon as it is posted. However, Medwave reserves the right to remove it later if the editors consider your comment to be: offensive in some sense, irrelevant, trivial, contains grammatical mistakes, contains political harangues, appears to be advertising, contains data from a particular person or suggests the need for changes in practice in terms of diagnostic, preventive or therapeutic interventions, if that evidence has not previously been published in a peer-reviewed journal.
No comments on this article.
To comment please log in
 Medwave provides HTML and PDF download counts as well as other harvested interaction metrics.
Medwave provides HTML and PDF download counts as well as other harvested interaction metrics. There may be a 48-hour delay for most recent metrics to be posted.

- Organization WH. Coronavirus disease 2019 (COVID-19): situation report, 82. 2020 | Link |
- Kallem VR, Sharma D. COVID 19 in neonates. J Matern Fetal Neonatal Med 2020;1–9. | Link |
- Wilde AH de, Snijder EJ, Kikkert M, Hemert MJ van. Host factors in coronavirus replication. Curr Top Microbiol Immunol. 2018; 419:1–42. | CrossRef |
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020; 382(8):727–733. https://doi.org/10.1056/NEJMoa2001017
- Kyle MH, Glassman ME, Khan A, Fernández CR, Hanft E, Emeruwa UN, et al. A review of newborn outcomes during the COVID-19 pandemic. Semin Perinatol. 2020; 44(7):151286. | CrossRef |
- Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. The Lancet. 2020; 395 (10226):809–815. | CrossRef |
- Chen X, Li Y, Wang J, Cai H, Cao H, Sheng J. Pregnant women complicated with COVID-19: a clinical analysis of 3 cases. Univ Med Sci. 2020;49(2):240–244. | CrossRef |
- Walker KF, O'Donoghue K, Grace N, Dorling J, Comeau JL, Li W, et al. Maternal transmission of SARS‐COV‐2 to the neonate, and possible routes for such transmission: a systematic review and critical analysis. BJOG Int J Obstet Gynaecol 2020;127(11):1324–1336. | CrossRef |
- Dong L, Tian J, He S, Zhu C, Wang J, Liu C, et al. Possible Vertical Transmission of SARS-CoV-2 From an Infected Mother to Her Newborn. JAMA. 2020; 323(18): 1846-1848. | CrossRef |
- Wang L, Shi Y, Xiao T, Fu J, Feng X, Mu D, et al. Chinese expert consensus on the perinatal and neonatal management for the prevention and control of the 2019 novel coronavirus infection (First edition). Ann Transl Med. 2020; 8(3):47. | CrossRef |
- Cui X, Zhao Z, Zhang T, Guo W, Guo W, Zheng J, et al. A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19). J Med Virol. 2021; 93(2): 1057-1069. | CrossRef |
- Denina M, Pruccoli G, Scolfaro C, Mignone F, Zoppo M, Giraudo I, et al. Sequelae of COVID-19 in Hospitalized Children: A 4-Months Follow-Up. Pediatr Infect Dis J. 2020; 39(12): e458-e459. | CrossRef |
- Dávila-Aliaga C, Hinojoza-Pérez R, Espinola-Sánchez M, et al. Resultados materno-perinatales en gestantes con COVID-19 en un hospital nivel III del Perú. Rev Peru Med Exp Salud Pública 2021;38(1):58–63. | CrossRef |
- Giuliani C, Li Volsi P, Brun E, Chiambretti A, Giandalia A, Tonutti L, et al. Breastfeeding during the COVID-19 pandemic: Suggestions on behalf of woman study group of AMD. Diabetes Res Clin Pract. 2020; 165: 108239. | CrossRef |
- Barrero-Castillero A, Beam KS, Bernardini LB, Ramos EGC, Davenport PE, Duncan AR, et al. COVID-19: neonatal-perinatal perspectives. J Perinatol. 2021; 41(5): 940-951. | CrossRef |
- Chawla D, Chirla D, Dalwai S, Deorari AK, Ganatra A, Gandhi A, et al. Perinatal-Neonatal Management of COVID-19 Infection - Guidelines of the Federation of Obstetric and Gynaecological Societies of India (FOGSI), National Neonatology Forum of India (NNF), and Indian Academy of Pediatrics (IAP). Indian Pediatr. 2020; 57(6): 536-548. | CrossRef |
- Raschetti R, Vivanti AJ, Vauloup-Fellous C, Loi B, Benachi A, De Luca D. Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat Commun 2020;11(1):5164. | CrossRef |
- Dhir SK, Kumar J, Meena J, Kumar P. Clinical Features and Outcome of SARS-CoV-2 Infection in Neonates: A Systematic Review. J Trop Pediatr 2020; fmaa059. | CrossRef |
- Ayala FD, Guevara E, Carranza C, Luna A, Espinola-Sanchez M, Racchumí A, et al. Factores asociados a malformaciones congénitas. Rev Peru Investig Matern Perinat. 2019;8(4): 30-40. | CrossRef |
- Marín Gabriel MA, Reyne Vergeli M, Caserío Carbonero S, Sole L, Carrizosa Molina T, Rivero Calle I, et al. Maternal, Perinatal and Neonatal Outcomes With COVID-19: A Multicenter Study of 242 Pregnancies and Their 248 Infant Newborns During Their First Month of Life. Pediatr Infect Dis J. 2020; 39(12):e393-e397. | CrossRef |
- Dávila-Aliaga C, Espínola-Sánchez M, Mendoza-Ibáñez E, et al. Perinatal outcomes and serological results in neonates of pregnant women seropositive to SARS-CoV-2: A cross-sectional descriptive study. Medwave 2020;20(11):e8084–e8084. | CrossRef |
- Gale C, Quigley MA, Placzek A, Knight M, Ladhani S, Draper ES, et al. Characteristics and outcomes of neonatal SARS-CoV-2 infection in the UK: a prospective national cohort study using active surveillance. Lancet Child Adolesc Health. 2021; 5(2):113-121. | CrossRef |
- Dumitriu D, Emeruwa UN, Hanft E, Liao GV, Ludwig E, Walzer L, et al. Outcomes of Neonates Born to Mothers With Severe Acute Respiratory Syndrome Coronavirus 2 Infection at a Large Medical Center in New York City. JAMA Pediatr. 2021;175(2):157-167. | CrossRef |
- Asociación Española de Pediatría. Recomendaciones para el manejo del recién nacido en relación con la infección por SARS-CoV-2. 2020 | Link |
- Sola A, Rodríguez S, Cardetti M, Dávila C. COVID-19 perinatal en América Latina. Rev Panam Salud Pública 2020;44:1. | CrossRef |
- Salvatore CM, Han JY, Acker KP, Tiwari P, Jin J, Brandler M, et al. Neonatal management and outcomes during the COVID-19 pandemic: an observation cohort study. Lancet Child Adolesc Health. 2020; 4(10):721-727. | CrossRef |
- Saini L, Madaan P, Bhagwat C, Einspieler C. Home-Videos for Neurodevelopmental Follow-Up of High-Risk Infants during COVID-19 Pandemic: A Simple and Inexpensive Tool. J Trop Pediatr. 2021 Jan 29;67(1): fmaa088. | CrossRef |
- Guo L, Ren L, Yang S, Xiao M, Chang D, Yang F, et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin Infect Dis. 2020; 71(15):778-785. | CrossRef |
 Wilde AH de, Snijder EJ, Kikkert M, Hemert MJ van. Host factors in coronavirus replication. Curr Top Microbiol Immunol. 2018; 419:1–42. | CrossRef |
Wilde AH de, Snijder EJ, Kikkert M, Hemert MJ van. Host factors in coronavirus replication. Curr Top Microbiol Immunol. 2018; 419:1–42. | CrossRef | Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020; 382(8):727–733. https://doi.org/10.1056/NEJMoa2001017
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020; 382(8):727–733. https://doi.org/10.1056/NEJMoa2001017  Kyle MH, Glassman ME, Khan A, Fernández CR, Hanft E, Emeruwa UN, et al. A review of newborn outcomes during the COVID-19 pandemic. Semin Perinatol. 2020; 44(7):151286. | CrossRef |
Kyle MH, Glassman ME, Khan A, Fernández CR, Hanft E, Emeruwa UN, et al. A review of newborn outcomes during the COVID-19 pandemic. Semin Perinatol. 2020; 44(7):151286. | CrossRef | Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. The Lancet. 2020; 395 (10226):809–815. | CrossRef |
Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. The Lancet. 2020; 395 (10226):809–815. | CrossRef | Chen X, Li Y, Wang J, Cai H, Cao H, Sheng J. Pregnant women complicated with COVID-19: a clinical analysis of 3 cases. Univ Med Sci. 2020;49(2):240–244. | CrossRef |
Chen X, Li Y, Wang J, Cai H, Cao H, Sheng J. Pregnant women complicated with COVID-19: a clinical analysis of 3 cases. Univ Med Sci. 2020;49(2):240–244. | CrossRef | Walker KF, O'Donoghue K, Grace N, Dorling J, Comeau JL, Li W, et al. Maternal transmission of SARS‐COV‐2 to the neonate, and possible routes for such transmission: a systematic review and critical analysis. BJOG Int J Obstet Gynaecol 2020;127(11):1324–1336. | CrossRef |
Walker KF, O'Donoghue K, Grace N, Dorling J, Comeau JL, Li W, et al. Maternal transmission of SARS‐COV‐2 to the neonate, and possible routes for such transmission: a systematic review and critical analysis. BJOG Int J Obstet Gynaecol 2020;127(11):1324–1336. | CrossRef | Dong L, Tian J, He S, Zhu C, Wang J, Liu C, et al. Possible Vertical Transmission of SARS-CoV-2 From an Infected Mother to Her Newborn. JAMA. 2020; 323(18): 1846-1848. | CrossRef |
Dong L, Tian J, He S, Zhu C, Wang J, Liu C, et al. Possible Vertical Transmission of SARS-CoV-2 From an Infected Mother to Her Newborn. JAMA. 2020; 323(18): 1846-1848. | CrossRef | Wang L, Shi Y, Xiao T, Fu J, Feng X, Mu D, et al. Chinese expert consensus on the perinatal and neonatal management for the prevention and control of the 2019 novel coronavirus infection (First edition). Ann Transl Med. 2020; 8(3):47. | CrossRef |
Wang L, Shi Y, Xiao T, Fu J, Feng X, Mu D, et al. Chinese expert consensus on the perinatal and neonatal management for the prevention and control of the 2019 novel coronavirus infection (First edition). Ann Transl Med. 2020; 8(3):47. | CrossRef | Cui X, Zhao Z, Zhang T, Guo W, Guo W, Zheng J, et al. A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19). J Med Virol. 2021; 93(2): 1057-1069. | CrossRef |
Cui X, Zhao Z, Zhang T, Guo W, Guo W, Zheng J, et al. A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19). J Med Virol. 2021; 93(2): 1057-1069. | CrossRef | Denina M, Pruccoli G, Scolfaro C, Mignone F, Zoppo M, Giraudo I, et al. Sequelae of COVID-19 in Hospitalized Children: A 4-Months Follow-Up. Pediatr Infect Dis J. 2020; 39(12): e458-e459. | CrossRef |
Denina M, Pruccoli G, Scolfaro C, Mignone F, Zoppo M, Giraudo I, et al. Sequelae of COVID-19 in Hospitalized Children: A 4-Months Follow-Up. Pediatr Infect Dis J. 2020; 39(12): e458-e459. | CrossRef | Dávila-Aliaga C, Hinojoza-Pérez R, Espinola-Sánchez M, et al. Resultados materno-perinatales en gestantes con COVID-19 en un hospital nivel III del Perú. Rev Peru Med Exp Salud Pública 2021;38(1):58–63. | CrossRef |
Dávila-Aliaga C, Hinojoza-Pérez R, Espinola-Sánchez M, et al. Resultados materno-perinatales en gestantes con COVID-19 en un hospital nivel III del Perú. Rev Peru Med Exp Salud Pública 2021;38(1):58–63. | CrossRef | Giuliani C, Li Volsi P, Brun E, Chiambretti A, Giandalia A, Tonutti L, et al. Breastfeeding during the COVID-19 pandemic: Suggestions on behalf of woman study group of AMD. Diabetes Res Clin Pract. 2020; 165: 108239. | CrossRef |
Giuliani C, Li Volsi P, Brun E, Chiambretti A, Giandalia A, Tonutti L, et al. Breastfeeding during the COVID-19 pandemic: Suggestions on behalf of woman study group of AMD. Diabetes Res Clin Pract. 2020; 165: 108239. | CrossRef | Barrero-Castillero A, Beam KS, Bernardini LB, Ramos EGC, Davenport PE, Duncan AR, et al. COVID-19: neonatal-perinatal perspectives. J Perinatol. 2021; 41(5): 940-951. | CrossRef |
Barrero-Castillero A, Beam KS, Bernardini LB, Ramos EGC, Davenport PE, Duncan AR, et al. COVID-19: neonatal-perinatal perspectives. J Perinatol. 2021; 41(5): 940-951. | CrossRef | Chawla D, Chirla D, Dalwai S, Deorari AK, Ganatra A, Gandhi A, et al. Perinatal-Neonatal Management of COVID-19 Infection - Guidelines of the Federation of Obstetric and Gynaecological Societies of India (FOGSI), National Neonatology Forum of India (NNF), and Indian Academy of Pediatrics (IAP). Indian Pediatr. 2020; 57(6): 536-548. | CrossRef |
Chawla D, Chirla D, Dalwai S, Deorari AK, Ganatra A, Gandhi A, et al. Perinatal-Neonatal Management of COVID-19 Infection - Guidelines of the Federation of Obstetric and Gynaecological Societies of India (FOGSI), National Neonatology Forum of India (NNF), and Indian Academy of Pediatrics (IAP). Indian Pediatr. 2020; 57(6): 536-548. | CrossRef | Raschetti R, Vivanti AJ, Vauloup-Fellous C, Loi B, Benachi A, De Luca D. Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat Commun 2020;11(1):5164. | CrossRef |
Raschetti R, Vivanti AJ, Vauloup-Fellous C, Loi B, Benachi A, De Luca D. Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat Commun 2020;11(1):5164. | CrossRef | Dhir SK, Kumar J, Meena J, Kumar P. Clinical Features and Outcome of SARS-CoV-2 Infection in Neonates: A Systematic Review. J Trop Pediatr 2020; fmaa059. | CrossRef |
Dhir SK, Kumar J, Meena J, Kumar P. Clinical Features and Outcome of SARS-CoV-2 Infection in Neonates: A Systematic Review. J Trop Pediatr 2020; fmaa059. | CrossRef | Ayala FD, Guevara E, Carranza C, Luna A, Espinola-Sanchez M, Racchumí A, et al. Factores asociados a malformaciones congénitas. Rev Peru Investig Matern Perinat. 2019;8(4): 30-40. | CrossRef |
Ayala FD, Guevara E, Carranza C, Luna A, Espinola-Sanchez M, Racchumí A, et al. Factores asociados a malformaciones congénitas. Rev Peru Investig Matern Perinat. 2019;8(4): 30-40. | CrossRef | Marín Gabriel MA, Reyne Vergeli M, Caserío Carbonero S, Sole L, Carrizosa Molina T, Rivero Calle I, et al. Maternal, Perinatal and Neonatal Outcomes With COVID-19: A Multicenter Study of 242 Pregnancies and Their 248 Infant Newborns During Their First Month of Life. Pediatr Infect Dis J. 2020; 39(12):e393-e397. | CrossRef |
Marín Gabriel MA, Reyne Vergeli M, Caserío Carbonero S, Sole L, Carrizosa Molina T, Rivero Calle I, et al. Maternal, Perinatal and Neonatal Outcomes With COVID-19: A Multicenter Study of 242 Pregnancies and Their 248 Infant Newborns During Their First Month of Life. Pediatr Infect Dis J. 2020; 39(12):e393-e397. | CrossRef | Dávila-Aliaga C, Espínola-Sánchez M, Mendoza-Ibáñez E, et al. Perinatal outcomes and serological results in neonates of pregnant women seropositive to SARS-CoV-2: A cross-sectional descriptive study. Medwave 2020;20(11):e8084–e8084. | CrossRef |
Dávila-Aliaga C, Espínola-Sánchez M, Mendoza-Ibáñez E, et al. Perinatal outcomes and serological results in neonates of pregnant women seropositive to SARS-CoV-2: A cross-sectional descriptive study. Medwave 2020;20(11):e8084–e8084. | CrossRef | Gale C, Quigley MA, Placzek A, Knight M, Ladhani S, Draper ES, et al. Characteristics and outcomes of neonatal SARS-CoV-2 infection in the UK: a prospective national cohort study using active surveillance. Lancet Child Adolesc Health. 2021; 5(2):113-121. | CrossRef |
Gale C, Quigley MA, Placzek A, Knight M, Ladhani S, Draper ES, et al. Characteristics and outcomes of neonatal SARS-CoV-2 infection in the UK: a prospective national cohort study using active surveillance. Lancet Child Adolesc Health. 2021; 5(2):113-121. | CrossRef | Dumitriu D, Emeruwa UN, Hanft E, Liao GV, Ludwig E, Walzer L, et al. Outcomes of Neonates Born to Mothers With Severe Acute Respiratory Syndrome Coronavirus 2 Infection at a Large Medical Center in New York City. JAMA Pediatr. 2021;175(2):157-167. | CrossRef |
Dumitriu D, Emeruwa UN, Hanft E, Liao GV, Ludwig E, Walzer L, et al. Outcomes of Neonates Born to Mothers With Severe Acute Respiratory Syndrome Coronavirus 2 Infection at a Large Medical Center in New York City. JAMA Pediatr. 2021;175(2):157-167. | CrossRef | Asociación Española de Pediatría. Recomendaciones para el manejo del recién nacido en relación con la infección por SARS-CoV-2. 2020 | Link |
Asociación Española de Pediatría. Recomendaciones para el manejo del recién nacido en relación con la infección por SARS-CoV-2. 2020 | Link | Sola A, Rodríguez S, Cardetti M, Dávila C. COVID-19 perinatal en América Latina. Rev Panam Salud Pública 2020;44:1. | CrossRef |
Sola A, Rodríguez S, Cardetti M, Dávila C. COVID-19 perinatal en América Latina. Rev Panam Salud Pública 2020;44:1. | CrossRef | Salvatore CM, Han JY, Acker KP, Tiwari P, Jin J, Brandler M, et al. Neonatal management and outcomes during the COVID-19 pandemic: an observation cohort study. Lancet Child Adolesc Health. 2020; 4(10):721-727. | CrossRef |
Salvatore CM, Han JY, Acker KP, Tiwari P, Jin J, Brandler M, et al. Neonatal management and outcomes during the COVID-19 pandemic: an observation cohort study. Lancet Child Adolesc Health. 2020; 4(10):721-727. | CrossRef | Saini L, Madaan P, Bhagwat C, Einspieler C. Home-Videos for Neurodevelopmental Follow-Up of High-Risk Infants during COVID-19 Pandemic: A Simple and Inexpensive Tool. J Trop Pediatr. 2021 Jan 29;67(1): fmaa088. | CrossRef |
Saini L, Madaan P, Bhagwat C, Einspieler C. Home-Videos for Neurodevelopmental Follow-Up of High-Risk Infants during COVID-19 Pandemic: A Simple and Inexpensive Tool. J Trop Pediatr. 2021 Jan 29;67(1): fmaa088. | CrossRef | Guo L, Ren L, Yang S, Xiao M, Chang D, Yang F, et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin Infect Dis. 2020; 71(15):778-785. | CrossRef |
Guo L, Ren L, Yang S, Xiao M, Chang D, Yang F, et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin Infect Dis. 2020; 71(15):778-785. | CrossRef |Systematization of initiatives in sexual and reproductive health about good practices criteria in response to the COVID-19 pandemic in primary health care in Chile
Clinical, psychological, social, and family characterization of suicidal behavior in Chilean adolescents: a multiple correspondence analysis









